Eradication of Staphylococcus aureus Biofilm Infection by Persister Drug Combination
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Media, Antibiotics, and Chemicals
2.2. Microtiter Plate Biofilm Drug Exposure Assay and the SYBR Green I/PI Assay
2.3. Mouse Skin Infection Model
2.4. Histopathology
2.5. Statistical Analyses
3. Results
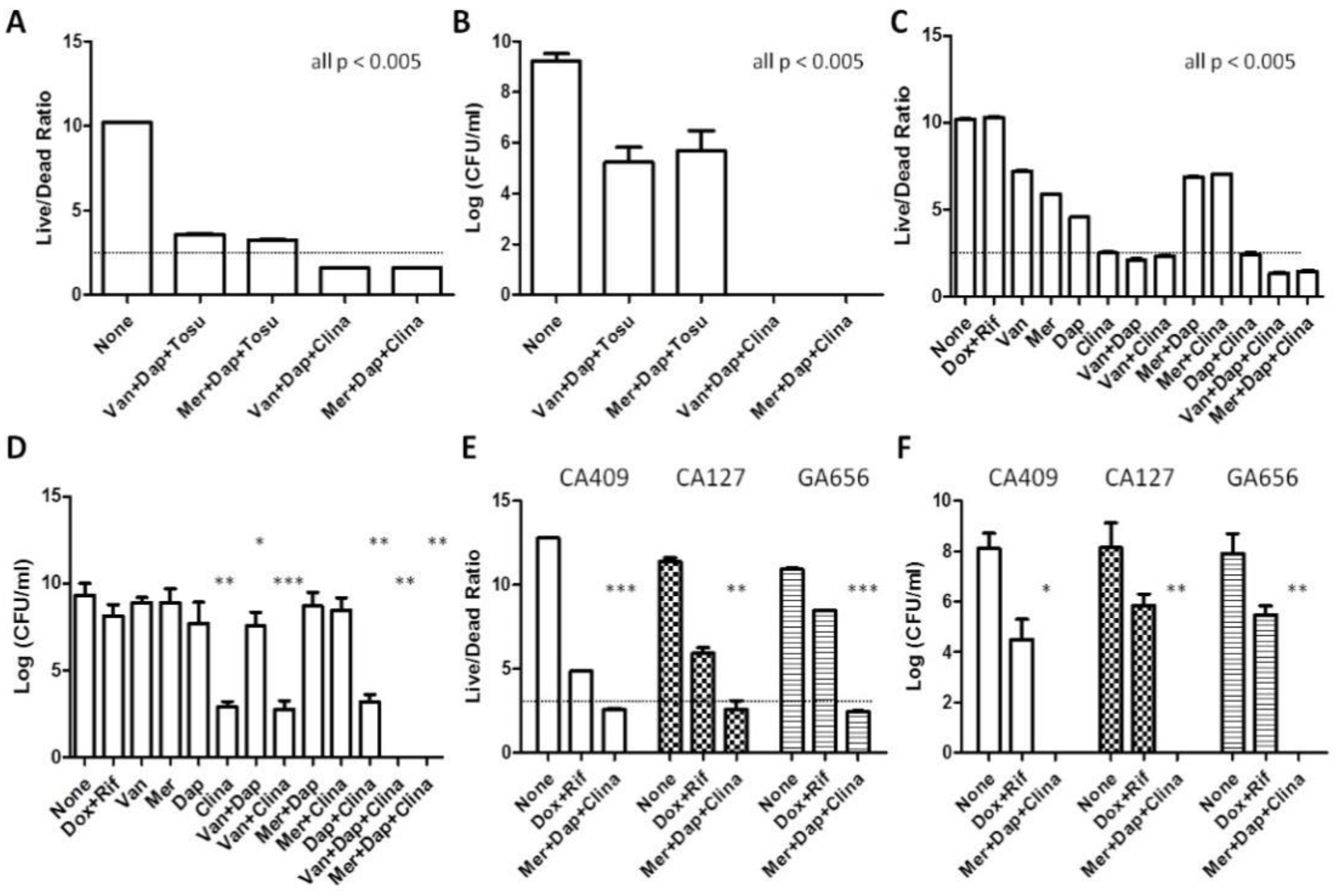
3.1. Commonly Used Treatments for MRSA Have Poor Activity against Biofilms In Vitro
3.2. Identification of Drug Combinations with Strong Anti-Biofilm Activity
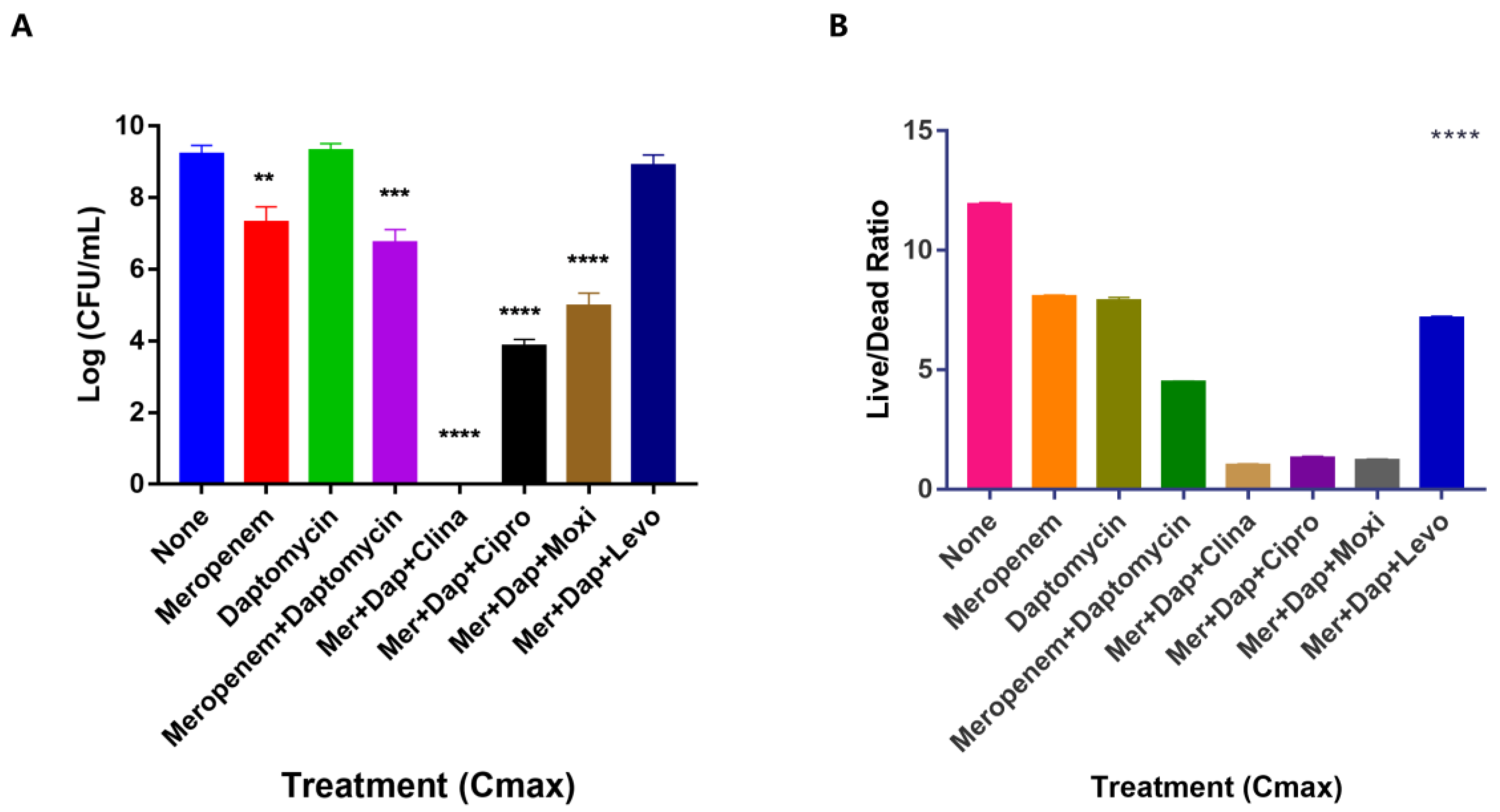
3.3. Unique Anti-Persister Activity of Clinafloxacin Could Not Be Replaced by Other Fluoroquinolone Drugs
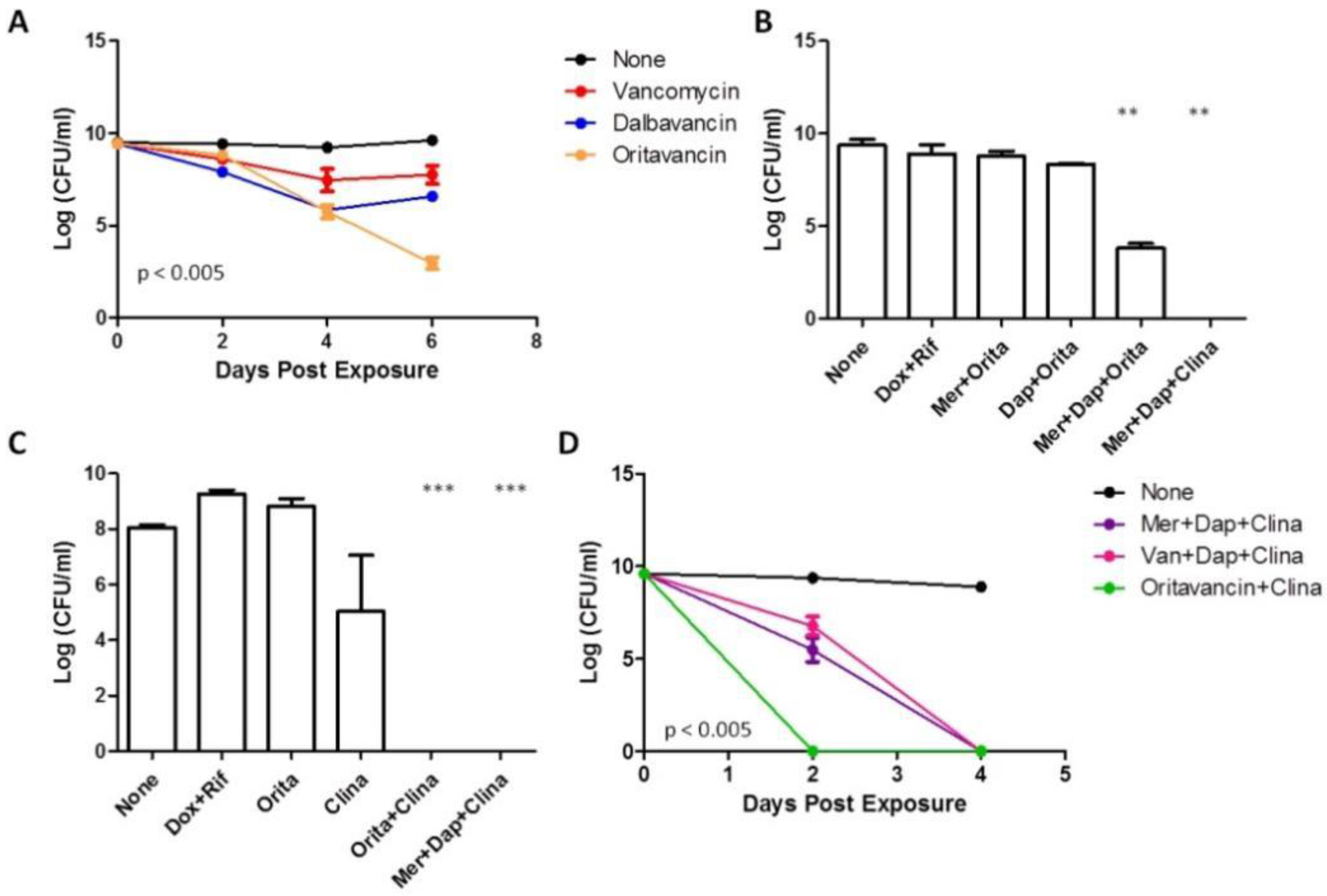
3.4. Anti-Biofilm Activity of Oritavancin and Dalbavancin
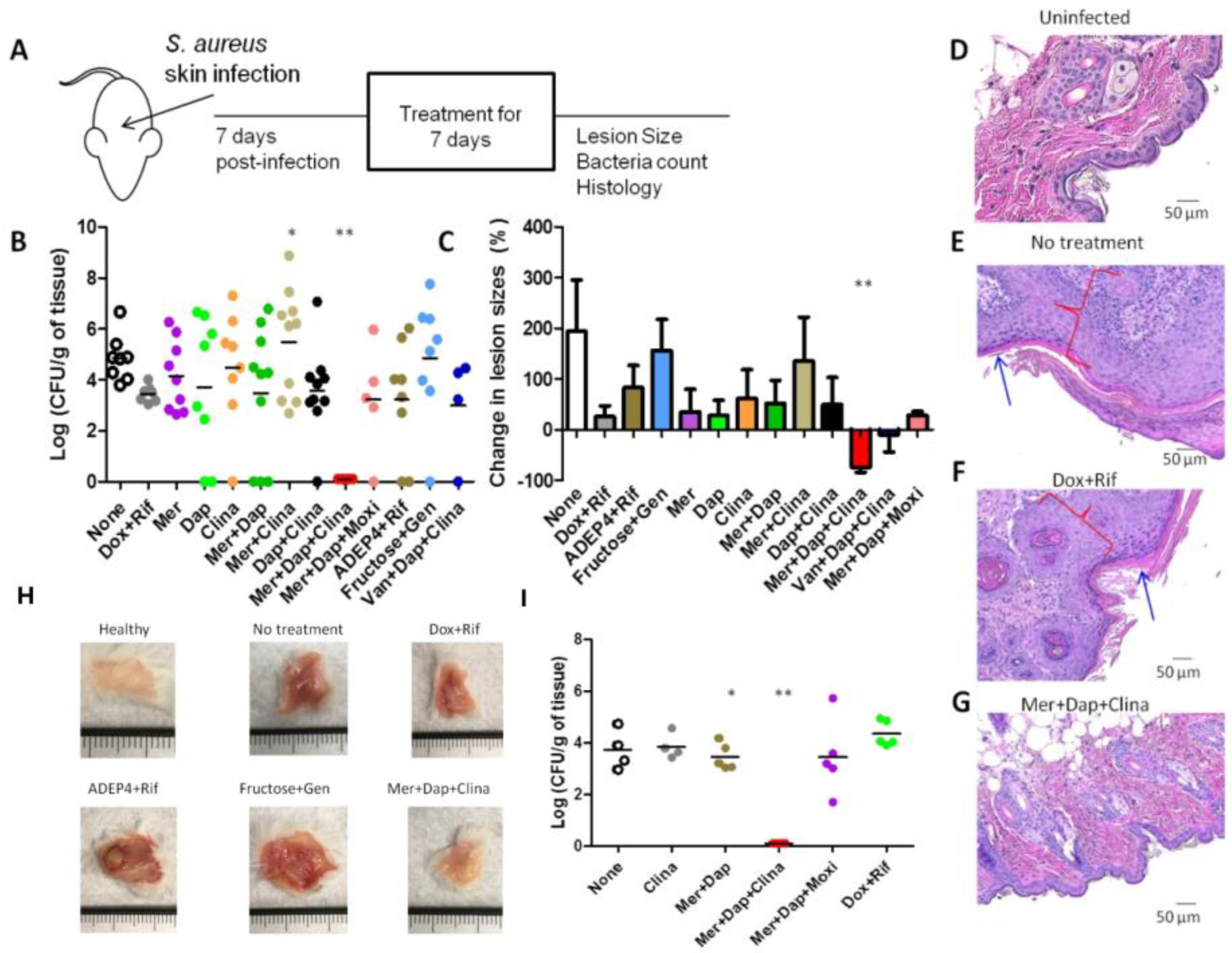
3.5. The Drug Combination Meropenem + Daptomycin +Clinafloxacin Eradicated Biofilm Infections in the Mouse Skin Persistent Infection Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, L.C.; Chaili, S.; Filler, S.G.; Miller, L.S.; Solis, N.V.; Wang, H.; Johnson, C.W.; Lee, H.K.; Diaz, L.F.; Yeaman, M.R. Innate Immune Memory Contributes to Host Defense against Recurrent Skin and Skin Structure Infections Caused by Methicillin-Resistant Staphylococcus aureus. Infect. Immun. 2017, 85, e00876-16. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65, iii35–iii44. [Google Scholar] [CrossRef]
