Evaluating the Efficacy of Eravacycline and Omadacycline against Extensively Drug-Resistant Acinetobacter baumannii Patient Isolates
Abstract
1. Introduction
2. Results
2.1. Determination of Antibiotic Susceptibilities of Standard-of-Care Drugs against Clinical Isolates of A. baumannii
2.2. Determination of the Minimum Inhibitory Concentrations of Eravacycline
2.3. Determination of the Minimum Inhibitory Concentrations of Omadacycline
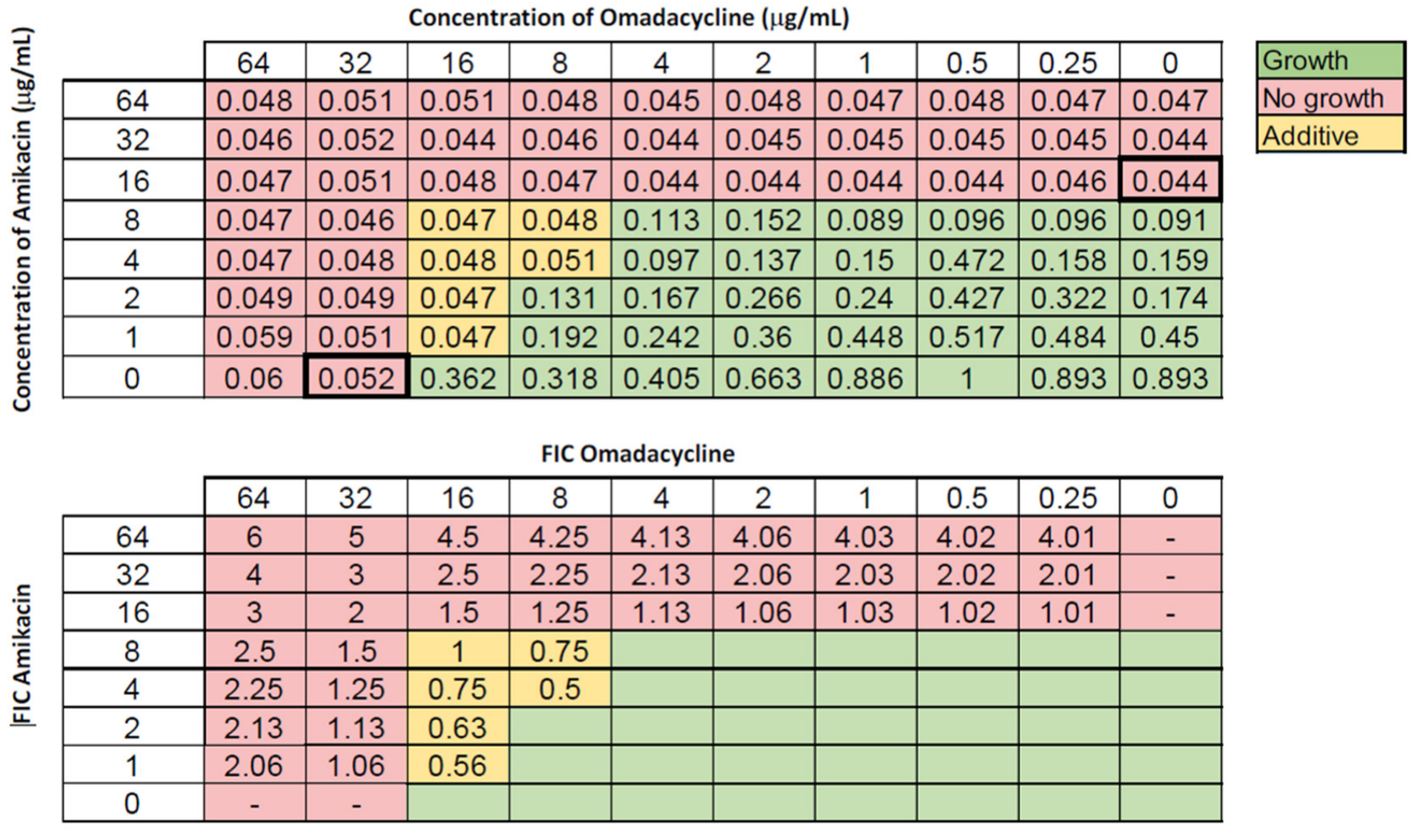
2.4. Determination of the Synergistic or Additive Effects of Eravacycline and Omadacycline Combined with Standard-of-Care Antibiotics
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Media, and Growth Conditions
4.2. Kirby–Bauer Disc Diffusion Assay
4.3. Broth Microdilution Assay
4.4. Checkerboard Assay
4.5. Interpretation of Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dettori, M.; Piana, A.; Deriu, M.G.; Curto, A.L.; Cossu, A.; Musumeci, R.; Cocuzza, C.; Astone, V.; Contu, M.A.; Sotgiu, G. Outbreak of multidrug-resistant Acinetobacter baumannii in an intensive care unit. New Microbiol. 2014, 37, 185–191. [Google Scholar] [PubMed]
- Chung, D.R.; Song, J.H.; Kim, S.H.; Thamlikitkul, V.; Huang, S.G.; Wang, H.; So, T.M.K.; Yasin, R.M.D.; Hsueh, P.R.; Carlos, C.C.; et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am. J. Resp. Crit. Care Med. 2011, 184, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Consales, G.; Gramigni, E.; Zamidei, L.; Bettocchi, D.; De Gaudio, A.R. A multidrug-resistant Acinetobacter baumannii outbreak in intensive care unit: Antimicrobial and organizational strategies. J. Crit. Care 2011, 26, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Con. Hosp. Epid. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Agodi, A.; Auxilia, F.; Barchitta, M.; Brusaferro, S.; D’Alessandro, D.; Montagna, M.T.; Orsi, G.B.; Pasquarella, C.; Torregrossa, V.; Suetens, C.; et al. Building a benchmark through active surveillance of intensive care unit-acquired infections: The Italian network SPIN-UTI. J. Hosp. Infect. 2010, 74, 258–265. [Google Scholar] [CrossRef]
- Martins, A.F.; Kuchenbecker, R.S.; Pilger, K.O.; Pagano, M.; Barth, A.L. High endemic levels of multidrug-resistant Acinetobacter baumannii among hospitals in southern Brazil. Am. J. Infect. Cont. 2012, 40, 108–112. [Google Scholar] [CrossRef]
- Luna, C.M.; Rodriguez-Noriega, E.; Bavestrello, L.; Guzmán-Blanco, M. Gram-negative infections in adult intensive care units of Latin America and the Caribbean. Crit. Care Res. Pract. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization report: Current crisis of antibiotic resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Biggest Threats and Data. Antibiotic. Antimicrobial Resistance. CDC. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html#acine (accessed on 16 April 2022).
