Contribution of Symptomatic, Herbal Treatment Options to Antibiotic Stewardship and Microbiotic Health
Abstract
1. Introduction
2. Antibiotic Treatment of Infections: Inappropriate Use and Risks
3. Common Treatment Alternatives to Antibiotics
4. Herbal Drugs: A Safe Treatment Alternative for Uncomplicated Infections
5. Biologically Active Compounds of Herbal Preparations
6. Treatment of Respiratory Infections with Herbal Medicinal Products: Bronchipret® and Sinupret®
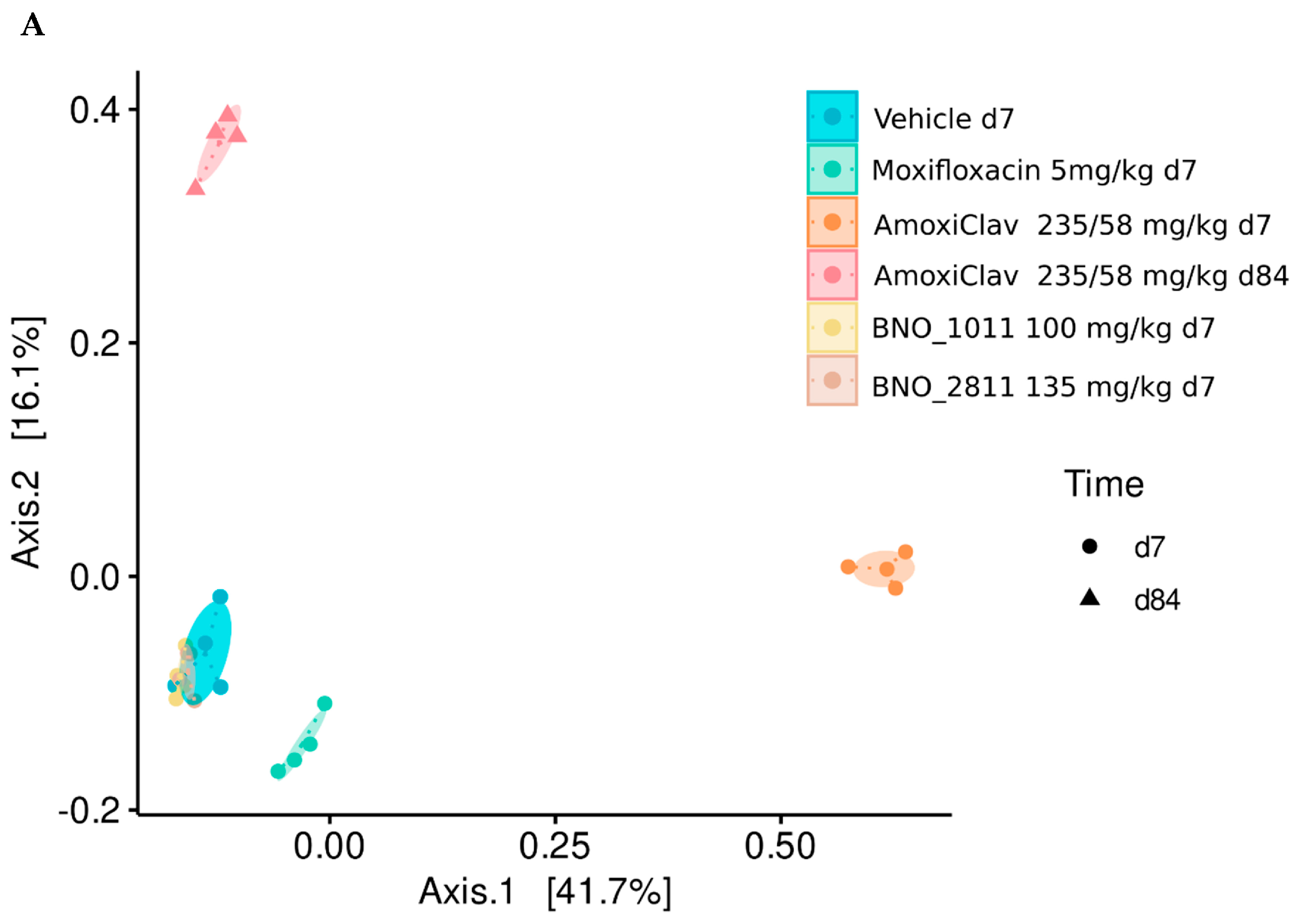
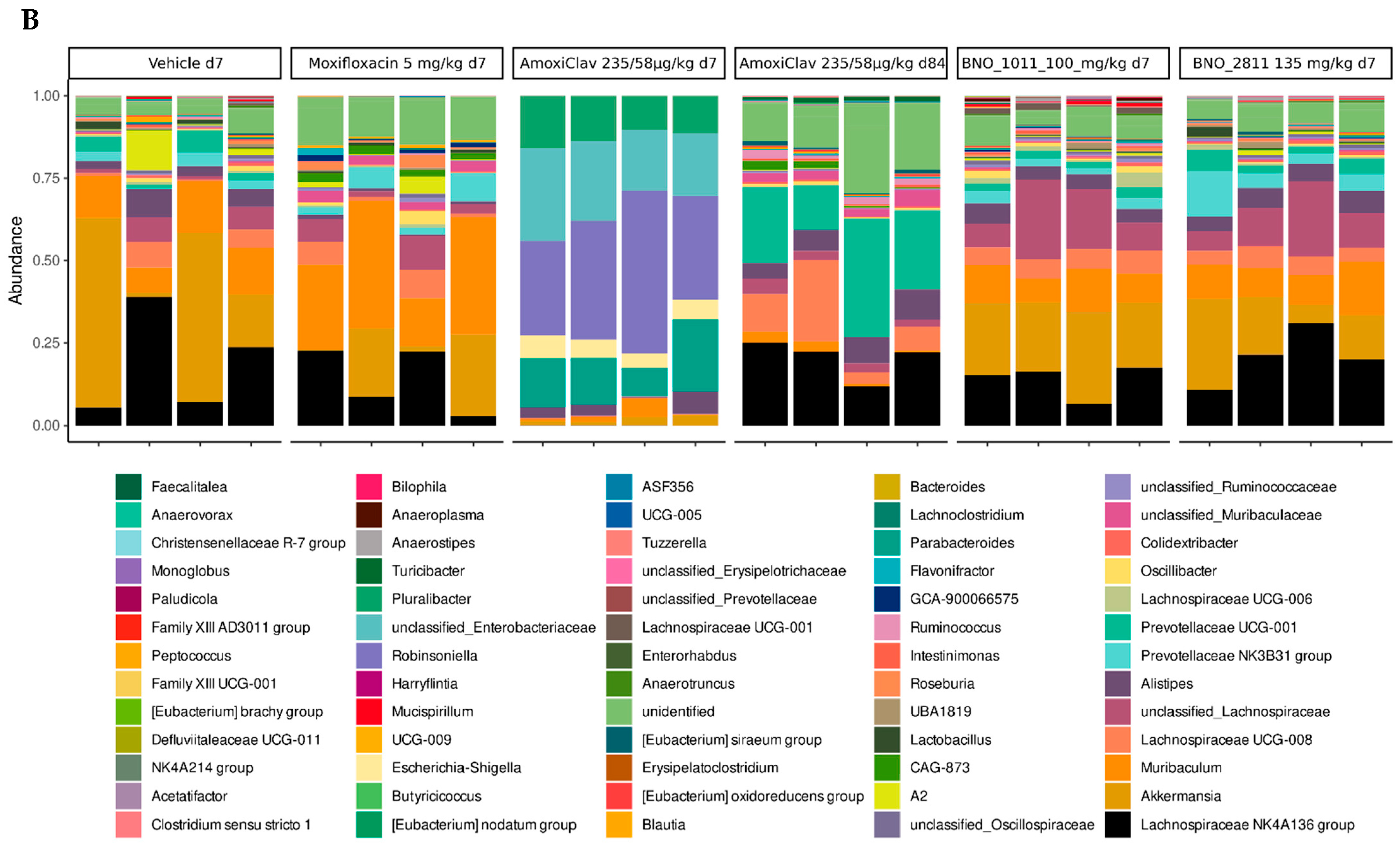
7. Preservation of the Gut Microbiome under BNO 2811 and BNO 1011: Results of a Mouse Model
8. Treatment of Urogenital Infections with an Herbal Medicinal Product: Canephron®
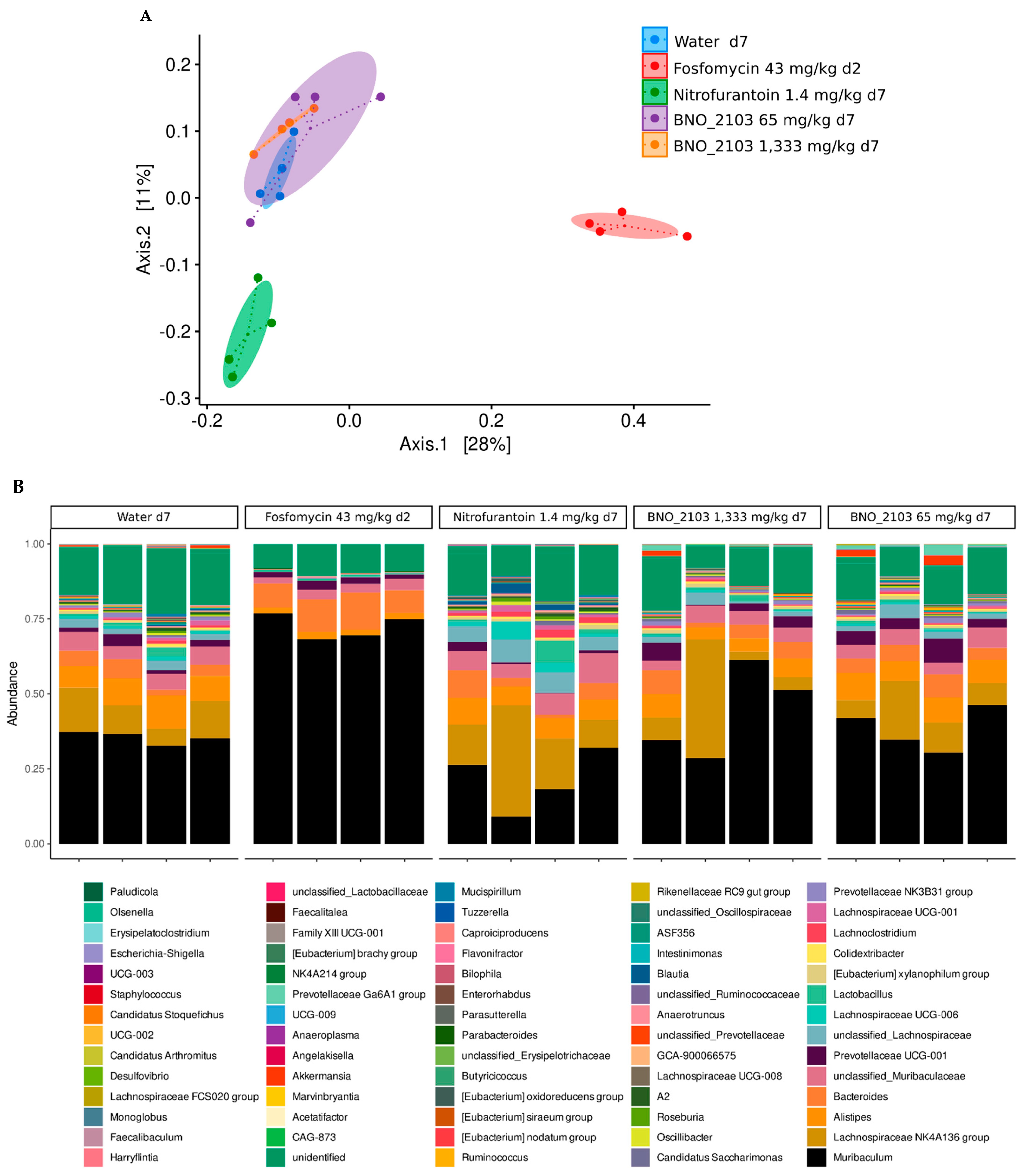
9. Preservation of the Gut Microbiome under BNO 2103: Results in a Mouse Model
10. Future and Prospects for Application
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moens, E.; Veldhoen, M. Epithelial barrier biology: Good fences make good neighbours. Immunology 2011, 135, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Vaga, S.; Lee, S.; Ji, B.; Andreasson, A.; Talley, N.J.; Agréus, L.; Bidkhori, G.; Kovatcheva-Datchary, P.; Park, J.; Lee, D.; et al. Compositional and functional differences of the mucosal microbiota along the intestine of healthy individuals. Sci. Rep. 2020, 10, 14977. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef]
- Bomar, L.; Brugger, S.D.; Lemon, K.P. Bacterial microbiota of the nasal passages across the span of human life. Curr. Opin. Microbiol. 2018, 41, 8–14. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Jones-Freeman, B.; Chonwerawong, M.; Marcelino, V.R.; Deshpande, A.V.; Forster, S.C.; Starkey, M.R. The microbiome and host mucosal interactions in urinary tract diseases. Mucosal Immunol. 2021, 14, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Gottschick, C.; Deng, Z.L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Rohde, M.; Mendling, W.; Wagner-Dobler, I. Treatment of biofilms in bacterial vaginosis by an amphoteric tenside pessary-clinical study and microbiota analysis. Microbiome 2017, 5, 119. [Google Scholar] [CrossRef]
- Gottschick, C.; Deng, Z.L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sitarik, A.R.; Woodcroft, K.; Johnson, C.C.; Zoratti, E. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations with the Gut Microbiome and Sensitization in Children. Curr. Allergy Asthma Rep. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Raplee, I.; Walker, L.; Xu, L.; Surathu, A.; Chockalingam, A.; Stewart, S.; Han, X.; Rouse, R.; Li, Z. Emergence of nosocomial associated opportunistic pathogens in the gut microbiome after antibiotic treatment. Antimicrob. Resist. Infect. Control 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Surathu, A.; Raplee, I.; Chockalingam, A.; Stewart, S.; Walker, L.; Sacks, L.; Patel, V.; Li, Z.; Rouse, R. The effect of antibiotics on the gut microbiome: A metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genom. 2020, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162 Pt A, 22–38. [Google Scholar] [CrossRef]
