Optimized Chemical Extraction Methods of Antimicrobial Peptides from Roots and Leaves of Extremophilic Plants: Anthyllis sericea and Astragalus armatus Collected from the Tunisian Desert
Abstract
1. Introduction
2. Results
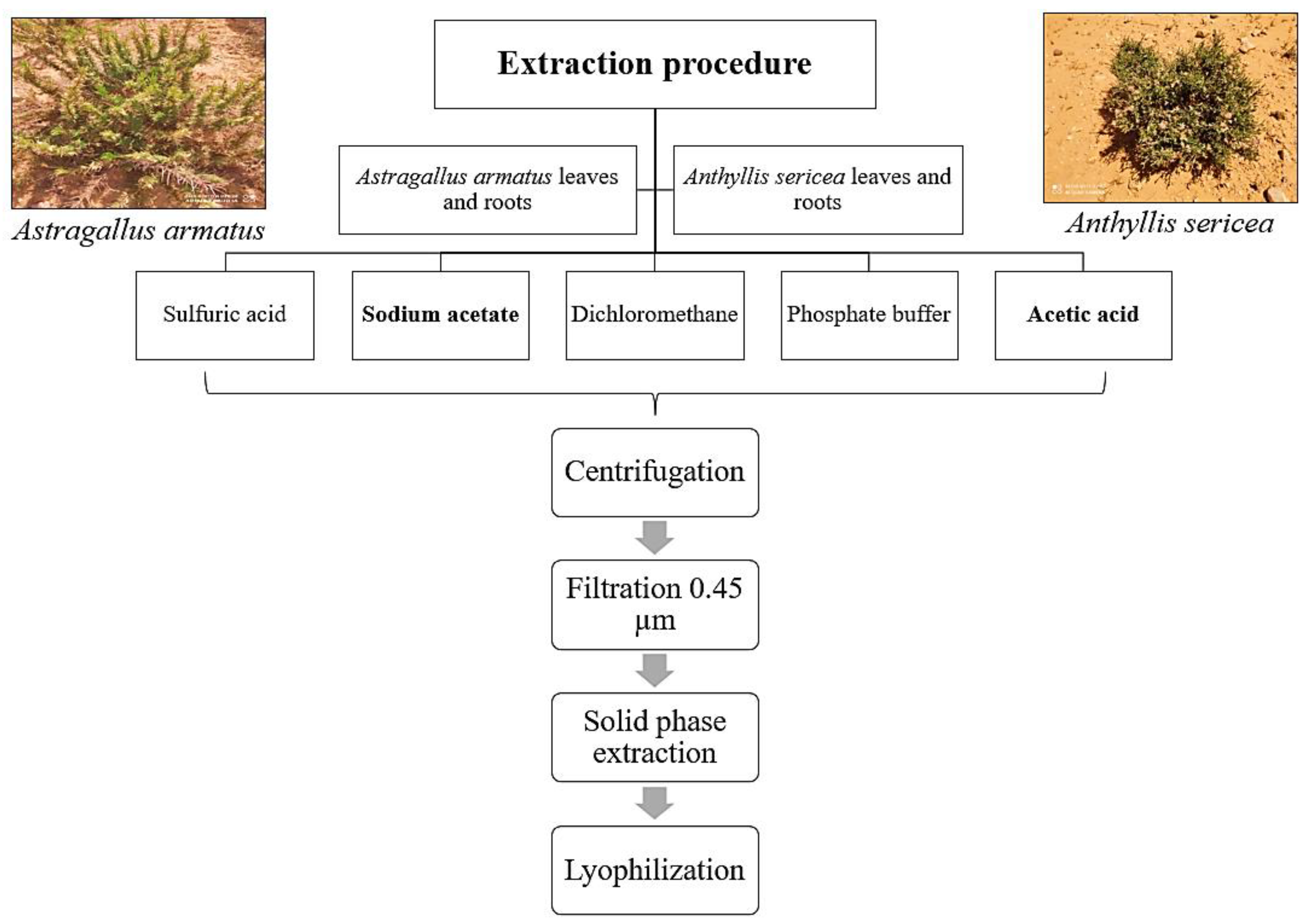
2.1. Extraction
2.2. Antimicrobial Activity
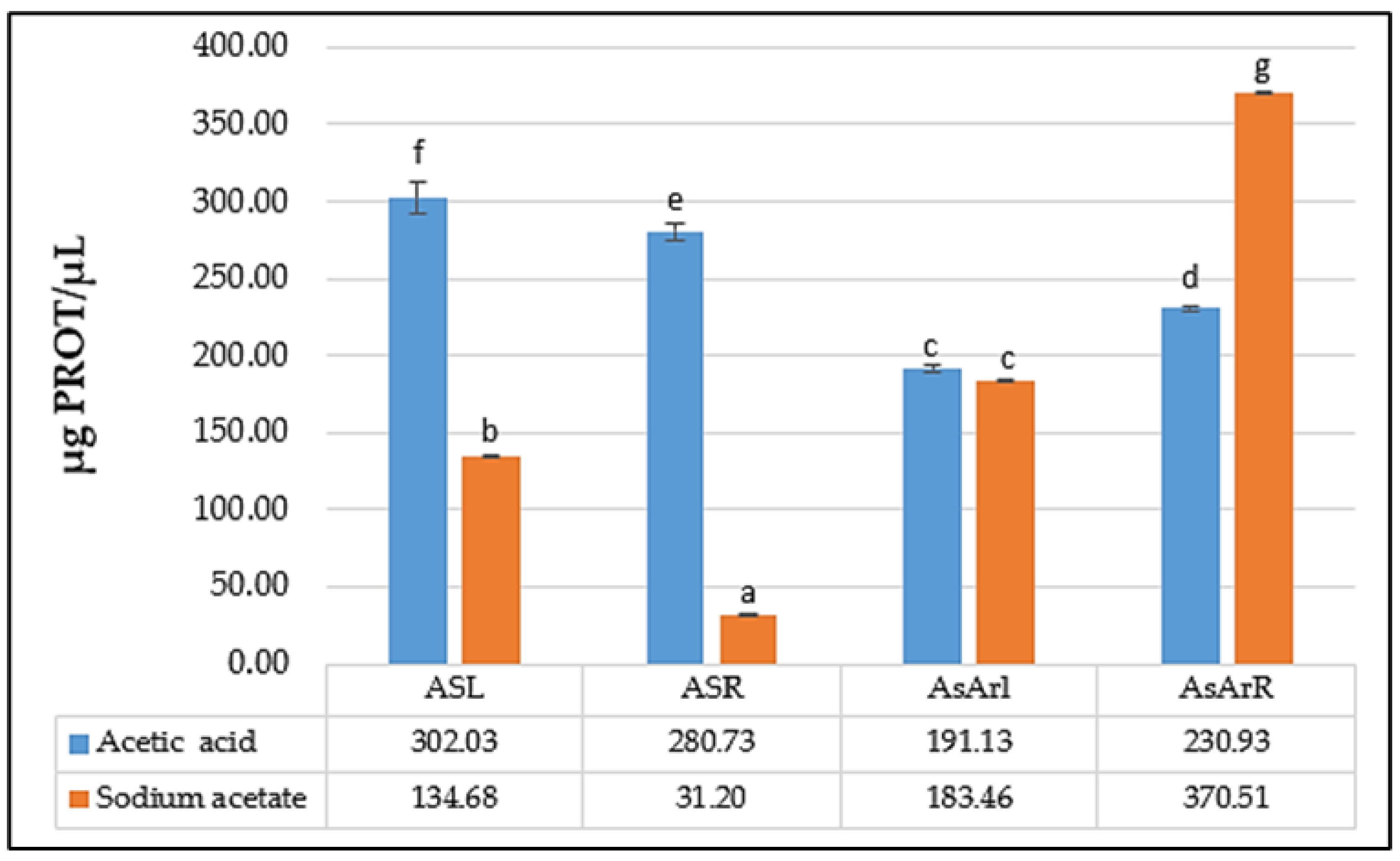
2.3. Protein Content of the Extracts
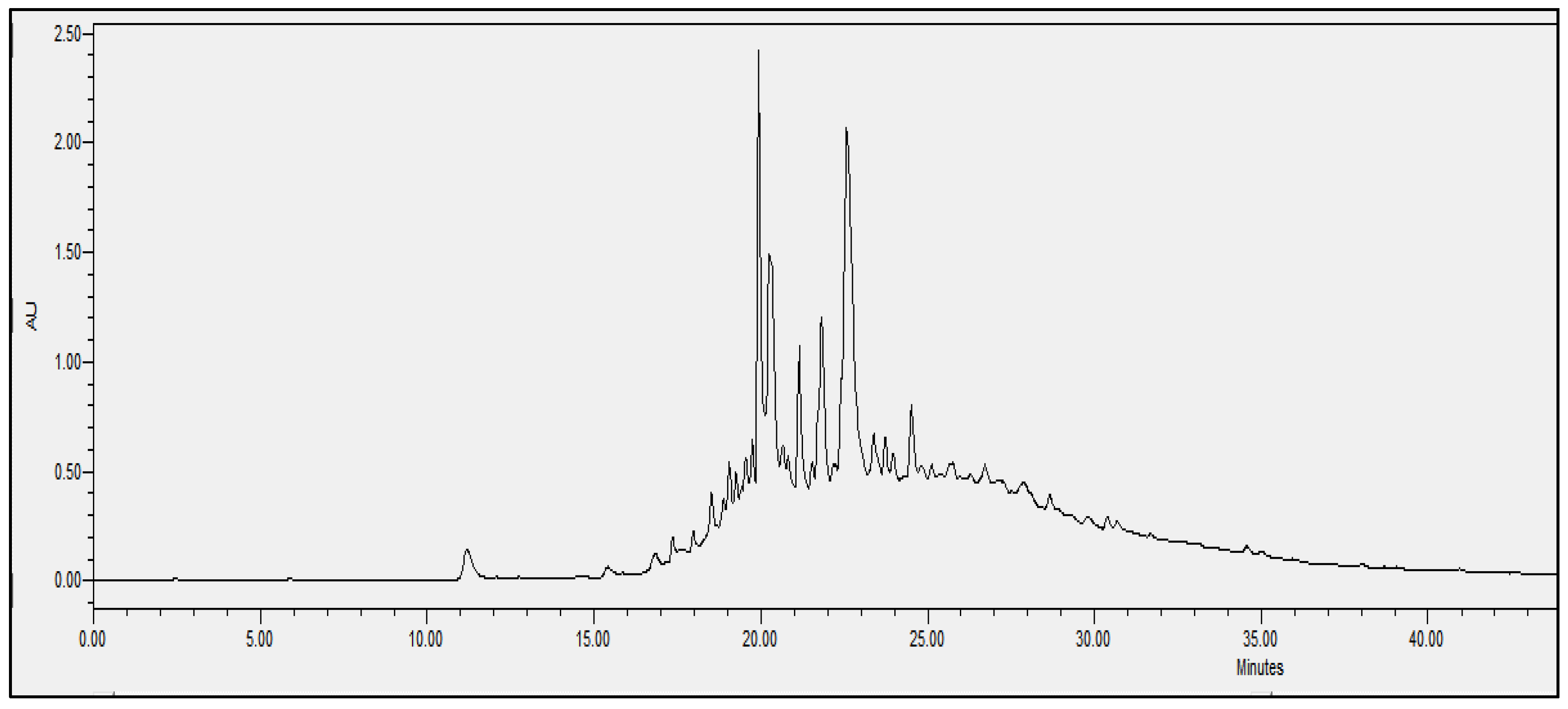
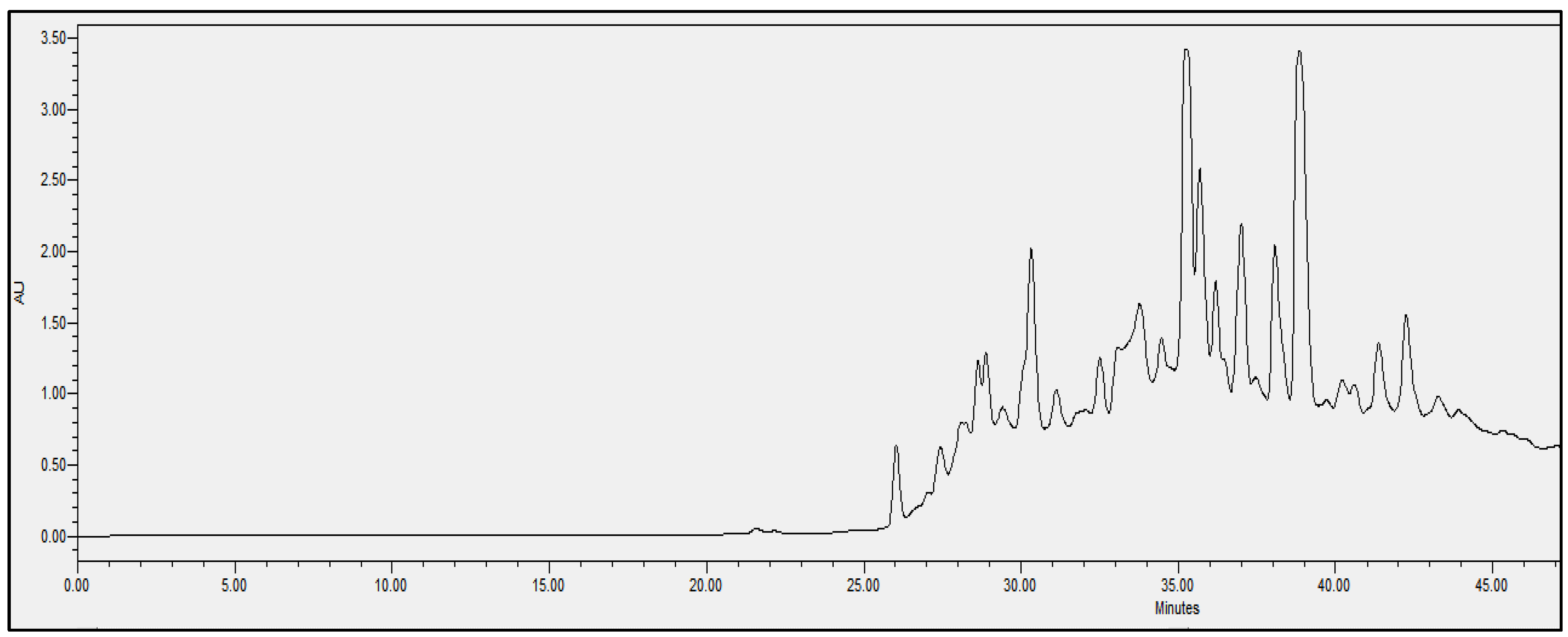
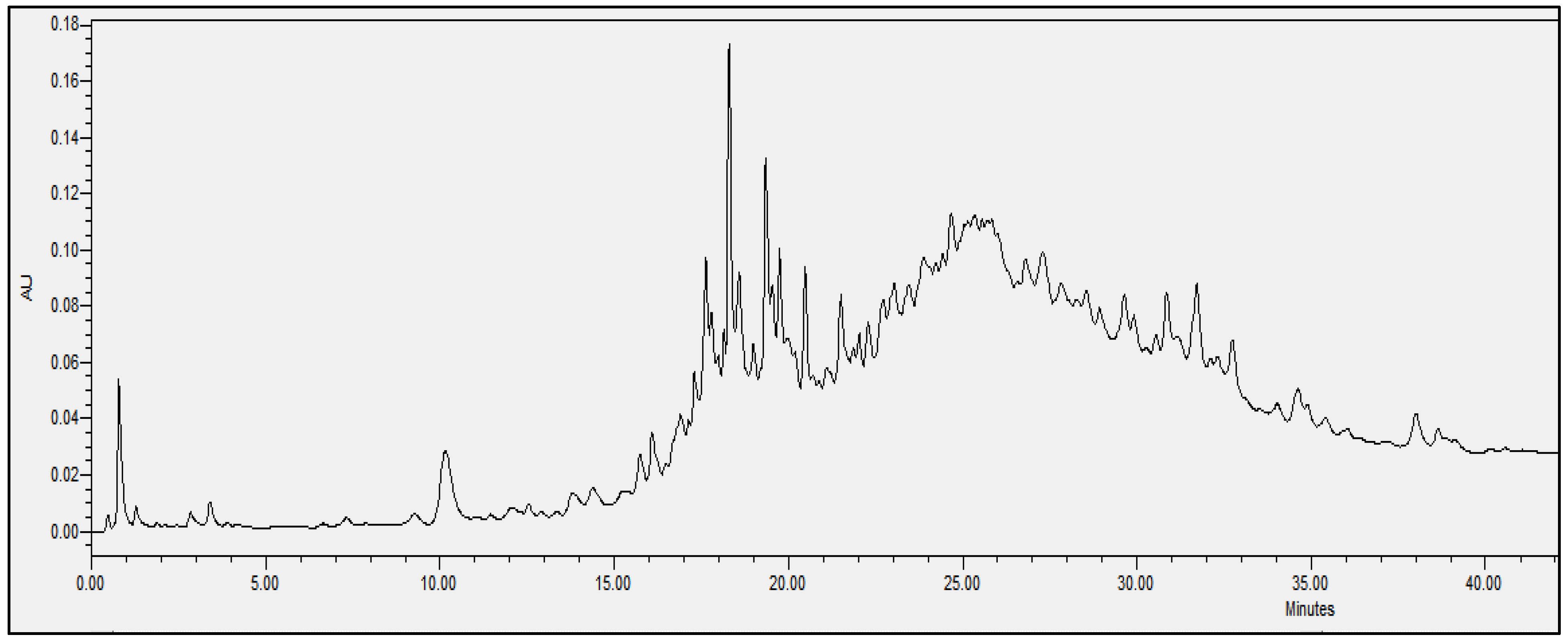
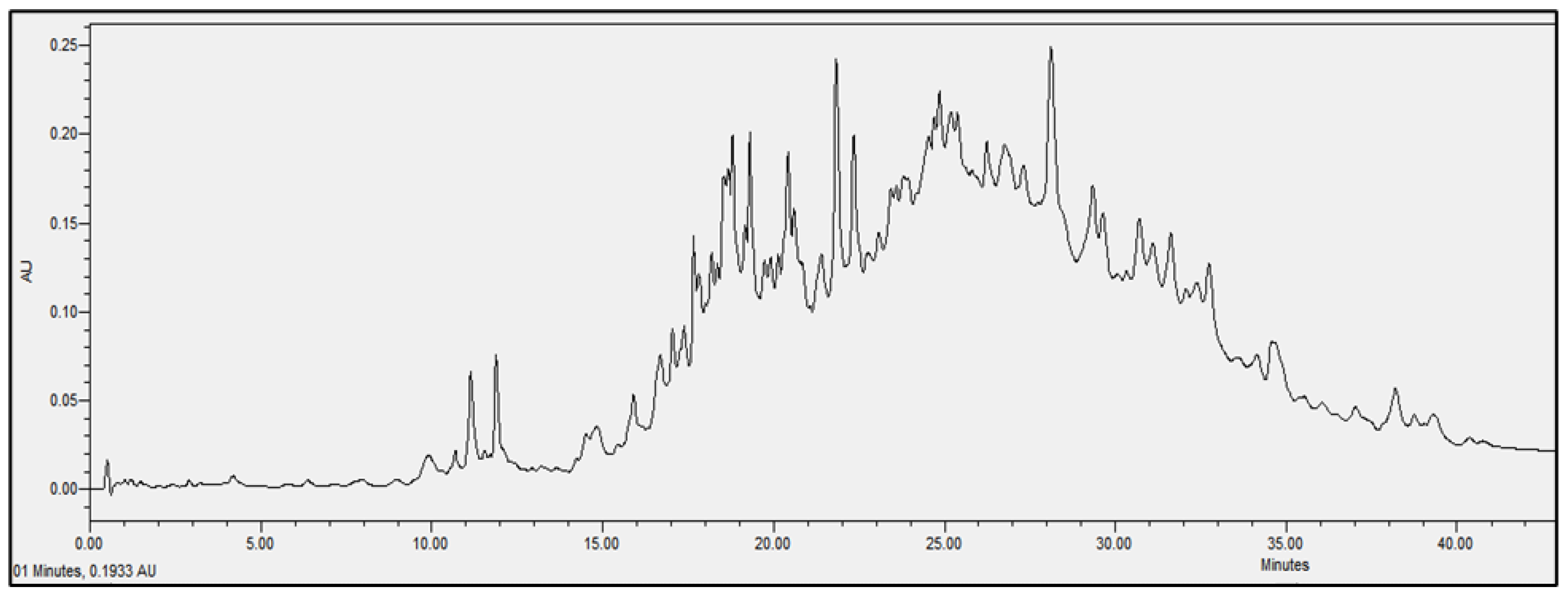
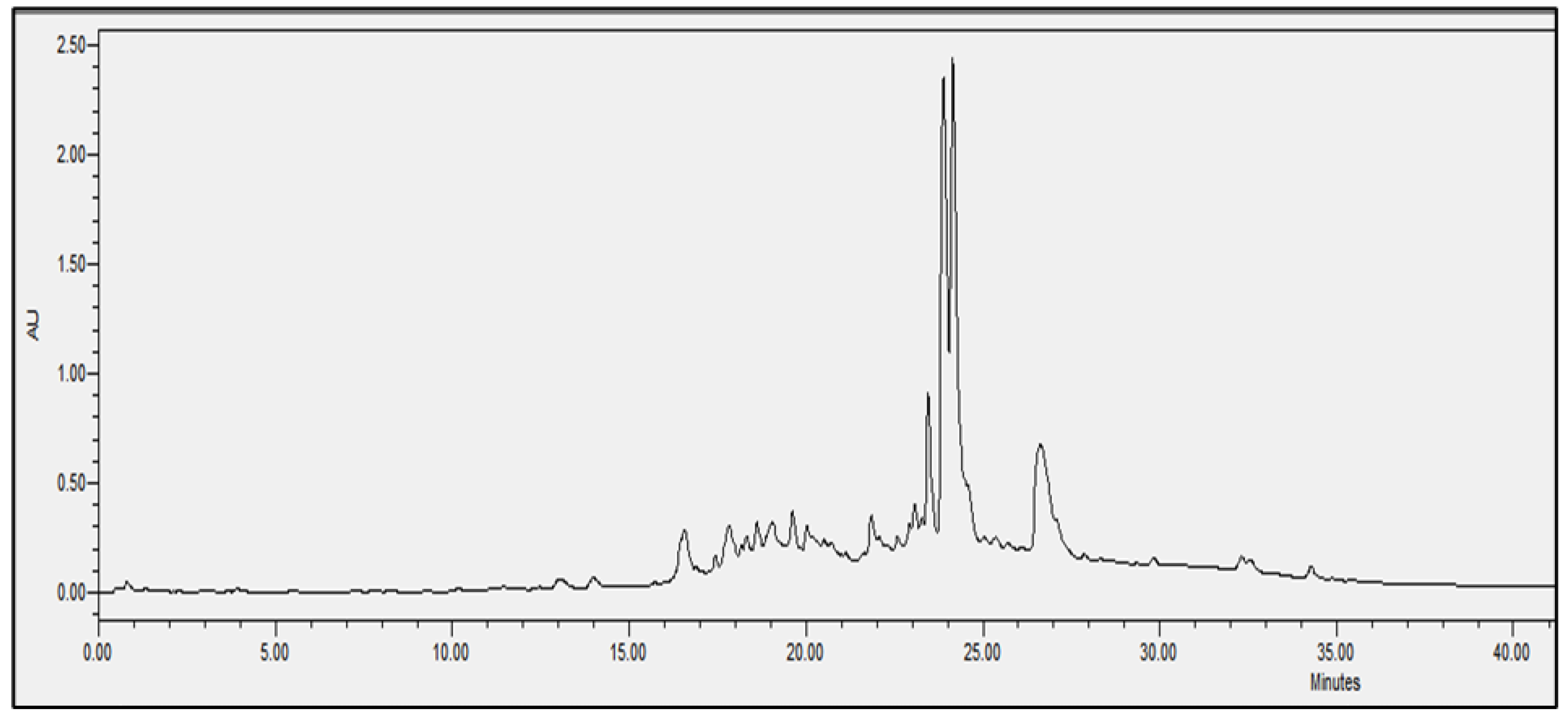
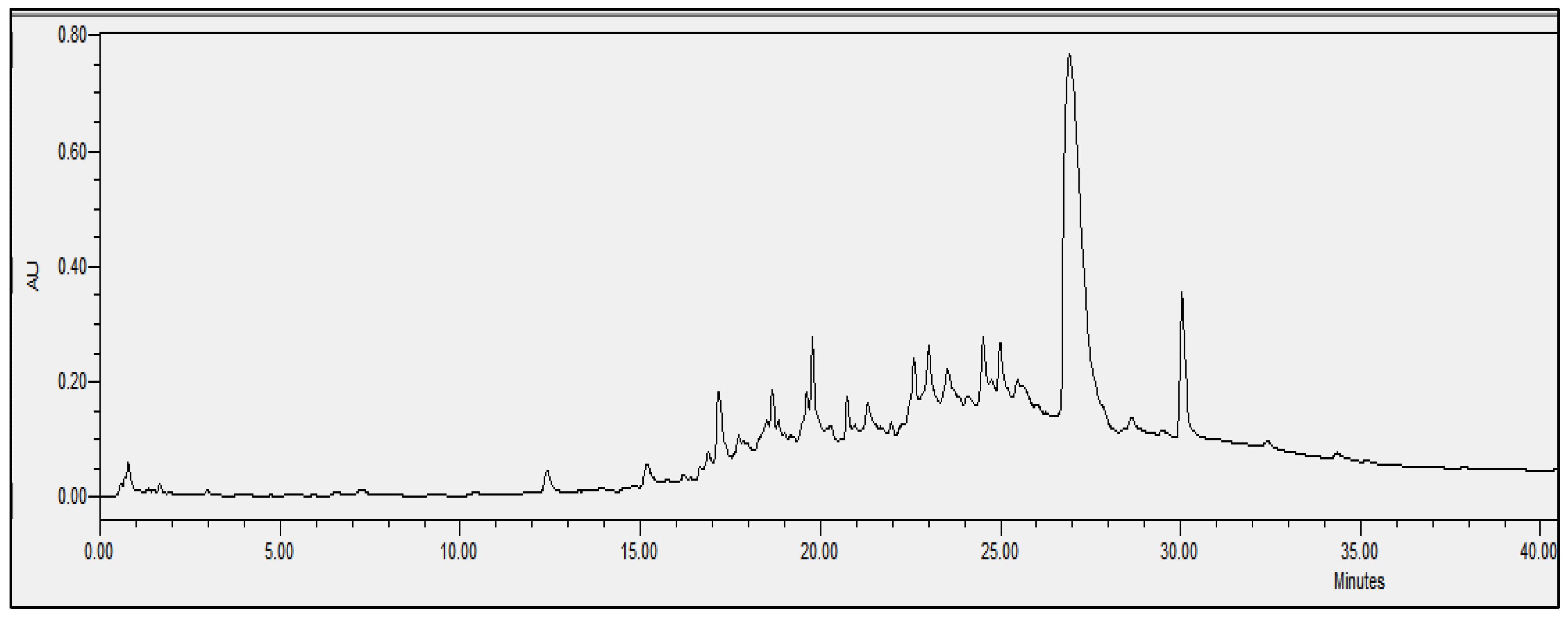
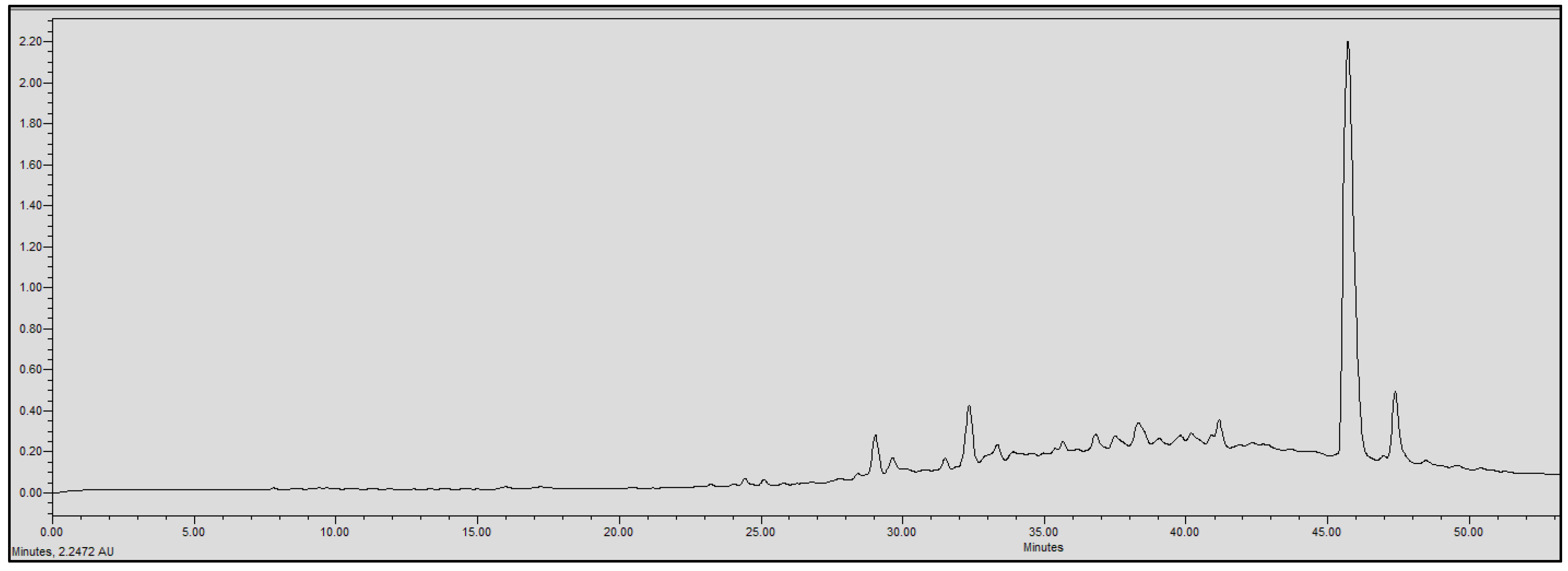
2.4. Chromatographic Analysis
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Bacterial Strains
4.3. Extraction of Antimicrobial Peptides
4.4. Antimicrobial Activity Tests
4.5. Protein Quantification
4.6. Liquid Chromatography (UPLC)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, M.; Tse, M.W.; Weller, J.; Chen, J.; Blainey, P.C. The future of antibiotics begins with discovering new combinations. Ann. N. Y. Acad. Sci. 2021, 1496, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Bartlett, J.G. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Infect. Dis. Clin. Pr. 2004, 12, 375. [Google Scholar] [CrossRef]
