In Vitro Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Mangifera indica Aqueous Leaf Extract against Multidrug-Resistant Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Plant Material (Mangifera indica) and Chemicals
2.2. Preparation of the M. indica Aqueous Leaf Extract
2.3. Green Synthesis of Silver Nanoparticles (AgNPs) Using M. indica Aqueous Leaf Extract
2.4. Characterization of Green Synthesized Silver Nanoparticles (AgNPs)
2.5. Biochemical Analysis
2.6. Extraction of Enzyme and Determination of Protein Content
2.7. Antioxidant Enzyme Assays
2.7.1. Catalase
2.7.2. Superoxide Dismutase
2.7.3. Peroxidase Activity
2.7.4. Glutathione Reductase
2.7.5. Phenylalanine Ammonia Lyase
2.8. Estimation of Reducing the Sugar
2.9. Estimation of Total Phenols
2.10. Bacterial Strains Used in This Study
2.11. In Vitro Antibiotic Susceptibility Test of M. indica Nano Particles (MNPs) against MDR Bacteria
Disc Diffusion Method
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Green Synthesized AgNPs
3.1.1. The UV-Visible Absorption Spectrum of Green Synthesized AgNPs
3.1.2. AgNPs Particle Size Distribution Study by Particle Size Analyzer (PSA)
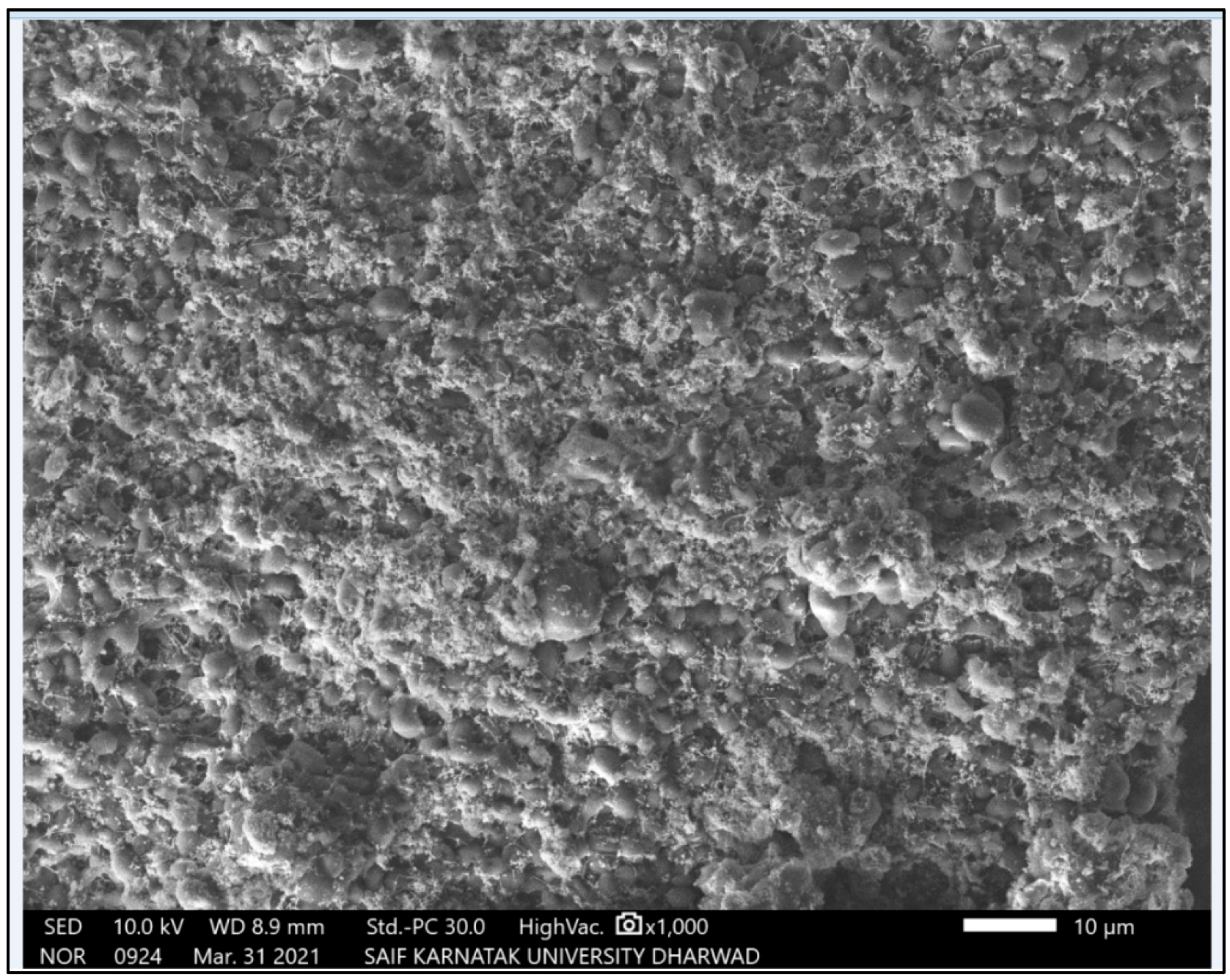
3.1.3. SEM Micrographs of Green Synthesized AgNPs
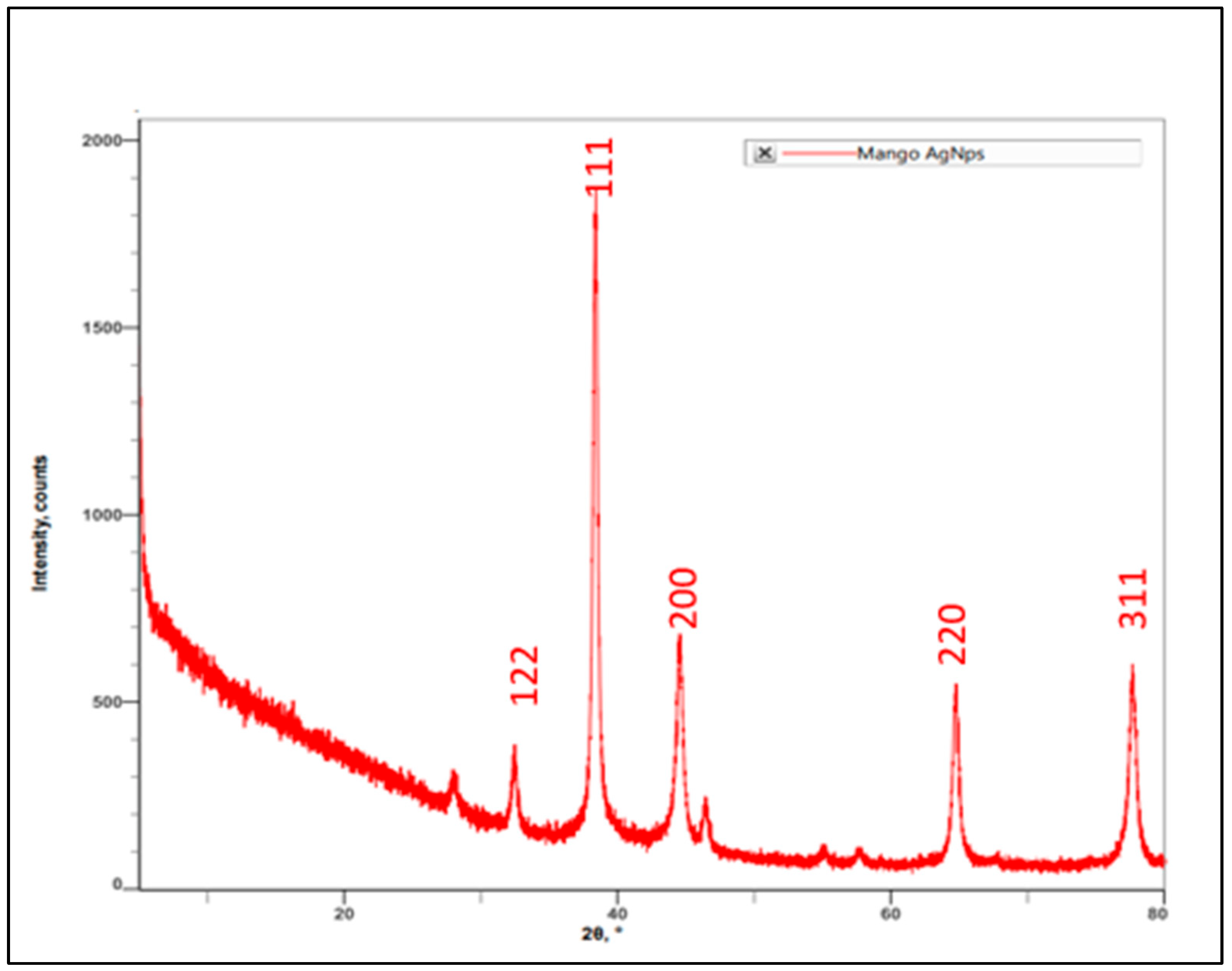
3.1.4. XRD Spectrum of AgNPs Synthesized by Using M. indica Leaf Extract
3.2. Estimation of Primary and Secondary Metabolites in AgNPs Treated Wheat Plants Infected with Spot Blotch Infection (Bipolaris sorokiniana)
3.2.1. Reducing Sugar
3.2.2. Total Phenol
3.3. Estimation of Stress Response Enzyme Activities in AgNPs Treated Wheat Plants Infected with Bipolaris sorokiniana
3.3.1. Estimation of SOD Activity
3.3.2. Estimation of CAT Activity
3.3.3. Estimation of POX Activity
3.3.4. Estimation of GR Activity
3.3.5. Estimation of PAL Activity
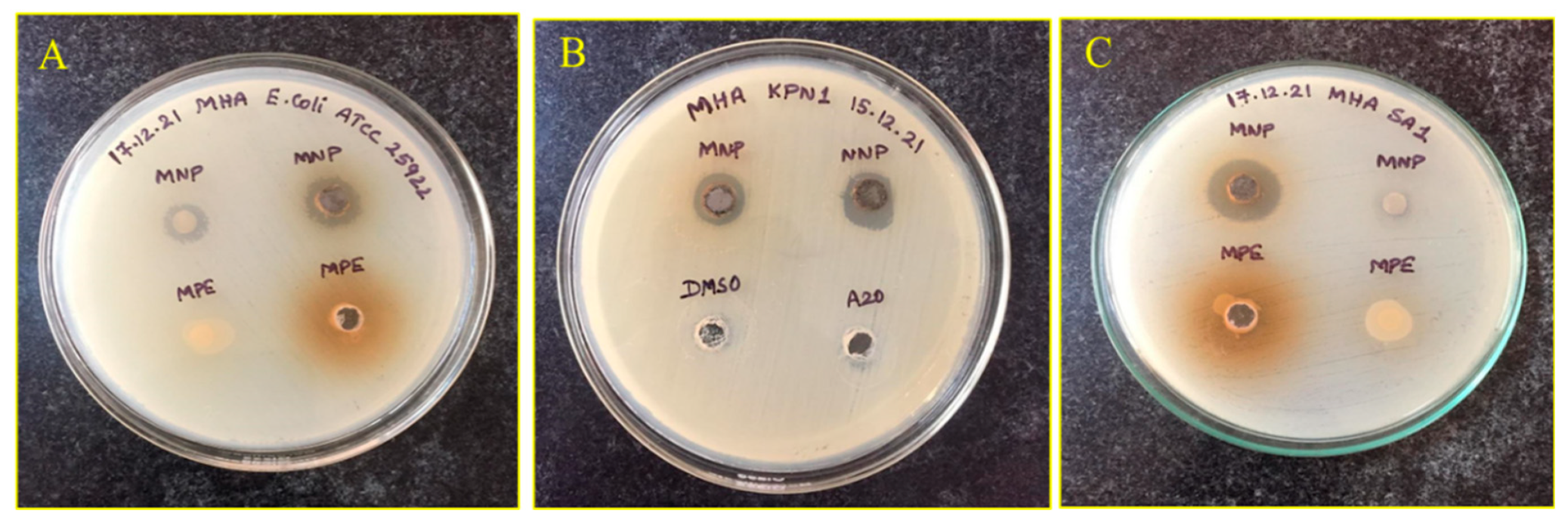
3.4. Antibacterial Activity against MDRs
3.5. Green Synthesis of AgNPs by Using M. indica Leaf Extract and Their Characterization
3.6. Studies on Biochemical Changes in Wheat Plants Infected with Spot Blotch (Bipolaris sorokiniana)
3.6.1. Reducing Sugars
3.6.2. Total Phenol
3.6.3. Superoxide Dismutase
3.6.4. Catalase
3.6.5. Peroxidase (POX)
3.6.6. Glutathione Reductase