- Schulz, C.; Schütte, K.; Mayerle, J.; Malfertheiner, P. The role of the gastric bacterial microbiome in gastric cancer: Helicobacter pylori and beyond. Therap. Adv. Gastroenterol. 2019, 12, 1756284819894062. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Contijoch, E.J.; Britton, G.J.; Yang, C.; Mogno, I.; Li, Z.; Ng, R.; Llewellyn, S.R.; Hira, S.; Johnson, C.; Rabinowitz, K.M.; et al. Gut microbiota density influences host physiology and is shaped by host and microbial factors. Elife 2019, 8, e40553. [Google Scholar] [CrossRef]
- Saus, E.; Iraola-Guzmán, S.; Willis, J.R.; Brunet-Vega, A.; Gabaldón, T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol. Asp. Med. 2019, 69, 93–106. [Google Scholar] [CrossRef]
- de Steenhuijsen Piters, W.A.; Sanders, E.A.; Bogaert, D. The role of the local microbial ecosystem in respiratory health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140294. [Google Scholar] [CrossRef]
- Stapleton, A.E. The Vaginal Microbiota and Urinary Tract Infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Goldfarb, D.S.; Nazzal, L. The role of the microbiome in kidney stone formation. Int. J. Surg. 2016, 36 Pt D, 607–612. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57 (Suppl. S1), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Lynch, S.V. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe 2015, 17, 592–602. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 108. [Google Scholar] [CrossRef]
- Dickerson, F.; Severance, E.; Yolken, R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav. Immun. 2017, 62, 46–52. [Google Scholar] [CrossRef]
- Agorastos, A.; Bozikas, V.P. Gut microbiome and adaptive immunity in schizophrenia. Psychiatriki 2019, 30, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zeng, T.; Deligios, M.; Milanesi, L.; Langille, M.G.I.; Zinellu, A.; Rubino, S.; Carru, C.; Kelvin, D.J. Age-Related Variation of Bacterial and Fungal Communities in Different Body Habitats across the Young, Elderly, and Centenarians in Sardinia. Msphere 2020, 5, e00558-19. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, K.B.; Hopkins, S.; Llewelyn, M.J.; Walker, A.S.; McNulty, C.A.; Robotham, J.V. Duration of antibiotic treatment for common infections in English primary care: Cross sectional analysis and comparison with guidelines. BMJ 2019, 364, l440. [Google Scholar] [CrossRef]
- O’Connor, R.; O’Doherty, J.; O’Regan, A.; Dunne, C. Antibiotic use for acute respiratory tract infections (ARTI) in primary care; what factors affect prescribing and why is it important? A narrative review. Ir. J. Med. Sci. 2018, 187, 969–986. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of antibiotics on gut microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Wilton, L.; Kollarova, M.; Heeley, E.; Shakir, S. Relative risk of vaginal candidiasis after use of antibiotics compared with antidepressants in women. Drug Saf. 2003, 26, 589–597. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017, 15, 422–434. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Zweigner, J.; Meyer, E.; Gastmeier, P.; Schwab, F. Rate of antibiotic prescriptions in German outpatient care-are the guidelines followed or are they still exceeded? GMS Hyg. Infect. Control. 2018, 13, Doc04. [Google Scholar] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)-Annual Epidemiological Report 2020; ECDC: Stockholm, Sweden, 2021. [Google Scholar]