- Bourlioux, P.; Bourlioux, P. De quelles alternatives notre arsenal thérapeutique anti-infectieux dispose-t-il face aux bactéries multi-résistantes? Ann. Pharm. Fr. 2013, 71, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M.; Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Portieles, R.C.; Ayraand, O.; BorrásPortieles, R.C.; Ayraand, O.B. Basic insight on plant defensins. Biotecnol. Apl. 2006, 23, 75–78. [Google Scholar]
- Thevissen, K.; Kristensen, H.-H.; Thomma, B.P.; Cammue, B.P.; François, I.E. Therapeutic potential of antifungal plant and insect defensins. Drug Discov. Today 2007, 12, 966–971. [Google Scholar] [CrossRef]
- Park, I.Y.; Cho, J.H.; Kim, K.S.; Kim, Y.-B.; Kim, M.S.; Kim, S.C. Helix Stability Confers Salt Resistance upon Helical Antimicrobial Peptides. J. Biol. Chem. 2004, 279, 13896–13901. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial peptides from plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Tang, S.-S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.-F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Castro, M.S.; Fontes, M.S.C.A.W. Plant Defense and Antimicrobial Peptides. Protein Pept. Lett. 2005, 12, 11–16. [Google Scholar] [CrossRef]
- Fujimura, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization, and sequencing of a novel type of antimicrobial peptides, fa-amp1 and fa-amp2, from seeds of buckwheat (fagopyrum esculentum moench.). Biosci. Biotechnol. Biochem. 2003, 67, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, N. Antimicrobial Peptides with Unusual Amino Acid Compositions and Unusual Structures; Centre for cellular and Molecular Biology: Hyderabad, India, 2006. [Google Scholar]
- Fungal Pathogen Protection in Potato by Expression of a Plant Defensin Peptide; Nature America Inc.: Chinatown, NY, USA, 2000; pp. 1307–1310.
- Wijaya, R.G.M.; Neumann, R.; Condron, A.B.; Hughesand, G.M. Defense proteins from seed of Cassia fistula include a lipid transfer protein homologue and a protease inhibitory plant defensin. Plant Sci. 2000, 159, 243–255. [Google Scholar] [CrossRef]
- Salas, C.E.; Badillo-Corona, J.A.; Ramírez-Sotelo, G.; Oliver-Salvador, C. Biologically active and antimicrobial peptides from plants. BioMed Res. Int. 2015, 2015, 102129. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Amils, R.; Wagner, D.; McGenity, T. Life and applications of extremophiles. Environ. Microbiol. 2011, 13, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- First report of the in vitro antileishmanial properties of extremophile plants from the Algarve Coast. Nat. Prod. Res. 2017, 32, 600–604. [CrossRef]
