Do Anti-Phage Antibodies Persist after Phage Therapy? A Preliminary Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Plate Phage Neutralization Test
2.3. Categories of the Results of PT
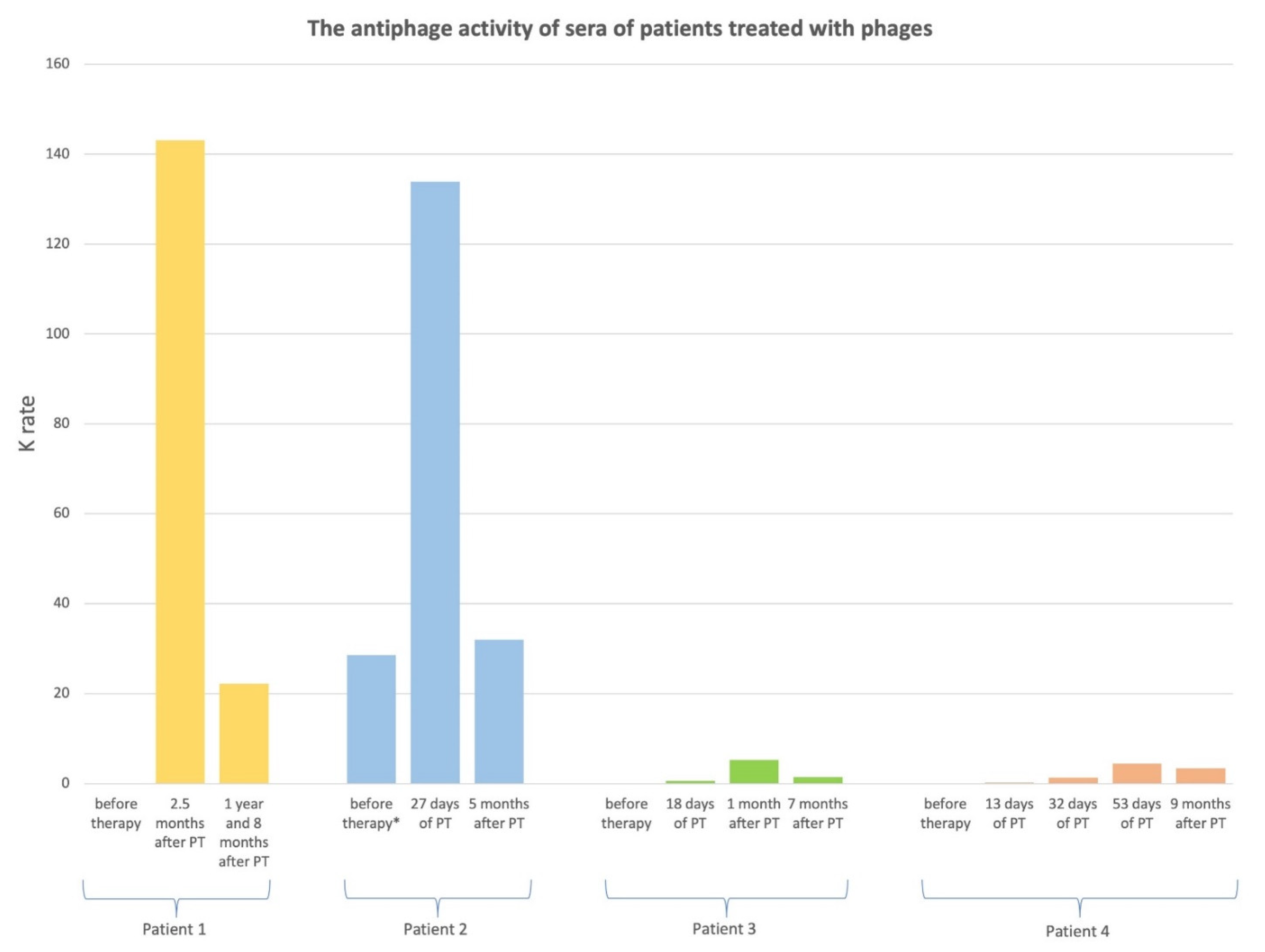
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Krut, O.; Bekeredjian-Ding, I. Contribution of the immune response to phage therapy. J. Immunol. 2018, 200, 3037–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gembara, K.; Dąbrowska, K. Phage-specific antibodies. Curr. Opin. Biotechnol. 2021, 68, 186–192. [Google Scholar] [CrossRef]
- Kaźmierczak, Z.; Majewska, J.; Miernikiewicz, P.; Międzybrodzki, R.; Nowak, S.; Harhala, M.; Lecion, D.; Kęska, W.; Owczarek, B.; Ciekot, J.; et al. Immune response to therapeutic staphylococcal bacteriophages in mammals: Kinetics of induction, immunogenic structural proteins, natural and induced antibodies. Front. Immunol. 2021, 12, 639570. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Letkiewicz, S.; Fortuna, W.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; Olchawa, E.; et al. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol. 2017, 12, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Żaczek, M.; Łusiak-Szelachowska, M.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Owczarek, B.; Kopciuch, A.; Fortuna, W.; Rogóż, P.; Górski, A. Antibody production in response to staphylococcal MS-1 phage cocktail in patients undergoing phage therapy. Front. Microbiol. 2016, 7, 1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedrick, R.M.; Freeman, K.G.; Nguyen, J.A.; Bahadirli-Talbott, A.; Cardin, M.E.; Cristinziano, M.; Smith, B.E.; Jeong, S.; Ignatius, E.H.; Lin, C.T.; et al. Nebulized bacteriophage in a patient with refractory Mycobacterium abscessus lung disease. Open Forum Infect. Dis. 2022, 9, ofac194. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.H. Methods of study of bacterial viruses. In Bacteriophages; Adams, M.H., Ed.; Interscience: New York, NY, USA, 1959; pp. 443–522. [Google Scholar]
- Dan, J.M.; Lehman, S.M.; Al-Kolla, R.; Penziner, S.; Afshar, K.; Yung, G.; Golts, E.; Law, N.; Logan, C.; Kovach, Z.; et al. Development of host immune response to bacteriophage in a lung transplant recipient on adjunctive phage therapy for a multidrug resistant pneumonia. J. Infect. Dis. 2022, jiac368. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.A. Virus-induced immune complex formation and disease: Definition, regulation, importance. In Concepts in Viral Pathogenesis; Notkins, A.L., Oldstone, M.B.A., Eds.; Springer: New York, NY, USA, 1984. [Google Scholar] [CrossRef]