- Shlaes, D.M.; Moellering, R.C. The United States Food and Drug Administration and the end of antibiotics. Clin. Infect. Dis. 2002, 34, 430. [Google Scholar] [CrossRef][Green Version]
- Zilberberg, M.D.; Kollef, M.H.; Shorr, A.F. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: A survey study. J. Hosp. Med. 2016, 11, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, V.; Sanchaita, S.; Singh, N. Multidrug resistant Acinetobacter. J. Glob. Infect. Dis. 2010, 2, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Cheong, H.J.; Choi, W.S.; Heo, J.Y.; Noh, J.Y.; Kim, W.J. Clinical and microbiological characterization of carbapenem-resistant Acinetobacter baumannii bloodstream infections. J. Med. Microbiol. 2011, 60, 605–611. [Google Scholar] [CrossRef]
- Evans, B.A.; Hamouda, A.; Amyes, S.G. The rise of carbapenem-resistant Acinetobacter baumannii. Curr. Pharm. Des. 2013, 19, 223–238. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Hittle, L.E.; O’Hara, J.A.; Rivera, J.I.; Syed, A.; Shields, R.K.; Pasculle, A.W.; Ernst, R.K.; Doi, Y. Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin. Infect. Dis. 2015, 60, 1295–1303. [Google Scholar] [CrossRef]
- Tsioutis, C.; Kritsotakis, E.I.; Karageorgos, S.A.; Stratakou, S.; Psarologakis, C.; Kokkini, S.; Gikas, A. Clinical epidemiology, treatment and prognostic factors of extensively drug-resistant Acinetobacter baumannii ventilator-associated pneumonia in critically ill patients. Int. J. Antimicrob. Agents 2016, 48, 492–497. [Google Scholar] [CrossRef]
- Lee, Y.R.; Burton, C.E. Eravacycline, a newly approved fluorocycline. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1787–1794. [Google Scholar] [CrossRef]
- Seifert, H.; Stefanik, D.; Sutcliffe, J.A.; Higgins, P.G. In vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Deresinski, S. Omadacycline: A novel tetracycline derivative with oral and intravenous formulations. Clin. Infect. Dis. 2019, 69, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Villano, S.; Steenbergen, J.; Loh, E. Omadacycline: Development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol. 2016, 11, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Ozger, H.S.; Cuhadar, T.; Yildiz, S.S.; Demirbas Gulmez, Z.; Dizbay, M.; Guzel Tunccan, O.; Kalkanci, A.; Simsek, H.; Unaldi, O. In vitro activity of eravacycline in combination with colistin against carbapenem-resistant A. baumannii isolates. J. Antibiot. 2019, 72, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, L.; Xue, F.; Wang, Q.; Zheng, B. Synergism of eravacycline combined with other antimicrobial agents against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. J. Glob. Antimicrob. Res. 2022, 30, 56–59. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Document M100 ED32:2022; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Landman, D.; Quale, J. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob. Agents Chemother. 2015, 59, 1802–1805. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. In vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 3840–3844. [Google Scholar] [CrossRef]