3.6.7. Phenylalanine Ammonia-Lyase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food & Agriculture Organization of the United Nations (FAO). Faostat: FAO Statistical Databases; Food & Agriculture Organization of the United Nations (FAO): Rome, Italy, 2000; ISBN 9780119861105. [Google Scholar]
- Mielgo-Ayuso, J.; Aparicio-Ugarriza, R.; Olza, J.; Aranceta-Bartrina, J.; Gil, A.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; González-Gross, M. Dietary Intake and Food Sources of Niacin, Riboflavin, Thiamin and Vitamin B6 in a Representative Sample of the Spanish Population. The ANthropometry, Intake, and Energy Balance in Spain (ANIBES) Study dagger. Nutrients 2018, 10, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.K.; Chand, R. Variation and Inheritance of Leaf Angle, and Its Association with Spot Blotch (B. Sorokiniana) Severity in Wheat (Triticum Aestivum). Euphytica 2002, 124, 283–290. [Google Scholar] [CrossRef]
- Rajan, M.S. Nano: The next Revolution. Natl. Book Trust 2004, 15–72. [Google Scholar]
- Mousavi, S.R.; Rezaei, M. Nanotechnology in Agriculture and Food Production. J. App Environ. Biol. Sci. 2011, 1, 414–419. [Google Scholar]
- Mishra, S.; Singh, H. Silver nanoparticles mediated altered gene expression of melanin biosynthesis genes in Bipolaris sorokiniana. Microbiol. Res. 2015, 172, 16–18. [Google Scholar] [CrossRef]
- Boller, T. Chemoperception of Microbial Signals in Plant Cells. Annu. Rev. Plant Biol. 1995, 46, 189–214. [Google Scholar] [CrossRef]
- Kobayashi, I.; Murdoch, L.J.; Hardham, A.R.; Kunoh, H. Cell biology of early events in the plant resistance response to infection by pathogenic fungi. Can. J. Bot. 1995, 73, 418–425. [Google Scholar] [CrossRef]
- Brisson, L.F.; Tenhaken, R.; Lamb, C. Function of Oxidative Cross-Linking of Cell Wall Structural Proteins in Plant Disease Resistance. Plant Cell 1994, 6, 1703. [Google Scholar] [CrossRef] [Green Version]
- Bolwell, G.P. Role of active oxygen species and NO in plant defence responses. Curr. Opin. Plant Biol. 1999, 2, 287–294. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and Molecular Biology of Phenylpropanoid Metabolism. Annu. Rev. Plant Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Peltonen, S.; Karjalainen, R. Phenylalanine Ammonia Lyase Activity in Barlay after Infection with Bipolaris Sorokiniana or Treatment with Its Purified Baralay. J. Phytopathol. 1995, 143, 239–245. [Google Scholar] [CrossRef]