- Shekhar, S.; Petersen, F.C. The Dark Side of Antibiotics: Adverse Effects on the Infant Immune Defense Against Infection. Front. Pediatr. 2020, 8, 544460. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Forsythe, P.; Uzan, A.; Avni, O.; Koren, O. Antibiotics in early life: Dysbiosis and the damage done. FEMS Microbiol. Rev. 2018, 42, 489–499. [Google Scholar] [CrossRef]
- Machowska, A.; Lundborg, C.S. Drivers of irrational use of antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyere, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilaz, A.; Veeratterapillay, R.; Wagenlehner, F. EAU guidelines on urological infections 2022. In European Association of Urology Guidelines; EAU Guidelines Office: Arnhem, The Netherlands, 2022. [Google Scholar]
- World Health Organization. Antimicrobial Stewardship Interventions: A Practical Guide; WHO Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef]
- Leis, J.A.; Born, K.B.; Theriault, G.; Ostrow, O.; Grill, A.; Johnston, K.B. Using antibiotics wisely for respiratory tract infection in the era of COVID-19. BMJ 2020, 371, m4125. [Google Scholar] [CrossRef]
- Harris, A.M.; Hicks, L.A.; Qaseem, A. Appropriate Antibiotic Use for Acute Respiratory Tract Infection in Adults: Advice for High-Value Care From the American College of Physicians and the Centers for Disease Control and Prevention. Ann. Intern. Med. 2016, 164, 425–434. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kotsantis, I.K.; Vouloumanou, E.K.; Rafailidis, P.I. Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: A meta-analysis of randomized controlled trials. J. Infect. 2009, 58, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Bleidorn, J.; Gágyor, I.; Kochen, M.M.; Wegscheider, K.; Hummers-Pradier, E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?—Results of a randomized controlled pilot trial. BMC Med. 2010, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Vik, I.; Bollestad, M.; Grude, N.; Bærheim, A.; Damsgaard, E.; Neumark, T.; Bjerrum, L.; Cordoba, G.; Olsen, I.C.; Lindbæk, M. Ibuprofen versus pivmecillinam for uncomplicated urinary tract infection in women—A double-blind, randomized non-inferiority trial. PLoS Med. 2018, 15, e1002569. [Google Scholar] [CrossRef]
- Kronenberg, A.; Butikofer, L.; Odutayo, A.; Muhlemann, K.; da Costa, B.R.; Battaglia, M.; Meli, D.N.; Frey, P.; Limacher, A.; Reichenbach, S.; et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: Randomised, double blind trial. BMJ 2017, 359, j4784. [Google Scholar] [CrossRef]
- Gágyor, I.; Bleidorn, J.; Kochen, M.M.; Schmiemann, G.; Wegscheider, K.; Hummers-Pradier, E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: Randomised controlled trial. BMJ 2015, 351, h6544. [Google Scholar] [CrossRef]
- Bjarnason, I.; Scarpignato, C.; Holmgren, E.; Olszewski, M.; Rainsford, K.D.; Lanas, A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology 2018, 154, 500–514. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Aronoff, D.M. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 2016, 22, 178.e1–178.e9. [Google Scholar] [CrossRef]
- Maseda, D.; Ricciotti, E. NSAID-gut microbiota interactions. Front. Pharmacol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Lasek, R.; Adam, D.; Barker, M. Empfehlungen zur Therapie Akuter Atemwegsinfektionen und der Ambulant Erworbenen Pneumonie (3. Auflage). Arzneiverordnung in der Praxis Band 40 Sonderheft 1 (Therapie Empfehlungen); Arzneimittelkommission der Deutschen Ärzteschaft (AkdÄ): Berlin, Germany, 2013. [Google Scholar]