- Morrissey, I.; Olesky, M.; Hawser, S.; Lob Sibylle, H.; Karlowsky James, A.; Corey, G.R.; Bassetti, M.; Fyfe, C. In vitro activity of eravacycline against Gram-negative bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob. Agents Chemother. 2020, 64, e01699-19. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Zhang, Y.; Wang, R.; Wang, Q.; Li, H.; Wang, H. In vitro activities of eravacycline against 336 isolates collected from 2012 to 2016 from 11 teaching hospitals in China. BMC Infect. Dis. 2019, 19, 508. [Google Scholar] [CrossRef]
- Chiwunze, T.E.; Azumah, R.; Ramtahal, M.A.; Somboro, A.M.; Arvidsson, P.I.; Kruger, H.G.; Govender, T.; Naicker, T. Organocatalyzed mannich reactions on minocycline: Towards novel tetracycline antibiotics. S. Afr. J. Chem. 2016, 69, 72–78. [Google Scholar] [CrossRef]
- Iregui, A.; Landman, D.; Quale, J. Activity of omadacycline and other tetracyclines against contemporary Gram-negative pathogens from New York City hospitals. Microb. Drug. Resist. 2021, 27, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Huband, M.D.; Shortridge, D.; Flamm, R.K. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as part of the 2016 SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2018, 62, e02327-17. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, B.; Bazgir, Z.N.; Goli, H.R.; Iranpour, F.; Mohammadi, F.; Babaei, R. Prevalence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) phenotypes of Pseudomonas aeruginosa and Acinetobacter baumannii isolated in clinical samples from Northeast of Iran. BMC Res. Notes 2020, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Villedieu, A.; Bagdasarian, N.; Karah, N.; Teare, L.; Elamin, W.F. Control and management of multidrug resistant Acinetobacter baumannii: A review of the evidence and proposal of novel approaches. Infect. Prev. Pract. 2020, 2, 100077. [Google Scholar] [CrossRef] [PubMed]
- Navon-Venezia, S.; Leavitt, A.; Carmeli, Y. High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2007, 59, 772–774. [Google Scholar] [CrossRef]
- Hua, X.; He, J.; Wang, J.; Zhang, L.; Zhang, L.; Xu, Q.; Shi, K.; Leptihn, S.; Shi, Y.; Fu, X.; et al. Novel tigecycline resistance mechanisms in Acinetobacter baumannii mediated by mutations in adeS, rpoB and rrf. Emer. Microbes Infect. 2021, 10, 1404–1417. [Google Scholar] [CrossRef]
- Justo, J.A.; Bosso, J.A. Adverse reactions associated with systemic polymyxin therapy. Pharmacother 2015, 35, 28–33. [Google Scholar] [CrossRef]
- Rose, W.E.; Rybak, M.J. Tigecycline: First of a new class of antimicrobial agents. Pharmacother 2006, 26, 1099–1110. [Google Scholar] [CrossRef]