- Adell, J.; Barbera, O.; Marco, J.A. Flavonoid glycosides from Anthyllis sericea. Phytochemistry 1988, 27, 2967–2970. [Google Scholar] [CrossRef]
- Selmi, A.H.; Debaya, T.; Trikiand, A.; FerchichiSelmi, A.H.; Debaya, T.; Trikiand, A.F. Effect of water deficiency on the cellular status and antioxidant defences in anthyllis sericea, a saharian plant. Pakistan J. Bot. 2017, 49, 1231–1237. [Google Scholar]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Hirche, A.; Salamani, M.; Abdellaoui, A.; Benhouhou, S.; Martinez-Valderrama, J. Landscape changes of desertification in arid areas: The case of south-west Algeria. Environ. Monit. Assess. 2010, 179, 403–420. [Google Scholar] [CrossRef]
- Chaudhary, L.B.T.S.; Ranaand, K.K. Current status of the systematics of Astragalus L. (Fabaceae) with special reference to the Himalayan species in India. Taiwania 2008, 53, 338–355. [Google Scholar] [CrossRef]
- Wojciechowski, M.F. Astragalus (Fabaceae): A molecular phylogenetic perspective. Brittonia 2005, 57, 382–396. [Google Scholar] [CrossRef]
- Labed, A.; Ferhat, M.; Labed-Zouad, I.; Kaplaner, E.; Zerizer, S.; Voutquenne-Nazabadioko, L.; Magid, A.A.; Semra, Z.; Kabouche, A.; Kabouche, Z.; et al. Compounds from the pods of Astragalus armatus with antioxidant, anticholinesterase, antibacterial and phagocytic activities. Pharm. Biol. 2016, 54, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Abdellaoui, R.; Boughalleb, F.; Yahia, B.; Mabrouk, M.; Nasri, N. Characterization of lipids, proteins, and bioactive compounds in the seeds of three Astragalus species. Food Chem. 2020, 339, 127824. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.; Limas, G.G. Isolation and characterization of thirteen new salt-soluble proteins from barley by reversed-phase high performance liquid chromatography. Planta 1988, 176, 221–229. [Google Scholar] [CrossRef]
- Okada, T.H.; Yoshizumiand, Y. A lethal toxic substance for brewing yeast in wheat and barley: Part I. assay of toxicity on various grains, and sensitivity of various yeast strains. Agric. Biol. Chem. 1970, 34, 1084–1088. [Google Scholar] [CrossRef]
- Okada, T.; Yoshizumi, H. A Lethal Toxic Substance for Brewing Yeast in Wheat and Barley: Part II. Isolation and Some Properties of Toxic Principle. Agric. Biol. Chem. 1970, 34, 1089–1094. [Google Scholar] [CrossRef]
