Zinc Oxide Nanoconjugates against Brain-Eating Amoebae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Henrietta Lacks (HeLa) Cervical Cancer Cells
2.2. Naegleria fowleri Culture
2.3. Balamuthia mandrillaris Culture
2.4. Amoebicidal Assay
2.5. Cytotoxicity Assay
2.6. Cytopathogenicity Assay
2.7. Statistical Analysis
3. Results
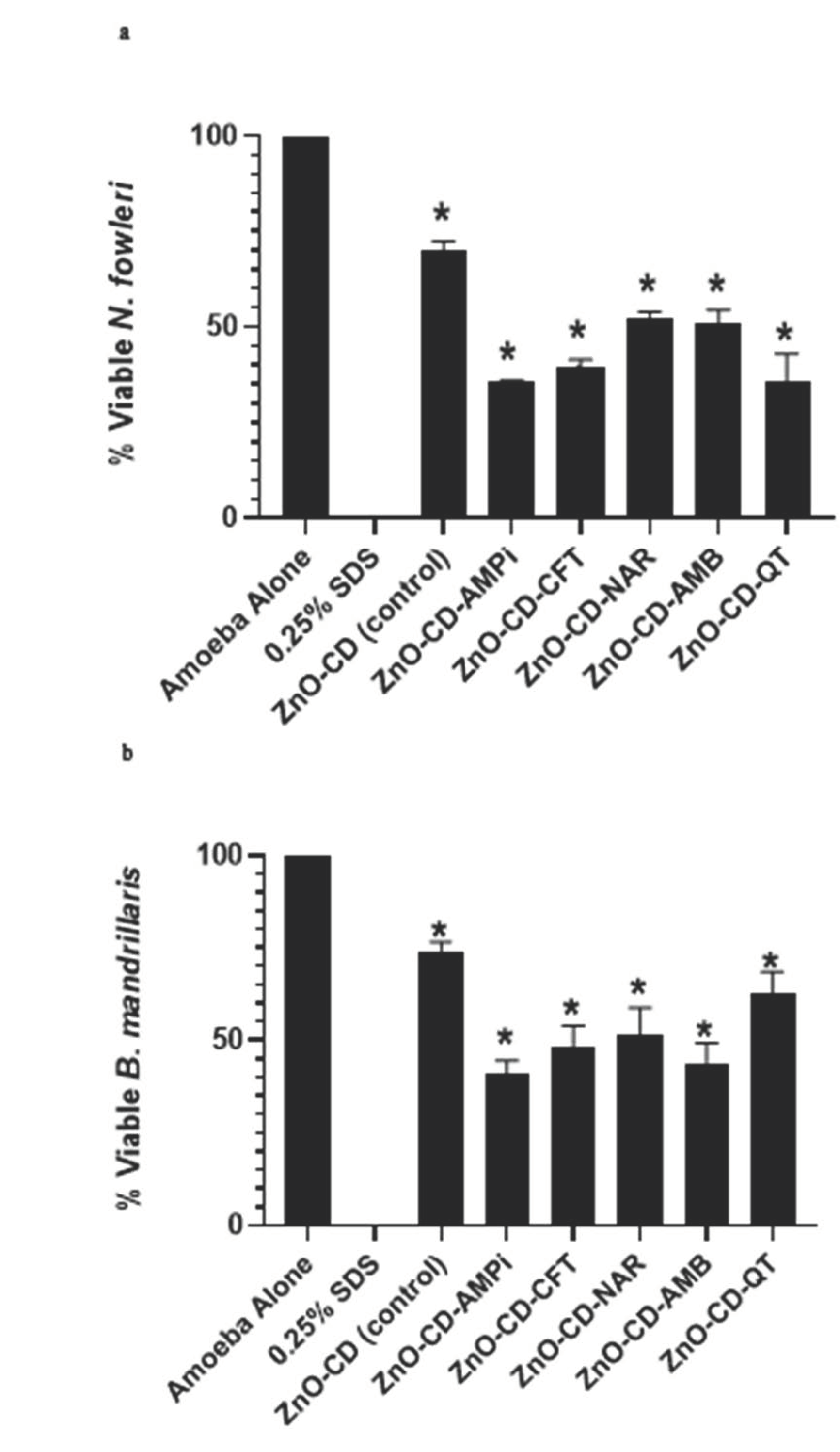
3.1. The Conjugates of Zinc Oxide Showed Significant Amoebicidal Properties against N. fowleri and B. mandrillaris
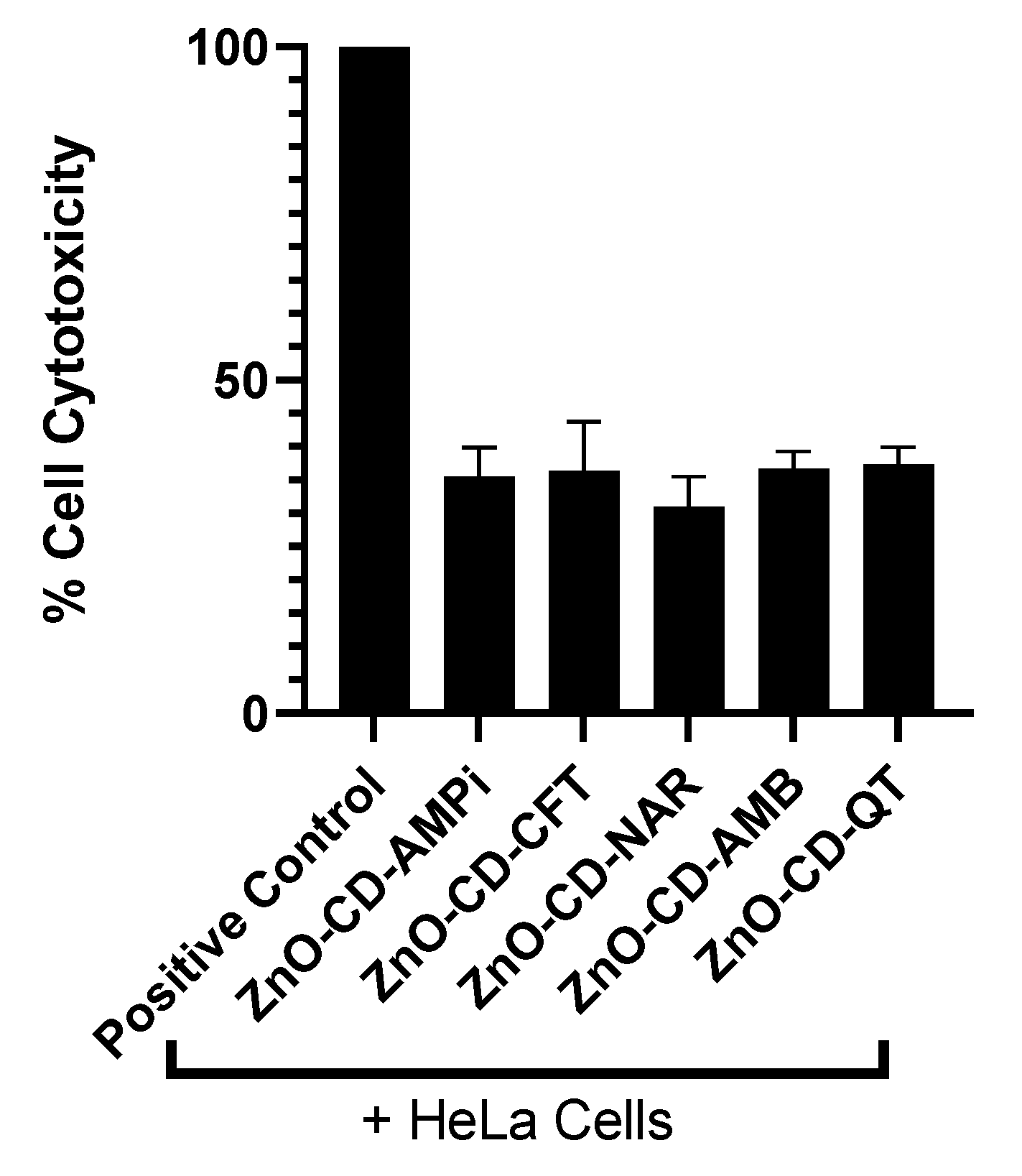
3.2. Limited Cytotoxic Activity Was Observed against Human Cells
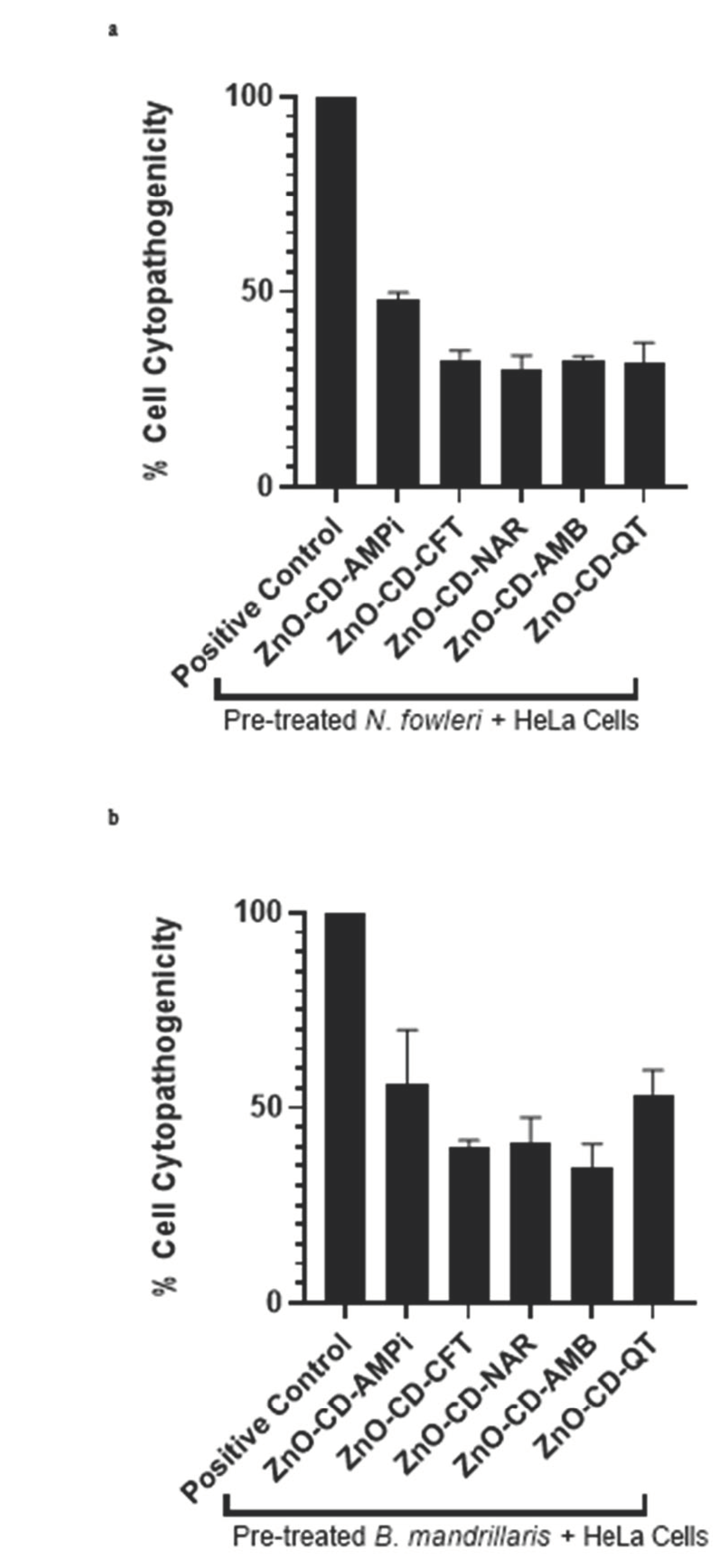
3.3. All Compounds Promoted a Decrease in Amoeba-Mediated Cytotoxicity in Human Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taravaud, A.; Fechtali-Moute, Z.; Loiseau, P.M.; Pomel, S. Drugs used for the treatment of cerebral and disseminated infections caused by free-living amoebae. Clin. Transl. Sci. 2021, 14, 791–805. [Google Scholar] [CrossRef]
- Schuster, F.L. Cultivation of Pathogenic and Opportunistic Free-Living Amebas. Clin. Microbiol. Rev. 2002, 15, 342–354. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yi, M.H.; Kim, M.; Yeom, J.S.; Yoo, H.D.; Kim, S.M.; Yong, T.S. Diagnosis of Balamuthia mandrillaris encephalitis by Thymine-Adenine cloning using universal eukaryotic primers. Ann. Lab. Med. 2022, 42, 196–202. [Google Scholar] [CrossRef]
- Mungroo, M.R.; Anwar, A.; Khan, N.A.; Siddiqui, R. Gold-conjugated curcumin as a novel therapeutic agent against brain-eating amoebae. ACS Omega 2020, 5, 12467–12475. [Google Scholar] [CrossRef]
- Król-Turmińska, K.; Olender, A. Human infections caused by free-living amoebae. Ann. Agric. Environ. Med. 2017, 24, 254–260. [Google Scholar] [CrossRef]