- Lee, J.Y.; Jun, S.A.; Hong, S.S.; Ahn, Y.C.; Lee, D.S.; Son, C.G. Systematic Review of Adverse Effects from Herbal Drugs Reported in Randomized Controlled Trials. Phytother. Res. 2016, 30, 1412–1419. [Google Scholar] [CrossRef]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lim, D.J.; Yang, H.J.; Choi, E.K.; Shin, M.H.; Ahn, K.S.; Jung, S.H.; Um, J.Y.; Jung, H.J.; Lee, J.H.; et al. The multi-targeted effects of Chrysanthemum herb extract against Escherichia coli O157:H7. Phytother. Res. 2013, 27, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Amparo, T.R.; Seibert, J.B.; Vieira, P.M.A.; Teixeira, L.F.M.; Santos, O.; de Souza, G.H.B. Herbal medicines to the treatment of skin and soft tissue infections: Advantages of the multi-targets action. Phytother. Res. 2020, 34, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, F.; Hollywood, A.; Ryan, K. A systematic review of non-antibiotic measures for the prevention of urinary tract infections in pregnancy. BMC Pregnancy Childbirth 2018, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Wawrysiuk, S.; Naber, K.; Rechberger, T.; Miotla, P. Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance-non-antibiotic approaches: A systemic review. Arch. Gynecol. Obstet. 2019, 300, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Palm, J.; Steiner, I.; Abramov-Sommariva, D.; Ammendola, A.; Mitzenheim, S.; Steindl, H.; Wonnemann, M.; Bachert, C. Assessment of efficacy and safety of the herbal medicinal product BNO 1016 in chronic rhinosinusitis. Rhinology 2017, 55, 142–151. [Google Scholar] [CrossRef]
- Kardos, P.; Bittner, C.B.; Seibel, J.; Abramov-Sommariva, D.; Birring, S.S. Effectiveness and tolerability of the thyme/ivy herbal fluid extract BNO 1200 for the treatment of acute cough: An observational pharmacy-based study. Curr. Med. Res. Opin. 2021, 37, 1837–1844. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Abramov-Sommariva, D.; Holler, M.; Steindl, H.; Naber, K.G. Non-Antibiotic Herbal Therapy (BNO 1045) versus Antibiotic Therapy (Fosfomycin Trometamol) for the Treatment of Acute Lower Uncomplicated Urinary Tract Infections in Women: A Double-Blind, Parallel-Group, Randomized, Multicentre, Non-Inferiority Phase III Trial. Urol. Int. 2018, 101, 327–336. [Google Scholar]
- Albrecht, U.; Goos, K.H.; Schneider, B. A randomised, double-blind, placebo-controlled trial of a herbal medicinal product containing Tropaeoli majoris herba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections. Curr. Med. Res. Opin. 2007, 23, 2415–2422. [Google Scholar]
- Stange, R.; Schneider, B.; Albrecht, U.; Mueller, V.; Schnitker, J.; Michalsen, A. Results of a randomized, prospective, double-dummy, double-blind trial to compare efficacy and safety of a herbal combination containing Tropaeoli majoris herba and Armoraciae rusticanae radix with co-trimoxazole in patients with acute and uncomplicated cystitis. Res. Rep. Urol. 2017, 14, 43–50. [Google Scholar]