- López-García, B.; Segundo, B.S.; Coca, M. Antimicrobial peptides as a promising alternative for plant disease protection. ACS Symp. Ser. 2012, 1095, 263–294. [Google Scholar] [CrossRef]
- Marshall, R.L.; Bavro, V.N. Multidrug resistance. Bact. Resist. Antibiot. Mol. Man 2017, 2, 201–237. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 1–27. [Google Scholar] [CrossRef]
- Crowe-McAuliffe, C.; Graf, M.; Huter, P.; Takada, H.; Abdelshahid, M.; Nováček, J.; Murina, V.; Atkinson, G.C.; Hauryliuk, V.; Wilson, D.N. Structural basis for antibiotic resistance mediated by the Bacillus subtilis ABCF ATPase VmlR. Proc. Natl. Acad. Sci. USA 2018, 115, 8978–8983. [Google Scholar] [CrossRef] [PubMed]
- Schito, G.C. The importance of the development of antibiotic resistance in Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12 (Suppl. S1), 3–8. [Google Scholar] [CrossRef] [PubMed]
- Catherinen, K. Antibiotic Resistance in Candida Albicans and Staphylococcus Aureus. CAOBIOS 2007, 20–25. [Google Scholar]
- Zhang, Y.; Lewis, K. Fabatins: New antimicrobial plant peptides. FEMS Microbiol. Lett. 1997, 149, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Atindehou, M.; Lagnika, L.; Guérold, B.; Strub, J.M.; Zhao, M.; Van Dorsselaer, A.; Marchioni, E.; Prévost, G.; Haikel, Y.; Taddéi, C.; et al. Isolation and Identification of Two Antibacterial Agents from Chromolaena odorata L. Active against Four Diarrheal Strains. Adv. Microbiol. 2013, 3, 115–121. [Google Scholar] [CrossRef]