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.; Cataneli, P.V.; Pinheiro, L.; Moraes Riboli, D.F.; Benini Martins, K.; de Souza da Cunha, M.L.R. Antimicrobial Resistance Profile of Planktonic and Biofilm Cells of Staphylococcus aureus and Coagulase-Negative Staphylococci. Int. J. Mol. Sci. 2016, 17, 1423. [Google Scholar] [CrossRef]
- Stewart, P.S.; Davison, W.M.; Steenbergen, J.N. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 2009, 53, 3505–3507. [Google Scholar] [CrossRef]
- Kirker, K.R.; Fisher, S.T.; James, G.A. Potency and penetration of telavancin in staphylococcal biofilms. Int. J. Antimicrob. Agents 2015, 46, 451–455. [Google Scholar] [CrossRef]
- Kavanaugh, J.S.; Horswill, A.R. Impact of Environmental Cues on Staphylococcal Quorum Sensing and Biofilm Development. J. Biol. Chem. 2016, 291, 12556–12564. [Google Scholar] [CrossRef]
- Hobby, G.L.; Meyer, K.; Chaffee, E. Observations on the mechanism of action of penicillin. Proc. Soc. Exp. Biol. Med. 1942, 50, 281–285. [Google Scholar] [CrossRef]
- Bigger, J.W. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 1944, 244, 4. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Chen, G.; Du, X.; Cui, P.; Wu, J.; Zhao, J.; Wu, N.; Zhang, W.; Li, M.; et al. Transposon Mutagenesis Identifies Novel Genes Associated with Staphylococcus aureus Persister Formation. Front. Microbiol. 2015, 6, 1437. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.; Cui, P.; Shi, W.; Feng, J.; Zhang, Y. Genetic Screen Reveals the Role of Purine Metabolism in Staphylococcus aureus Persistence to Rifampicin. Antibiotics 2015, 4, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.Y.; Cui, P.; Zhang, Y.M.; Zhang, W.H.; Zhang, Y. The Agr Quorum Sensing System Represses Persister Formation through Regulation of Phenol Soluble Modulins in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2189. [Google Scholar] [CrossRef]
- Sahukhal, G.S.; Pandey, S.; Elasri, M.O. msaABCR operon is involved in persister cell formation in Staphylococcus aureus. BMC Microbiol. 2017, 17, 218. [Google Scholar] [CrossRef]
- Zhang, Y.; Mitchison, D. The curious characteristics of pyrazinamide: A review. Int. J. Tuberc. Lung Dis. 2003, 7, 6–21. [Google Scholar]