- Matin, A.; Siddiqui, R.; Jayasekera, S.; Khan, N.A. Increasing Importance of Balamuthia mandrillaris. Clin. Microbiol. Rev. 2008, 21, 435–448. [Google Scholar] [CrossRef]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Siddiqui, R. Brain-Eating Amoebae: Silver nanoparticle conjugation enhanced efficacy of anti-amoebic drugs against Naegleria fowleri. ACS Chem. Neurosci. 2017, 8, 2626–2630. [Google Scholar] [CrossRef]
- Da Rocha-Azevedo, B.; Tanowitz, H.B.; Marciano-Cabral, F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 251406. [Google Scholar] [CrossRef]
- Anwar, A.; Mungroo, M.R.; Khan, S.; Fatima, I.; Rafique, R.; Kanwal; Khan, K.M.; Siddiqui, R.; Khan, N.A. Novel azoles as antiparasitic remedies against brain-eating amoebae. Antibiotics 2020, 9, 188. [Google Scholar] [CrossRef]
- De Jonckheere, J.F. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect. Genet. Evol. 2011, 11, 1520–1528. [Google Scholar] [CrossRef]
- John, D.T. Primary amebic meningoencephalitis and the Biology of Naegleria fowleri. Annu. Rev. Microbiol. 1982, 36, 101–123. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; Cárdenas-Zúñiga, R.; Coronado-Velázquez, D.; Debnath, A.; Serrano-Luna, J.; Shibayama, M. Naegleria fowleri after 50 years: Is it a neglected pathogen? J. Med. Microbiol. 2016, 65, 885–896. [Google Scholar] [CrossRef]
- Debnath, A.; Tunac, J.B.; Galindo-Gómez, S.; Silva-Olivares, A.; Shibayama, M.; McKerrow, J.H. Corifungin, a new drug lead against Naegleria, identified from a high-throughput screen. Antimicrob. Agents Chemother. 2012, 56, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Matanock, A.; Mehal, J.M.; Liu, L.; Blau, D.M.; Cope, J.R. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States. Emerg. Infect. Dis. 2018, 24, 162. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khamis, M.; Ibrahim, T.; Khan, N.A. Brain-Eating Amoebae in the United Arab Emirates? ACS Pharmacol. Transl. Sci. 2021, 4, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Contemporary approaches to treat Naegleria fowleri: A patent overview. Pharm. Pat. Anal. 2020, 10, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Maciver, S.K.; Piñero, J.E.; Lorenzo-Morales, J. Is Naegleria fowleri an Emerging Parasite? Trends Parasitol. 2020, 36, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M. Global Warming Favors Pathogenicity of the Brain-Eating Amoebae. Anti-Infect. Agents 2019, 17, 2–3. [Google Scholar] [CrossRef]
- Rizo-Liendo, A.; Sifaoui, I.; Reyes-Batlle, M.; Chiboub, O.; Rodríguez-Expósito, R.L.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Hendiger, E.B.; López-Arencibia, A.; Rocha-Cabrera, P.; et al. In Vitro Activity of Statins against Naegleria fowleri. Pathogens 2019, 8, 122. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.; Li, B.; Jian, Z.; Qi, X.; Sun, D.; Gao, J.; Lu, X.; Yang, Y.; Lin, K.; et al. Balamuthia mandrillaris infection in China: A retrospective report of 28 cases. Emerg. Microbes Infect. 2020, 9, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Khurana, S.; Mahadevan, A.; John, D.V. Central nervous system infections caused by pathogenic free-living amoebae: An Indian perspective. Trop. Biomed. 2022, 39, 265–280. [Google Scholar] [PubMed]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, Diagnosis, and Treatment Options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.A.; Colon, B.L.; Chen, E.; Hull, M.V.; Kyle, D.E. Discovery of repurposing drug candidates for the treatment of diseases caused by pathogenic free-living amoebae. PLoS Negl. Trop. Dis. 2020, 14, e0008353. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, H.; Dendana, F.; Sellami, A.; Cheikhrouhou, F.; Neji, S.; Makni, F.; Ayadi, A. Pathogenic free-living amoebae: Epidemiology and clinical review. Pathol. Biol. 2012, 60, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.; Beg, M.A.; Mahmood, S.F.; Bandea, R.; Sriram, R.; Noman, F.; Ali, F.; Visvesvara, G.S.; Zafar, A. Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg. Infect. Dis. 2011, 17, 258. [Google Scholar] [CrossRef]