- Projan, S.J. Preclinical pharmacology of GAR-936, a novel glycylcycline antibacterial agent. Pharmacother 2000, 20, 219–223. [Google Scholar] [CrossRef]
- Sum, P.E.; Petersen, P. Synthesis and structure-activity relationship of novel glycylcycline derivatives leading to the discovery of GAR-936. Bioorg. Med. Chem. Lett. 1999, 9, 1459–1462. [Google Scholar] [CrossRef]
- Parveen, A. and Bhat, P. Evaluation of tigecycline and minocycline susceptibility among clinical isolates of carbapenem-resistant Acinetobacter species. J. Evolution Med. Dent. Sci. 2021, 10, 1408–1412. [Google Scholar] [CrossRef]
- Jo, J.; Ko, K.S. Tigecycline heteroresistance and resistance mechanism in clinical isolates of Acinetobacter baumannii. Microbiol. Spectr. 2021, 9, e0101021. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.-X.; Chen, C.; Cui, C.-Y.; Li, X.-P.; Zhang, Y.; Liao, X.-P.; Sun, J.; Liu, Y.-H. Emerging high-level tigecycline resistance: Novel tetracycline destructases spread via the mobile Tet(X). BioEssays 2020, 42, 2000014. [Google Scholar] [CrossRef] [PubMed]
- Heaney, M.; Mahoney, M.V.; Gallagher, J.C. Eravacycline: The Tetracyclines Strike Back. Ann. Pharmacother. 2019, 53, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Alosaimy, S.; Molina, K.C.; Claeys, K.C.; Andrade, J.; Truong, J.; King, M.A.; Pullinger, B.M.; Huang, G.; Morrisette, T.; Lagnf, A.M.; et al. Early experience with eravacycline for complicated infections. Open For. Infect. Dis. 2020, 7, ofaa071. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hua, X.; Xu, Q.; Yang, Y.; Zhang, L.; He, J.; Mu, X.; Hu, L.; Leptihn, S.; Yu, Y. Mechanism of eravacycline resistance in Acinetobacter baumannii mediated by a deletion mutation in the sensor kinase adeS, leading to elevated expression of the efflux pump AdeABC. Infect. Gen. Evol. 2020, 80, 104185. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Rechenmacher, A.; Böck, A. Interaction between aminoglycoside uptake and ribosomal resistance mutations. Antimicrob. Agents Chemother. 1980, 18, 798–806. [Google Scholar] [CrossRef]
- El’Garch, F.; Jeannot, K.; Hocquet, D.; Llanes-Barakat, C.; Plésiat, P. Cumulative effects of several nonenzymatic mechanisms on the resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 2007, 51, 1016–1021. [Google Scholar] [CrossRef]
- Mogre, A.; Sengupta, T.; Veetil, R.T.; Ravi, P.; Seshasayee, A.S.N. Genomic analysis reveals distinct concentration-dependent evolutionary trajectories for antibiotic resistance in Escherichia coli. DNA Res. 2014, 21, 711–726. [Google Scholar] [CrossRef]
- Wang, J.-H.; Singh, R.; Benoit, M.; Keyhan, M.; Sylvester, M.; Hsieh, M.; Thathireddy, A.; Hsieh, Y.-J.; Matin, A.C. Sigma S-dependent antioxidant defense protects stationary-phase Escherichia coli against the bactericidal antibiotic gentamicin. Antimicrob. Agents Chemother. 2014, 58, 5964–5975. [Google Scholar] [CrossRef]