- Peltonen, S. Response of Barley and Wheat to Pathogen, Non- Pathogens and Wounding as Indicates by Induced Phenylalanine Ammonia Lyase Activity. Acta Agric. Scand Sect. B Soil Plant Sci. 1998, 48, 184–191. [Google Scholar]
- Narayan, A.; Jacob, R.; Paulsonb, J.M.; Dinesh, K.B.; Narayan, C.R. Mango Leaf Extract Synthesized Silver Nanorods Exert Anticancer Activity on Breast Cancer and Colorectal Carcinoma Cells. J. Drug. Deliv. Sci. Technol. 2018, 44, 8–12. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Mavis, R.D.; Stellwagen, E. Purification and Subunit Structure of Glutathione Reductase from Bakers’ Yeast. J. Biol. Chem. 1968, 243, 809–814. [Google Scholar] [CrossRef]
- Tewari, R.; Ganaie, F.; Venugopal, N.; Mitra, S.; Shome, R.; Shome, B.R. Occurrence and characterization of genetic determinants of β-lactam-resistance in Escherichia coli clinical isolates. Infect. Genet. Evol. 2022, 100, 105257. [Google Scholar] [CrossRef]
- Bauer, M.A.W.; Kirby, M.W.M.M.; Sherris, M.J.C.; Turck, M.M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Gomez, G.I.; Gonez, H.K. Analysis of CRD and DMRT’s Methods. Electron. J. Appl. Stat. Anal. 1984. [Google Scholar]
- Kora, A.J.; Mounika, J.; Jagadeeshwar, R. Rice leaf extract synthesized silver nanoparticles: An in vitro fungicidal evaluation against Rhizoctonia solani, the causative agent of sheath blight disease in rice. Fungal Biol. 2020, 124, 671–681. [Google Scholar] [CrossRef]
- Al Awadh, A.A.; Shet, A.R.; Patil, L.R.; Shaikh, I.A.; Alshahrani, M.M.; Nadaf, R.; Mahnashi, M.H.; Desai, S.V.; Muddapur, U.M.; Achappa, S.; et al. Sustainable Synthesis and Characterization of Zinc Oxide Nanoparticles Using Raphanus sativus Extract and Its Biomedical Applications. Crystals 2022, 12, 1142. [Google Scholar] [CrossRef]
- Nahari, M.H.; Al Ali, A.; Asiri, A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J. Green Synthesis and Characterization of Iron Nanoparticles Synthesized from Aqueous Leaf Extract of Vitex leucoxylon and Its Biomedical Applications. Nanomaterials 2022, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Zainab; Saeed, K.; Ammara; Ahmad, S.; Ahmad, H.; Ullah, F.; Sadiq, A.; Uddin, A.; Khan, I.; Ahmad, M. Green Synthesis, Characterization and Cholinesterase Inhibitory Potential of Gold Nanoparticles. J. Mex. Chem. Soc. 2021, 65, 416–423. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Bharathi, V.; Firdous, J.; Muhamad, N.; Mona, R. Green Synthesis of Mangifera Indica Silver Nanoparticles and Its Analysis Using Fourier Transform Infrared and Scanning Electron Microscopy. Natl. J. Physiol. Pharm. Pharm. 2017, 7, 83–94. [Google Scholar]
- Mishra, J.N.; Verma, N.K. A Brief Study on Catharanthus Roseus: A Review. Int. J. Res. Pharma Pharm. Sci. 2017, 2, 20–23. [Google Scholar]
- Ghozali, S.Z.; Vuanghao, L.; Ahmad, N.H. Biosynthesis and Characterization of Silver Nanoparticles Using Catharanthus Roseus Leaf Extract and Its Proliferative Effects on Cancer Cell Lines. J. Nanomed. Nanotechnol. 2015, 6, 4–11. [Google Scholar]
- Bhusnure, O.G.; Kuthar, V.S.; Gholve, S.B.; Giram, P.S.; Shembekar, V.S.; Zingade, S.G.; Jadhav, P.P. Green Synthesis of Silver Nanoparticle Using Catharanthus Roseus Extract for Pharmacological Activity. Int. J. Pharma Pharm. Res 2017, 10, 77–88. [Google Scholar]
- Banala, R.R.; Nagati, V.B.; Karnati, P.R. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci. 2015, 22, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Ajani, A.; Dada, E.; Olu-Arotiowa, O.; Okeowo, I.; Alade, A.; Afolabi, T. Adsorption of Methylene Blue from Aqueous Solution using Microwave-Assisted BaCl2 Modified Activated Carbon Produced from Mango Seed Shell. LAUTECH J. Civ. Environ. Stud. 2019, 3, 72–82. [Google Scholar] [CrossRef]
- Sundeep, D.; Vijaya Kumar, T.; Rao, P.S.S.; Ravikumar, R.V.S.S.N.; Gopala Krishna, A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog. Biomater. 2017, 6, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Hanady, S.A.; Wasnaa, H.; Ghassan, M.; Saadoon, A.H. Biosynthesis of Silver Nanoparticles from CatharanthusRoseus Leaf Extract and Assessing Their Antioxidant, Antimicrobial, and Wound-Healing Activities. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1234–1240. [Google Scholar]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Synthesis of Silver Nanoparticles Using Leaves of Catharanthus Roseus L. (G) Don. and Their Antiplasmodial Activities. Asian. Pac. J. Trop. Biomed. 2012, 2, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, T.; Jemimah, I.A.A.; Ponmanickam, P.; Ayyanar, M. Synthesis of Silver Nanoparticles Using Catharanthus Roseus Root Extract and Its Larvicidal Activities. J. Environ. Biol. 2015, 36, 1283–1289. [Google Scholar]