- Bellini, N.K.; Santos, T.M.; da Silva, M.T.A.; Thiemann, O.H. The therapeutic strategies against Naegleria fowleri. Exp. Parasitol. 2018, 187, 1–11. [Google Scholar] [CrossRef]
- Cunningham, A.A.; Daszak, P.; Wood, J.L. One Health, emerging infectious diseases and wildlife: Two decades of pro-gress? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160167. [Google Scholar] [CrossRef]
- Solis, A.; Nunn, C.L. One health disparities and COVID-19. Evol. Med. Public Health 2021, 9, 70–77. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, R.; Kumari, A. A review on biogenic synthesis, applications and toxicity aspects of zinc oxide nanoparticles. EXCLI J. 2020, 19, 1325. [Google Scholar]
- Akbar, N.; Aslam, Z.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Zinc oxide nanoparticles conjugated with clinically-approved medicines as potential antibacterial molecules. AMB Express 2021, 11, 104. [Google Scholar] [CrossRef]
- Mansur, F.A.; Sridewi, N.; Anwar, A.; Anwar, A.; Shahabuddin, S. Polypyrrole-conjugated zinc oxide nanoparticle as antiamoebic drugs against Acanthamoeba castellanii. Mater. Today Proc. 2022, 62, 7077–7081. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Wu, S.; Zhou, R.; Zhang, K.; Zhang, Z.; Liu, J.; Qin, S.; Shi, J. Nanomaterial-based zinc ion interference therapy to combat bacterial infections. Front. Immunol. 2022, 13, 899992. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Rahman, A.U.; Tajuddin; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Halioua, B.; Ziskind, B. Medicine in the Days of the Pharaohs; Harvard University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nano-Med. 2019, 14, 9395. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Khan, N.A. Antibacterial Activities of Selected Pure Compounds Isolated from Gut Bacteria of Animals Living in Polluted Environments. Antibiotics 2020, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Jeyamogan, S.; Khan, N.A.; Anwar, A.; Shah, M.R.; Siddiqui, R. Cytotoxic effects of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives on HeLa cells. SAGE Open Med. 2018, 6, 2050312118781962. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Anwar, A.; Mungroo, M.R.; Anwar, A.; Sullivan, W.J., Jr.; Khan, N.A.; Siddiqui, R. Repositioning of guanabenz in conjugation with gold and silver nanoparticles against pathogenic amoebae Acanthamoeba castellanii and Naegleria fowleri. ACS Infect. Dis. 2019, 5, 2039–2046. [Google Scholar] [CrossRef]
- Anwar, A.; Rajendran, K.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Clinically Approved Drugs against CNS Diseases as Potential Therapeutic Agents to Target Brain-Eating Amoebae. ACS Chem. Neurosci. 2018, 10, 658–666. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, J.; Le, T. Zinc oxide nanoparticle as a novel class of antifungal agents: Current advances and future perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.S. ZnO na-noparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B Biointerfaces 2014, 117, 267–276. [Google Scholar] [CrossRef] [PubMed]
| Naegleria fowleri | ||||
| % Of Amoeba Growth | ||||
| 50 µg/mL | 100 µg/mL | 150 µg/mL | MIC50 (µg/mL) | |
| ZnO-CD-AMPi | 65 ± 9.0 | 36 ± 0.4 | 13 ± 2.0 | 69.52 |
| ZnO-CD-CFT | 79 ± 6.4 | 40 ± 1.9 | 9 ± 4.5 | 82.05 |
| ZnO-CD-NAR | 74 ± 12.0 | 52 ± 2.0 | 14 ± 0.19 | 88.16 |
| ZnO-CD-AMB | 78 ± 8.3 | 51 ± 3.7 | 25 ± 0.67 | 95.61 |
| ZnO-CD-QT | 79 ± 10.4 | 36 ± 7.1 | 28 ± 0.51 | 85.69 |