- Mazurkiewicz, P.; Driessen, A.J.; Konings, W.N. What do proton motive force driven multidrug resistance transporters have in common? Curr. Issues Mol. Biol. 2005, 7, 7–21. [Google Scholar] [PubMed]
- Tanaka, S.K.; Steenbergen, J.; Villano, S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg. Med. Chem. 2016, 24, 6409–6419. [Google Scholar] [CrossRef] [PubMed]
- Abbey, T.; Vialichka, A.; Jurkovic, M.; Biagi, M.; Wenzler, E. Activity of omadacycline alone and in combination against carbapenem-nonsusceptible Acinetobacter baumannii with varying minocycline susceptibility. Microbiol. Spec. 2022, 10, e00542-22. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.; Putra, V.; Maring, B.L.; Ozer, E.A.; Belfiore, G.M.; Rhodes, N.J. Effect of omadacycline alone and in combination with meropenem against carbapenem-resistant Acinetobacter baumannii isolates. J. Glob. Antimicrob. Res. 2022, 29, 147–149. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disc Diffusion Susceptibility Test Protocol; American Society For Microbiology: Washington, DC, USA, 2012. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Bajaksouzian, S.; Visalli, M.A.; Jacobs, M.R.; Appelbaum, P.C. Activities of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with amikacin, against acinetobacters as determined by checkerboard and time-kill studies. Antimicrob. Agents Chemother. 1997, 41, 1073–1076. [Google Scholar] [CrossRef]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef]
- Thapa, B.; Tribuddharat, C.; Rugdeekha, S.; Techachaiwiwat, W.; Srifuengfung, S.; Dhiraputra, C. Rifampin resistance in carbapenem-resistant Acinetobacter baumannii in Siriraj Hospital, Thailand. Nepal Med. Coll. J. 2009, 11, 232–237. [Google Scholar]
- Jones, R.N.; Ferraro, M.J.; Relier, L.B.; Schreckenberger, P.C.; Swenson, J.M.; Sader, H.S. Multicenter studies of tigecycline disc diffusion susceptibility results for Acinetobacter spp. J. Clin. Microbiol. 2007, 45, 227–230. [Google Scholar] [CrossRef]

| Antibiotic | Susceptible | Intermediate | Resistant |
|---|---|---|---|
| Doripenem | 57.9% | 5.30% | 36.8% |
| Imipenem | 21.1% | 0 | 78.9% |
| Meropenem | 36.8% | 0 | 63.2% |
| Ampicillin-sulbactam | 42.1% | 0 | 57.9% |
| Piperacillin-tazobactam | 31.6% | 0 | 68.4% |
| Ticarcillin-clavulanate | 26.3% | 15.8% | 57.9% |
| Cefepime | 52.6% | 0 | 47.4% |
| Cefotaxime | 31.6% | 0 | 68.4% |
| Ceftazidime | 21.1% | 0 | 78.9% |
| Ceftriaxone | 26.3% | 0 | 73.7% |
| Doxycycline | 21.1% | 0 | 78.9% |
| Minocycline | 73.7% | 10.5% | 15.8% |
| Tetracycline | 1.10% | 0 | 98.9% |
| Tigecycline | 31.6% | 15.8% | 52.6% |
| Amikacin | 73.7% | 15.8% | 10.5% |
| Gentamicin | 26.3% | 0 | 73.7% |
| Netilmicin | 42.1% | 5.30% | 52.6% |
| Tobramycin | 42.1% | 0 | 57.9% |
| Ciprofloxacin | 26.3% | 0 | 73.7% |
| Levofloxacin | 26.3% | 26.3% | 47.4% |
| Trimethoprim-sulfamethoxazole | 0 | 0 | 100% |
| Rifampin | 52.6% | 0 | 47.4% |
| Colistin * | - | 31.6% | 68.4% |