- Morkunas, I.; Ratajczak, L. The Role of Suger Signalling in Plant Defence Response against Fungal Pathogen. Acta Physiol. Plant 2014, 36, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Kazemi-Shahandashti, S.-S.; Maali-Amiri, R. Global insights of protein responses to cold stress in plants: Signaling, defence, and degradation. J. Plant Physiol. 2018, 226, 123–135. [Google Scholar] [CrossRef]
- Alberto, R. Pathological response and biochemical changes in Allium cepa L. (bulb onions) infected with anthracnose-twister disease. Plant Pathol. Quar. 2014, 4, 23–31. [Google Scholar] [CrossRef]
- Kumar, P.; Kalappanavar, I.K.; Arukumar, G.S.; Pradeep, P.E. Studies on Selected Physiological and Biochemical Parameters Responsible for Resistance to Spot Blotch of Barlay. J. Wheat Res. 2011, 3, 25–29. [Google Scholar]
- Yanik, F.; Vardar, F. Assessment of Silver Nanoparticle-Induced Morphological, Biochemical and Physiological Alterations in Wheat Roots. Ann. Bot. 2019, 9, 83–94. [Google Scholar]
- Krishnaraj, C.; Jagan, E.; Ramachandran, R.; Abirami, S.; Mohan, N.; Kalaichelvan, P. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn) Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Before gene expression: Early events in plant–insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.T.; Anahousur, K.H.; Hegde, R.K. Role of Sugar Phenol and Amino Acid in Rust Resistance in Sorghum. J. Agril. Sci. 1981, 15, 282–288. [Google Scholar]
- Reddy, M.N. Changes in Phenolic Acids in Groundnut Leaves Infected with Rust. J. Phytopathol. 1984, 110, 78–81. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Foyer, C.; Noctor, G. Oxidant and Antioxidant Signaling in Plants, a Reevaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Du, W.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Zhu, J.; Peralta-Videa, J.R.; Guo, H. Physiological and Biochemical Changes Imposed by CeO2 Nanoparticles on Wheat: A Life Cycle Field Study. Environ. Sci. Technol. 2015, 49, 11884–11893. [Google Scholar] [CrossRef]