- Vahlensieck, W.; Lorenz, H.; Schumacher-Stimpfl, A.; Fischer, R.; Naber, K.G. Effect of a herbal therapy on clinical symptoms of acute lower uncomplicated urinary tract infections in women: Secondary analysis from a randomized controlled trial. Antibiotics 2019, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Schindler, G.; Patzak, U.; Brinkhaus, B.; von Nieciecki, A.; Wittig, J.; Krähmer, N.; Glöckl, I.; Veit, M. Urinary excretion and metabolism of arbutin after oral administration of arctostaphylos uvae ursi extract as film-coated tablets and aqueous solution in healthy humans. J. Clin. Pharmacol. 2002, 42, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Gbinigie, O.A.; Spencer, E.A.; Heneghan, C.J.; Lee, J.J.; Butler, C.C. Cranberry extract for symptoms of acute, uncomplicated urinary tract infection: A systematic review. Antibiotics 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Jund, R.; Mondigler, M.; Steindl, H.; Stammer, H.; Stierna, P.; Bachert, C. Clinical efficacy of a dry extract of five herbal drugs in acute viral rhinosinusitis. Rhinology 2012, 50, 417–426. [Google Scholar] [CrossRef]
- Jund, R.; Mondigler, M.; Stammer, H.; Stierna, P.; Bachert, C. Herbal drug BNO 1016 is safe and effective in the treatment of acute viral rhinosinusitis. Acta Otolaryngol. 2015, 135, 42–50. [Google Scholar] [CrossRef]
- Kehrl, W.; Sonnemann, U.; Dethlefsen, U. Therapy for acute nonpurulent rhinosinusitis with cineole: Results of a double-blind, randomized, placebo-controlled trial. Laryngoscope 2004, 114, 738–742. [Google Scholar] [CrossRef]
- Federspil, P.; Wulkow, R.; Zimmermann, T. Wirkung von Myrtol standardisiert* bei der Therapie der akuten Sinusitis-Ergebnisse einer doppelblinden, randomisierten Multicenterstudie gegen Plazebo. Laryngorhinootologie 1997, 76, 23–27. [Google Scholar] [CrossRef]
- Timmer, A.; Günther, J.; Motschall, E.; Rücker, G.; Antes, G.; Kern, W.V. Pelargonium sidoides extract for treating acute respiratory tract infections. Cochrane Database Syst. Rev. 2013, CD006323. [Google Scholar] [CrossRef]
- Bachert, C.; Schapowal, A.; Funk, P.; Kieser, M. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: A randomized, double-blind, placebo-controlled trial. Rhinology 2009, 47, 51–58. [Google Scholar]
- Perić, A.; Gaćeša, D.; Barać, A.; Sotirović, J.; Perić, A. Herbal drug EPs 7630 versus amoxicillin in patients with uncomplicated acute bacterial rhinosinusitis: A randomized, open-label study. Ann. Otol. Rhinol. Laryngol. 2020, 129, 969–976. [Google Scholar] [CrossRef]
- Kemmerich, B.; Eberhardt, R.; Stammer, H. Efficacy and tolerability of a fluid extract combination of thyme herb and ivy leaves and matched placebo in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled clinical trial. Arzneimittelforschung 2006, 56, 652–660. [Google Scholar] [PubMed]
- Kemmerich, B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of thyme herb and primrose root in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled multicentre clinical trial. Arzneimittelforschung 2007, 57, 607–615. [Google Scholar] [PubMed]
- Chuchalin, A.G.; Berman, B.; Lehmacher, W. Treatment of acute bronchitis in adults with a pelargonium sidoides preparation (EPs® 7630): A randomized, double-blind, placebo-controlled trial. EXPLORE 2005, 1, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Matthys, H.; Heger, M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs 7630): A randomised, double-blind, placebo-controlled, multicentre study. Curr. Med. Res. Opin. 2007, 23, 323–331. [Google Scholar] [CrossRef]
- Matthys, H.; Lizogub, V.G.; Malek, F.A.; Kieser, M. Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: A randomised, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides. Curr. Med. Res. Opin. 2010, 26, 1413–1422. [Google Scholar] [CrossRef]