| Balamuthia mandrillaris | ||||
| % Of Amoeba Growth | ||||
| 50 µg/mL | 100 µg/mL | 150 µg/mL | MIC50 (µg/mL) | |
| ZnO-CD-AMPi | 93 ± 3.7 | 53 ± 0.5 | 37 ± 0.7 | 113.9 |
| ZnO-CD-CFT | 99 ± 6.7 | 46 ± 4.3 | 27 ± 2.0 | 102.3 |
| ZnO-CD-NAR | 78 ± 0.9 | 59 ± 2.5 | 30 ± 1.8 | 106.9 |
| ZnO-CD-AMB | 86 ± 4.0 | 83 ± 10.4 | 46 ± 5.2 | 146.4 |
| ZnO-CD-QT | 74 ± 2.5 | 62 ± 6.0 | 43 ± 3.0 | 129.6 |
| Amoebicidal Activity against B. mandrillaris | Amoebicidal Activity against N. fowleri | Cytotoxic Activity against HeLa Cells | |
|---|---|---|---|
| ZnO-CD-AMPi | 35.5% | 40.9% | 35.5% |
| ZnO-CD-CFT | 39.6% | 48.2% | 36.4% |
| ZnO-CD-NAR | 52.0% | 51.6% | 30.9% |
| ZnO-CD-AMB | 50.8% | 43.8% | 36.6% |
| ZnO-CD-QT | 35.9% | 62.4% | 37.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, R.; Boghossian, A.; Akbar, N.; Jabri, T.; Aslam, Z.; Shah, M.R.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. Zinc Oxide Nanoconjugates against Brain-Eating Amoebae. Antibiotics 2022, 11, 1281. https://doi.org/10.3390/antibiotics11101281
Siddiqui R, Boghossian A, Akbar N, Jabri T, Aslam Z, Shah MR, Alharbi AM, Alfahemi H, Khan NA. Zinc Oxide Nanoconjugates against Brain-Eating Amoebae. Antibiotics. 2022; 11(10):1281. https://doi.org/10.3390/antibiotics11101281
Chicago/Turabian StyleSiddiqui, Ruqaiyyah, Anania Boghossian, Noor Akbar, Tooba Jabri, Zara Aslam, Muhammad Raza Shah, Ahmad M. Alharbi, Hasan Alfahemi, and Naveed Ahmed Khan. 2022. "Zinc Oxide Nanoconjugates against Brain-Eating Amoebae" Antibiotics 11, no. 10: 1281. https://doi.org/10.3390/antibiotics11101281
APA StyleSiddiqui, R., Boghossian, A., Akbar, N., Jabri, T., Aslam, Z., Shah, M. R., Alharbi, A. M., Alfahemi, H., & Khan, N. A. (2022). Zinc Oxide Nanoconjugates against Brain-Eating Amoebae. Antibiotics, 11(10), 1281. https://doi.org/10.3390/antibiotics11101281