- Cruz, C.M.H. Drought Stress and Reactive Oxygen Species: Production, Scavenging and Signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Mohamed, A.K.S.H.; Qayyum, M.F.; Abdel-Hadi, A.M.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Luthaje, S.; MeisrimLer, C.; Moller, B. Phylogenetic, Topology, Structural and Functions of Membrane Bound Peroxidase in Vascular Plant. Phytochemistry 2011, 72, 1124–1135. [Google Scholar] [CrossRef]
- Ye, X.; Ng, T. Isolation of a novel peroxidase from French bean legumes and first demonstration of antifungal activity of a non-milk peroxidase. Life Sci. 2002, 71, 1667–1680. [Google Scholar] [CrossRef]
- Ghosh, M. Antifungal Properties of Haem Peroxidase from Acorus calamus. Ann. Bot. 2006, 98, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.; Lee, R. Peroxidase changes in wheat isolines with compatible and incompatible leaf rust infections. Physiol. Plant Pathol. 1978, 13, 173–181. [Google Scholar] [CrossRef]
- Dixon, R.A.; Dey, P.M.; Lamb, C.J. Phytoalexins: Enzymology and Molecular Biology. Adv. Enzymol. Relat. Areas Mol. Biol. 1983, 55, 1–136. [Google Scholar] [CrossRef]
- Lawton, M.A.; Lamb, C.L. Transcriptional Activation of Plant Defense Gene by Fungus Elicitor, Wonding, and Infection. Mol. Cell Biol. 1987, 7, 335–341. [Google Scholar]


| Variety of Durum Wheat | Treatment | Replication |
|---|---|---|
| Bijaga Yellow | C: Control leaves T1: Diseased + 0 ppm AgNPs (Disease control) T2: Diseased + 5 ppm AgNPs T3: Diseased + 10 ppm AgNPs T4: Diseased + 15 ppm AgNPs T5: Diseased + 20 ppm AgNPs T6: Diseased + 30 ppm AgNPs T7: Diseased + 50 ppm AgNPs | Three |
| Treatments | Reducing Sugar | |
|---|---|---|
| g % Dry Weight | % Variation | |
| Control | 10.61 | - |
| T1: 0 ppm AgNPs | 2.73 | −288.645 |
| T2: 5 ppm AgNPs | 4.44 | −138.964 |
| T3: 10 ppm AgNPs | 5.00 | −112.20 |
| T4: 15 ppm AgNPs | 5.45 | −94.67 |
| T5: 20 ppm AgNPs | 5.87 | −80.74 |
| T6: 30 ppm AgNPs | 6.94 | −52.88 |
| T7: 50 ppm AgNPs | 7.82 | −35.67 |
| SE (m) | 0.22 | |
| C.D. | 0.67 | |
| Treatments | Total Phenol | |
|---|---|---|
| g % Dry Weight | % Variation | |
| Control | 1.2 | - |
| T1: 0 ppm AgNPs | 1.65 | 27.27 |
| T2: 5 ppm AgNPs | 1.72 | 30.23 |