- Fischer, J.; Dethlefsen, U. Efficacy of cineole in patients suffering from acute bronchitis: A placebo-controlled double-blind trial. Cough 2013, 9, 25. [Google Scholar] [CrossRef]
- Matthys, H.; de Mey, C.; Carls, C.; Ryś, A.; Geib, A.; Wittig, T. Efficacy and tolerability of myrtol standardized in acute bronchitis. A multi-centre, randomised, double-blind, placebo-controlled parallel group clinical trial vs. cefuroxime and ambroxol. Arzneimittelforschung 2000, 50, 700–711. [Google Scholar]
- Gillissen, A.; Wittig, T.; Ehmen, M.; Krezdorn, H.; de Mey, C. A multi-centre, randomised, double-blind, placebo-controlled clinical trial on the efficacy and tolerability of GeloMyrtol® forte in acute bronchitis. Drug Res. 2013, 63, 19–27. [Google Scholar] [CrossRef]
- Schaefer, A.; Kehr, M.S.; Giannetti, B.M.; Bulitta, M.; Staiger, C. A randomized, controlled, double-blind, multi-center trial to evaluate the efficacy and safety of a liquid containing ivy leaves dry extract (EA 575®) vs. placebo in the treatment of adults with acute cough. Pharmazie 2016, 71, 504–509. [Google Scholar]
- Cwientzek, U.; Ottillinger, B.; Arenberger, P. Acute bronchitis therapy with ivy leaves extracts in a two-arm study. A double-blind, randomised study vs. an other ivy leaves extract. Phytomedicine 2011, 18, 1105–1109. [Google Scholar] [CrossRef]
- Kruttschnitt, E.; Wegener, T.; Zahner, C.; Henzen-Bücking, S. Assessment of the efficacy and safety of ivy leaf (hedera helix) cough syrup compared with acetylcysteine in adults and children with acute bronchitis. Evid.-Based Complementary Altern. Med. 2020, 2020, 1910656. [Google Scholar] [CrossRef] [PubMed]
- Stuck, B.A.; Beule, A.; Jobst, D.; Klimek, L.; Laudien, M.; Lell, M.; Vogl, T.J.; Popert, U. Guideline for “rhinosinusitis”-long version: S2k guideline of the German College of General Practitioners and Family Physicians and the German Society for Oto-Rhino-Laryngology, Head and Neck Surgery. Hno 2018, 66, 38–74. [Google Scholar] [CrossRef] [PubMed]
- Kardos, P.; Dinh, Q.T.; Fuchs, K.H.; Gillissen, A.; Klimek, L.; Koehler, M.; Sitter, H.; Worth, H. German Respiratory Society guidelines for diagnosis and treatment of adults suffering from acute, subacute and chronic cough. Respir. Med. 2020, 170, 105939. [Google Scholar] [CrossRef]
- Krüger, K.; Holzinger, F.; Trauth, J.; Koch, M.; Heintze, C.; Gehrke-Beck, S. Clinical Practice Guideline: Chronic Cough. Dtsch. Arztebl. Int. 2022. Forthcoming. [Google Scholar] [CrossRef]
- German Society of Urology, D.G.U. Interdisziplinary S3 Guide Line: Epidemiology, Diagnostics, Therapy, Prevention and Management of Uncomplicated, Bacterial, Ambulantly Aquired Urinary Tract Infections in Adult Patients. Long Version 1.1-2, 2017. AWMF Registry Number: 043/044. 2017. Available online: https://www.awmf.org/uploads/tx_szleitlinien/043-044l_S3_Harnwegsinfektionen_2017-05.pdf (accessed on 18 July 2022).
- Cystitis: Are Herbal Medicinal Products Helpful in Cases of Recurrent Cystitis? [Blasenentzündung: Helfen pflanzliche Mittel bei wiederkehrender Blasenentzündung?, GERMAN], HTA-number: HT20-01, Version 1.0. IQWiG-Berichte. 2022. Available online: https://www.iqwig.de/sich-einbringen/themencheck-medizin-thema-vorschlagen/hta-berichte/ht20-01.html (accessed on 20 July 2022).