| Polymyxin B * | - | 36.8% | 63.2% |
| Eravacycline | Omadacycline | |
|---|---|---|
| Strain | MIC | MIC |
| ACB3 | 0.06 | 0.5 |
| ACB4 | 0.8 | 6.0 |
| ACB5 | 0.09 | 1.0 |
| ACB9 | 0.69 | 24 |
| ACB16 | 3.0 | 8.0 |
| ACB25 | 2.1 | 3.0 |
| ACB28 | 1.0 | 16 |
| ACB29 | 0.8 | 12 |
| ACB30 | 2.5 | 3.0 |
| ACB49 | 1.0 | 16 |
| ACB51 | 3.0 | 4.0 |
| ACB53 | 3.0 | 12 |
| ACB54 | 0.6 | 3.0 |
| ACB55 | 1.2 | 6.0 |
| ACB56 | 2.0 | 4.0 |
| ACB57 | 0.6 | 12 |
| ACB58 | 0.5 | 2.0 |
| ACB60 | 0.5 | 16 |
| ACB61 | 1.5 | 8.0 |
| Antibiotic | Amount (μg) |
|---|---|
| Meropenem | 10 |
| Imipenem | 10 |
| Ampicillin-sulbactam | 10/10 |
| Piperacillin-tazobactam | 100/10 |
| Ceftazidime | 30 |
| Ceftriaxone | 30 |
| Gentamicin | 10 |
| Amikacin | 30 |
| Tobramycin | 40 |
| Tetracycline | 30 |
| Doxycycline | 5 |
| Minocycline | 30 |
| Omadacycline | 30 |
| Eravacycline | 20 |
| Levofloxacin | 5 |
| Trimethoprim-sulfamethoxazole | 1.25/23.75 |
| Rifampin | 10 |
| Tigecycline | 15 |
| Antibiotic | X-Value (μg/mL) |
|---|---|
| Doripenem | 32 |
| Meropenem | 16 |
| Imipenem | 32 |
| Ampicillin-sulbactam | 128/64 |
| Piperacillin-tazobactam | 512/16 |
| Timentin * | 512/34 |
| Cefepime | 256 |
| Cefotaxime | 512 |
| Ceftazidime | 128 |
| Ceftriaxone | 128 |
| Gentamicin | 128 |
| Amikacin | 128 |
| Netilmicin | 128 |
| Tobramycin | 128 |
| Tetracycline | 128 |
| Doxycycline | 128 |
| Minocycline | 32 |
| Omadacycline | 64 |
| Eravacycline | 32 |
| Levofloxacin | 64 |
| Ciprofloxacin | 32 |
| Trimethoprim-sulfamethoxazole | 32/608 |
| Rifampin | 4 |
| Tigecycline | 16 |
| Colistin | 64 |
| Polymyxin B | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deolankar, M.S.; Carr, R.A.; Fliorent, R.; Roh, S.; Fraimow, H.; Carabetta, V.J. Evaluating the Efficacy of Eravacycline and Omadacycline against Extensively Drug-Resistant Acinetobacter baumannii Patient Isolates. Antibiotics 2022, 11, 1298. https://doi.org/10.3390/antibiotics11101298
Deolankar MS, Carr RA, Fliorent R, Roh S, Fraimow H, Carabetta VJ. Evaluating the Efficacy of Eravacycline and Omadacycline against Extensively Drug-Resistant Acinetobacter baumannii Patient Isolates. Antibiotics. 2022; 11(10):1298. https://doi.org/10.3390/antibiotics11101298
Chicago/Turabian StyleDeolankar, Manas S., Rachel A. Carr, Rebecca Fliorent, Sean Roh, Henry Fraimow, and Valerie J. Carabetta. 2022. "Evaluating the Efficacy of Eravacycline and Omadacycline against Extensively Drug-Resistant Acinetobacter baumannii Patient Isolates" Antibiotics 11, no. 10: 1298. https://doi.org/10.3390/antibiotics11101298
APA StyleDeolankar, M. S., Carr, R. A., Fliorent, R., Roh, S., Fraimow, H., & Carabetta, V. J. (2022). Evaluating the Efficacy of Eravacycline and Omadacycline against Extensively Drug-Resistant Acinetobacter baumannii Patient Isolates. Antibiotics, 11(10), 1298. https://doi.org/10.3390/antibiotics11101298