| T3: 10 ppm AgNPs | 1.76 | 31.81 |
| T4: 15 ppm AgNPs | 1.81 | 33.70 |
| T5: 20 ppm AgNPs | 1.85 | 35.13 |
| T6: 30 ppm AgNPs | 1.98 | 39.39 |
| T7: 50 ppm AgNPs | 2.09 | 42.58 |
| SE (m) | 0.05 | |
| C.D. | 0.16 | |
| Treatments | Superoxide Dismutase | |
|---|---|---|
| U/mg Protein | % Variation | |
| Control | 2.05 | - |
| T1: 0 ppm AgNPs | 3.18 | 35.53 |
| T2: 5 ppm AgNPs | 3.36 | 38.98 |
| T3: 10 ppm AgNPs | 3.35 | 38.80 |
| T4: 15 ppm AgNPs | 3.54 | 42.09 |
| T5: 20 ppm AgNPs | 3.56 | 42.41 |
| T6: 30 ppm AgNPs | 3.91 | 47.57 |
| T7: 50 ppm AgNPs | 4.42 | 53.61 |
| SE (m) | 0.11 | |
| C.D. | 0.34 | |
| Treatments | Catalase | |
|---|---|---|
| U/mg Protein | % Variation | |
| Control | 336 | - |
| T1: 0 ppm AgNPs | 432 | 22.22 |
| T2: 5 ppm AgNPs | 445 | 24.49 |
| T3: 10 ppm AgNPs | 435 | 22.76 |
| T4: 15 ppm AgNPs | 482 | 30.29 |
| T5: 20 ppm AgNPs | 461 | 28.05 |
| T6: 30 ppm AgNPs | 492 | 31.71 |
| T7: 50 ppm AgNPs | 583 | 42.37 |
| SE (m) | 14.77 | |
| C.D. | 44.68 | |
| Treatments | Peroxidase | |
|---|---|---|
| U/mg Protein | % Variation | |
| Control | 1.17 | - |
| T1: 0 ppm AgNPs | 1.97 | 40.61 |
| T2: 5 ppm AgNPs | 2.45 | 52.24 |
| T3: 10 ppm AgNPs | 2.69 | 56.51 |
| T4: 15 ppm AgNPs | 2.74 | 57.30 |
| T5: 20 ppm AgNPs | 2.89 | 59.52 |
| T6: 30 ppm AgNPs | 3.00 | 61.00 |
| T7: 50 ppm AgNPs | 3.24 | 63.89 |
| SE (m) | 0.07 | |
| C.D. | 0.22 | |
| Treatments | Glutathione Reductase | |
|---|---|---|
| U/mg Protein | % Variation | |
| Control | 1.54 | - |
| T1: 0 ppm AgNPs | 2.54 | 39.37 |
| T2: 5 ppm AgNPs | 3.02 | 49.00 |
| T3: 10 ppm AgNPs | 3.05 | 49.50 |
| T4: 15 ppm AgNPs | 3.28 | 53.04 |
| T5: 20 ppm AgNPs | 3.40 | 54.70 |
| T6: 30 ppm AgNPs | 3.79 | 59.36 |
| T7: 50 ppm AgNPs | 4.00 | 61.50 |
| SE (m) | 0.12 | |
| C.D. | 0.38 | |
| Treatments | Phenylalanine Ammonia Lyase | |
|---|---|---|
| U/mg Protein | % Variation | |
| Control | 3.82 | - |
| T1: 0 ppm AgNPs | 5.70 | 32.98 |
| T2: 5 ppm AgNPs | 5.87 | 34.92 |
| T3: 10 ppm AgNPs | 5.74 | 33.45 |
| T4: 15 ppm AgNPs | 6.13 | 37.68 |
| T5: 20 ppm AgNPs | 6.20 | 39.37 |
| T6: 30 ppm AgNPs | 6.36 | 39.94 |
| T7: 50 ppm AgNPs | 6.78 | 43.66 |
| SE (m) | 0.17 | |
| C.D. | 0.51 | |
| Bacteria | Diameter of Inhibition Zone (mm) | |
|---|---|---|
| MNP | ||
| 1. | ATCC 25922 E. coli | 12 |