- Molleston, J.M.; Holtz, L.R. Fighting the wrong enemy: Anti-bacteriophage immunity in phage therapy. J. Infect. Dis. 2022, jiac369. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage therapy of Mycobacterium infections: Compassionate-use of phages in twenty patients with drug-resistant mycobacterial disease. Clin. Infect. Dis. 2022, ciac453. [Google Scholar] [CrossRef]
- Nick, J.A.; Dedrick, R.M.; Gray, A.L.; Vladar, E.K.; Smith, B.E.; Freeman, K.G.; Malcolm, K.C.; Epperson, L.E.; Hasan, N.A.; Hendrix, J.; et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 2022, 185, 1860–1874. [Google Scholar] [CrossRef]
| Patient No. | Type of Infection | Route of Phage Administration | Target Pathogen | Phage Used in PT | Days of PT | Clinical Outcome of PT a |
|---|---|---|---|---|---|---|
| 1 | Inflammation of the left hip joint | local | S. aureus | Staph_1N | 24 | C |
| 2 | Inflammation of the left ankle joint | The third PT cycle: local and oral | S. aureus | Staph_1N | 44 | C |
| 3 | Inflammation of the left hip | local and oral | P.aeruginosa | Ps_2N | 25 | F |
| 4 | Inflammation of the right calcaneus | local | E. coli | Ecol_L-4 | 54 | D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łusiak-Szelachowska, M.; Międzybrodzki, R.; Rogóż, P.; Weber-Dąbrowska, B.; Żaczek, M.; Górski, A. Do Anti-Phage Antibodies Persist after Phage Therapy? A Preliminary Report. Antibiotics 2022, 11, 1358. https://doi.org/10.3390/antibiotics11101358
Łusiak-Szelachowska M, Międzybrodzki R, Rogóż P, Weber-Dąbrowska B, Żaczek M, Górski A. Do Anti-Phage Antibodies Persist after Phage Therapy? A Preliminary Report. Antibiotics. 2022; 11(10):1358. https://doi.org/10.3390/antibiotics11101358
Chicago/Turabian StyleŁusiak-Szelachowska, Marzanna, Ryszard Międzybrodzki, Paweł Rogóż, Beata Weber-Dąbrowska, Maciej Żaczek, and Andrzej Górski. 2022. "Do Anti-Phage Antibodies Persist after Phage Therapy? A Preliminary Report" Antibiotics 11, no. 10: 1358. https://doi.org/10.3390/antibiotics11101358
APA StyleŁusiak-Szelachowska, M., Międzybrodzki, R., Rogóż, P., Weber-Dąbrowska, B., Żaczek, M., & Górski, A. (2022). Do Anti-Phage Antibodies Persist after Phage Therapy? A Preliminary Report. Antibiotics, 11(10), 1358. https://doi.org/10.3390/antibiotics11101358