- Zitterl-Eglseer, K.; Marschik, T. Antiviral Medicinal Plants of Veterinary Importance: A Literature Review. Planta Med. 2020, 86, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds with Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Cela-Lopez, J.M.; Camacho Roldan, C.J.; Gomez-Lizarraga, G.; Martinez, V. A Natural Alternative Treatment for Urinary Tract Infections: Itxasol(c), the Importance of the Formulation. Molecules 2021, 26, 4564. [Google Scholar] [CrossRef]
- Dar, K.B.; Bhat, A.H.; Amin, S.; Masood, A.; Zargar, M.A.; Ganie, S.A. Inflammation: A Multidimensional Insight on Natural Anti-Inflammatory Therapeutic Compounds. Curr. Med. Chem. 2016, 23, 3775–3800. [Google Scholar]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C. Evidence-based management of acute rhinosinusitis with herbal products. Clin. Phytosci. 2020, 6, 85. [Google Scholar] [CrossRef]
- Martin, D.; Konrad, M.; Adarkwah, C.C.; Kostev, K. Reduced antibiotic use after initial treatment of acute respiratory infections with phytopharmaceuticals- a retrospective cohort study. Postgrad. Med. 2020, 132, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Murgia, V.; Manti, S.; Licari, A.; De Filippo, M.; Ciprandi, G.; Marseglia, G.L. Upper respiratory tract infection-associated acute cough and the urge to cough: New insights for clinical practice. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.; Thompson, M.; Buckley, D.I.; Heneghan, C.; Deyo, R.; Redmond, N.; Lucas, P.J.; Blair, P.S.; Hay, A.D. Interventions to influence consulting and antibiotic use for acute respiratory tract infections in children: A systematic review and meta-analysis. PLoS ONE 2012, 7, e30334. [Google Scholar] [CrossRef] [PubMed]
- Kuchar, E.; Miskiewicz, K.; Szenborn, L.; Kurpas, D. Respiratory tract infections in children in primary healthcare in Poland. Adv. Exp. Med. Biol. 2015, 835, 53–59. [Google Scholar] [PubMed]
- Kronman, M.P.; Gerber, J.S.; Grundmeier, R.W.; Zhou, C.; Robinson, J.D.; Heritage, J.; Stout, J.; Burges, D.; Hedrick, B.; Warren, L.; et al. Reducing antibiotic prescribing in primary care for respiratory illness. Pediatrics 2020, 146, e20200038. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Peterburs, P.; Seibel, J.; Abramov-Sommariva, D.; Lamy, E. In vitro screening of herbal medicinal products for their supportive curing potential in the context of SARS-CoV-2. bioRxiv 2021. [Google Scholar] [CrossRef]
- Seibel, J.; Kryshen, K.; Pongrácz, J.E.; Lehner, M.D. In vivo and in vitro investigation of anti-inflammatory and mucus-regulatory activities of a fixed combination of thyme and primula extracts. Pulm. Pharmacol. Ther. 2018, 51, 10–17. [Google Scholar] [CrossRef]
- Seibel, J.; Pergola, C.; Werz, O.; Kryshen, K.; Wosikowski, K.; Lehner, M.D.; Haunschild, J. Bronchipret® syrup containing thyme and ivy extracts suppresses bronchoalveolar inflammation and goblet cell hyperplasia in experimental bronchoalveolitis. Phytomedicine 2015, 22, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Seibel, J.; Wonnemann, M.; Werz, O.; Lehner, M.D. A tiered approach to investigate the mechanism of anti-inflammatory activity of an herbal medicinal product containing a fixed combination of thyme herb and primula root extracts. Clin. Phytosci. 2018, 4, 4. [Google Scholar] [CrossRef]
- Glatthaar-Saalmuller, B.; Rauchhaus, U.; Rode, S.; Haunschild, J.; Saalmuller, A. Antiviral activity in vitro of two preparations of the herbal medicinal product Sinupret(R) against viruses causing respiratory infections. Phytomedicine 2011, 19, 1–7. [Google Scholar] [CrossRef]
- Workman, A.D.; Maina, I.W.; Triantafillou, V.; Patel, N.N.; Tong, C.C.L.; Kuan, E.C.; Kennedy, D.W.; Palmer, J.N.; Adappa, N.D.; Cohen, N.A. Effects of BNO 1016 on ciliary transport velocity and cell culture surface liquid height of sinonasal epithelial cultures. Clin. Phytosci. 2021, 7, 35. [Google Scholar] [CrossRef]
- Cho, D.Y.; Skinner, D.; Mackey, C.; Lampkin, H.B.; Elder, J.B.; Lim, D.J.; Zhang, S.; McCormick, J.; Tearney, G.J.; Rowe, S.M.; et al. Herbal dry extract BNO 1011 improves clinical and mucociliary parameters in a rabbit model of chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2019, 9, 629–637. [Google Scholar] [CrossRef]
- Zhang, S.; Skinner, D.; Hicks, S.B.; Bevensee, M.O.; Sorscher, E.J.; Lazrak, A.; Matalon, S.; McNicholas, C.M.; Woodworth, B.A. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS ONE 2014, 9, e104090. [Google Scholar] [CrossRef] [PubMed]