- Zhang, Y.; Wade, M.M.; Scorpio, A.; Zhang, H.; Sun, Z. Mode of action of pyrazinamide: Disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 2003, 52, 790–795. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Jiang, X.; Yuan, H.; Lee, J.S.; Barry, C.E., 3rd; Wang, H.; Zhang, W.; Zhang, Y. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 2011, 333, 1630–1632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Shi, W.; Liu, W.; Zhang, W.; Zhang, Y. Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg. Microbes Infect. 2013, 2, e34. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, W.; Zhang, W.; Mitchison, D. Mechanisms of Pyrazinamide Action and Resistance. Microbiol. Spectr. 2013, 2, MGM2-0023-2013. [Google Scholar]
- Zhang, Y. Persisters, Persistent Infections and the Yin-Yang Model. Emerg. Microbes Infect. 2014, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Zhang, Y. Eradication of Biofilm-Like Microcolony Structures of Borrelia burgdorferi by Daunomycin and Daptomycin but not Mitomycin C in Combination with Doxycycline and Cefuroxime. Front. Microbiol. 2016, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, T.; Yee, R.; Yuan, Y.; Bai, C.; Cai, M.; Shi, W.; Embers, M.; Brayton, C.; Saeki, H.; et al. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: Implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov. Med. 2019, 27, 125–138. [Google Scholar]