- Song, X.; Zhou, Z.; Wang, J.; Wu, F.; Gong, W. Purification, characterization and preliminary crystallographic studies of a novel plant defensin from Pachyrrhizus erosus seeds. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Naouel, F.A.; Hellaland, A. Etude de l’activité antimicrobienne des souches de lactobacilles thermophiles utilisées dans l’ industrie laitière. Nature 2010, 13–20. Available online: https://www.asjp.cerist.dz/en/downArticle/47/2/2/41246 (accessed on 18 September 2022).
- Martí, M.; Frígols, B.; Serrano-Aroca, A. Antimicrobial characterization of advanced materials for bioengineering applications. J. Vis. Exp. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Mæhre, H.; Dalheim, L.; Edvinsen, G.; Elvevoll, E.; Jensen, I.-J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Brahim, R.; Ellouzi, H.; Fouzai, K.; Asses, N.; Neffati, M.; Sabatier, J.M.; Bulet, P.; Regaya, I. Optimized Chemical Extraction Methods of Antimicrobial Peptides from Roots and Leaves of Extremophilic Plants: Anthyllis sericea and Astragalus armatus Collected from the Tunisian Desert. Antibiotics 2022, 11, 1302. https://doi.org/10.3390/antibiotics11101302
Ben Brahim R, Ellouzi H, Fouzai K, Asses N, Neffati M, Sabatier JM, Bulet P, Regaya I. Optimized Chemical Extraction Methods of Antimicrobial Peptides from Roots and Leaves of Extremophilic Plants: Anthyllis sericea and Astragalus armatus Collected from the Tunisian Desert. Antibiotics. 2022; 11(10):1302. https://doi.org/10.3390/antibiotics11101302
Chicago/Turabian StyleBen Brahim, Raoua, Hasna Ellouzi, Khaoula Fouzai, Nedra Asses, Mohammed Neffati, Jean Marc Sabatier, Philippe Bulet, and Imed Regaya. 2022. "Optimized Chemical Extraction Methods of Antimicrobial Peptides from Roots and Leaves of Extremophilic Plants: Anthyllis sericea and Astragalus armatus Collected from the Tunisian Desert" Antibiotics 11, no. 10: 1302. https://doi.org/10.3390/antibiotics11101302
APA StyleBen Brahim, R., Ellouzi, H., Fouzai, K., Asses, N., Neffati, M., Sabatier, J. M., Bulet, P., & Regaya, I. (2022). Optimized Chemical Extraction Methods of Antimicrobial Peptides from Roots and Leaves of Extremophilic Plants: Anthyllis sericea and Astragalus armatus Collected from the Tunisian Desert. Antibiotics, 11(10), 1302. https://doi.org/10.3390/antibiotics11101302