| 2. | E. coli Ec1 | 11 |
| 3. | E. coli Ec2 | 13 |
| 4. | E. coli Ec3 | 11 |
| 5. | E. coli Ec4 | 12 |
| 6. | E. coli Ec5 | 12 |
| 7. | E. coli Ec6 | 11 |
| 8. | K. pneumoniae KP-1 | 10 |
| 9. | K. pneumoniae KP-2 | 11 |
| 10. | K. pneumoniae KP-3 | 12 |
| 11. | K. pneumoniae KP-4 | 11 |
| 12. | K. pneumoniae KP-5 | 11 |
| 13. | K. pneumoniae KP-6 | 8 |
| 14. | K. pneumoniae KP-7 | 11 |
| 15. | MRSA-1 | 15 |
| 16. | MRSA-2 | 16 |
| S. No. | Bacterial Strain | Gram Stain | ATCC Number |
|---|---|---|---|
| 1 | Klebsiella pneumoniae-KP1 | Gram negative | ATCC 13883 |
| 2 | Escherichia coli | Gram negative | ATCC 25922 |
| 3 | Methicillin-resistant Staphylococcus aureus MRSA-SA1 | Gram positive | ATCC BAA-41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, Y.S.; Bahafi, A.; Mirajkar, K.K.; Basavaraju, R.R.; Mitra, S.; S, S.; More, S.S.; Muddapur, U.M.; Khan, A.A.; Sudarshan, P.R.; et al. In Vitro Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Mangifera indica Aqueous Leaf Extract against Multidrug-Resistant Pathogens. Antibiotics 2022, 11, 1503. https://doi.org/10.3390/antibiotics11111503
Alqahtani YS, Bahafi A, Mirajkar KK, Basavaraju RR, Mitra S, S S, More SS, Muddapur UM, Khan AA, Sudarshan PR, et al. In Vitro Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Mangifera indica Aqueous Leaf Extract against Multidrug-Resistant Pathogens. Antibiotics. 2022; 11(11):1503. https://doi.org/10.3390/antibiotics11111503
Chicago/Turabian StyleAlqahtani, Yahya S., Amal Bahafi, Kiran K. Mirajkar, Rakshith Rudrapura Basavaraju, Susweta Mitra, Shailaja S, Sunil S. More, Uday M. Muddapur, Aejaz Abdullatif Khan, P. Renuka Sudarshan, and et al. 2022. "In Vitro Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Mangifera indica Aqueous Leaf Extract against Multidrug-Resistant Pathogens" Antibiotics 11, no. 11: 1503. https://doi.org/10.3390/antibiotics11111503
APA StyleAlqahtani, Y. S., Bahafi, A., Mirajkar, K. K., Basavaraju, R. R., Mitra, S., S, S., More, S. S., Muddapur, U. M., Khan, A. A., Sudarshan, P. R., & Shaikh, I. A. (2022). In Vitro Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Mangifera indica Aqueous Leaf Extract against Multidrug-Resistant Pathogens. Antibiotics, 11(11), 1503. https://doi.org/10.3390/antibiotics11111503