- Kreindler, J.L.; Chen, B.; Kreitman, Y.; Kofonow, J.; Adams, K.M.; Cohen, N.A. The novel dry extract BNO 1011 stimulates chloride transport and ciliary beat frequency in human respiratory epithelial cultures. Am. J. Rhinol. Allergy 2012, 26, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Dehm, F.; Kiesselbach, C.; Haunschild, J.; Sautebin, L.; Werz, O. The novel Sinupret® dry extract exhibits anti-inflammatory effectiveness in vivo. Fitoterapia 2012, 83, 715–720. [Google Scholar] [CrossRef]
- Stämmler, F.; Gläsner, J.; Hiergeist, A.; Holler, E.; Weber, D.; Oefner, P.J.; Gessner, A.; Spang, R. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 2016, 4, 28. [Google Scholar] [CrossRef]
- Höller, M.; Steindl, H.; Abramov-Sommariva, D.; Wagenlehner, F.; Naber, K.G.; Kostev, K. Treatment of urinary tract infections with Canephron(®) in Germany: A retrospective database analysis. Antibiotics 2021, 10, 685. [Google Scholar] [CrossRef]
- Mestrovic, T.; Matijasic, M.; Peric, M.; Cipcic Paljetak, H.; Baresic, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Brenneis, C.; Künstle, G.; Haunschild, J. Spasmolytic Activity of Canephron® N on the Contractility of Rate and Human Isolated Urinary Bladder. In Proceedings of the 13th International Congress of the Society for Ethnopharmacology, Graz, Austria, 2–6 September 2012. [Google Scholar]
- Haloui, M.; Louedec, L.; Michel, J.B.; Lyoussi, B. Experimental diuretic effects of Rosmarinus officinalis and Centaurium erythraea. J. Ethnopharmacol. 2000, 71, 465–472. [Google Scholar] [CrossRef]
- Nausch, B.; Künstle, G.; Mönch, B.; Koeberle, A.; Werz, O.; Haunschild, J.C. Canephron N alleviates pain in experimental cystitis and inhibits reactive oxygen/nitrogen species as well as microsomal prostaglandin E2 synthase-1. Der. Urol. 2015, 54, 28. [Google Scholar]
- Künstle, G.; Brenneis, C.; Haunschild, J. 671 Efficacy of Canephron® N against bacterial adhesion, inflammation and bladder hyperactivity. Eur. Urol. Suppl. 2013, 12, e671. [Google Scholar] [CrossRef]
- Shebeko, S.K.; Chernykh, V.V.; Zupanets, K.O. Nephroprotective Effect of the Herbal Composition BNO 2103 in Rats with Renal Failure. Sci. Pharm. 2020, 88, 47. [Google Scholar] [CrossRef]
- Nausch, B.; Pace, S.; Pein, H.; Koeberle, A.; Rossi, A.; Künstle, G.; Werz, O. The standardized herbal combination BNO 2103 contained in Canephron(®) N alleviates inflammatory pain in experimental cystitis and prostatitis. Phytomedicine 2019, 60, 152987. [Google Scholar] [CrossRef]
- Naber, K.G.; Kogan, M.; Wagenlehner, F.M.E.; Siener, R.; Gessner, A. How the microbiome is influenced by the therapy of urological diseases: Standard versus alternative approaches. Clin. Phytosci. 2017, 3, 8. [Google Scholar] [CrossRef]
- Zaniboni, D.; Ceretti, E.; Gelatti, U.; Pezzotti, M.; Covolo, L. Antibiotic resistance: Is knowledge the only driver for awareness and appropriate use of antibiotics? Ann Ig 2021, 33, 21–30. [Google Scholar]
- Cai, T.; Verze, P.; Palmieri, A.; Gacci, M.; Lanzafame, P.; Malossini, G.; Nesi, G.; Bonkat, G.; Wagenlehner, F.M.; Mirone, V.; et al. Is Preoperative Assessment and Treatment of Asymptomatic Bacteriuria Necessary for Reducing the Risk of Postoperative Symptomatic Urinary Tract Infections After Urologic Surgical Procedures? Urology 2017, 99, 100–105. [Google Scholar] [CrossRef]
- Little, P.; Moore, M.V.; Turner, S.; Rumsby, K.; Warner, G.; Lowes, J.A.; Smith, H.; Hawke, C.; Leydon, G.; Arscott, A.; et al. Effectiveness of five different approaches in management of urinary tract infection: Randomised controlled trial. BMJ 2010, 340, c199. [Google Scholar] [CrossRef]
- Spurling, G.K.; Del Mar, C.B.; Dooley, L.; Foxlee, R.; Farley, R. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst. Rev. 2017, 9, CD004417. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nausch, B.; Bittner, C.B.; Höller, M.; Abramov-Sommariva, D.; Hiergeist, A.; Gessner, A. Contribution of Symptomatic, Herbal Treatment Options to Antibiotic Stewardship and Microbiotic Health. Antibiotics 2022, 11, 1331. https://doi.org/10.3390/antibiotics11101331
Nausch B, Bittner CB, Höller M, Abramov-Sommariva D, Hiergeist A, Gessner A. Contribution of Symptomatic, Herbal Treatment Options to Antibiotic Stewardship and Microbiotic Health. Antibiotics. 2022; 11(10):1331. https://doi.org/10.3390/antibiotics11101331
Chicago/Turabian StyleNausch, Bernhard, Claudia B. Bittner, Martina Höller, Dimitri Abramov-Sommariva, Andreas Hiergeist, and André Gessner. 2022. "Contribution of Symptomatic, Herbal Treatment Options to Antibiotic Stewardship and Microbiotic Health" Antibiotics 11, no. 10: 1331. https://doi.org/10.3390/antibiotics11101331
APA StyleNausch, B., Bittner, C. B., Höller, M., Abramov-Sommariva, D., Hiergeist, A., & Gessner, A. (2022). Contribution of Symptomatic, Herbal Treatment Options to Antibiotic Stewardship and Microbiotic Health. Antibiotics, 11(10), 1331. https://doi.org/10.3390/antibiotics11101331