- Niu, H.; Cui, P.; Yee, R.; Shi, W.; Zhang, S.; Feng, J.; Sullivan, D.; Zhang, W.; Zhu, B.; Zhang, Y. A Clinical Drug Library Screen Identifies Tosufloxacin as Being Highly Active against Staphylococcus aureus Persisters. Antibiotics 2015, 4, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.; Yuan, Y.; Shi, W.; Brayton, C.; Tarff, A.; Feng, J.; Wang, J.; Behrens, A.; Zhang, Y. Infection with persister forms of Staphylococcus aureus causes a persistent skin infection with more severe lesions in mice: Failure to clear the infection by the current standard of care treatment. Discov. Med. 2019, 28, 7–16. [Google Scholar] [PubMed]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 30, 2437. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Sader, H.S.; Flamm, R.K.; Castanheira, M.; Mendes, R.E. Oritavancin in vitro activity against gram-positive organisms from European and United States medical centers: Results from the SENTRY Antimicrobial Surveillance Program for 2010-2014. Diagn. Microbiol. Infect. Dis. 2018, 91, 199–204. [Google Scholar] [CrossRef]
- Yan, Q.; Karau, M.J.; Patel, R. In vitro activity of oritavancin against biofilms of staphylococci isolated from prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2018, 92, 155–157. [Google Scholar] [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 2011, 473, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H.; Simões, M.; Borges, A. Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Webber, M.A. Novel approaches to the treatment of bacterial biofilm infections. Br. J. Pharm. 2017, 174, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob Chemother. 2014, 69 Suppl. S1, i37–i40. [Google Scholar] [CrossRef]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Abdelkhalek, A.; Seleem, M.N. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 29707. [Google Scholar] [CrossRef]
- Zhang, Y.; Yew, W.W.; Barer, M.R. Targeting persisters for tuberculosis control. Antimicrob. Agents Chemother. 2012, 56, 2223–2230. [Google Scholar] [CrossRef]
- Joers, A.; Kaldalu, N.; Tenson, T. The frequency of persisters in Escherichia coli reflects the kinetics of awakening from dormancy. J. Bacteriol. 2010, 192, 3379–3384. [Google Scholar] [CrossRef]
- Mandell, J.B.; Deslouches, B.; Montelaro, R.C.; Shanks, R.M.Q.; Doi, Y.; Urish, K.L. Elimination of Antibiotic Resistant Surgical Implant Biofilms Using an Engineered Cationic Amphipathic Peptide WLBU2. Sci. Rep. 2017, 7, 18098. [Google Scholar] [CrossRef]
- Humphries, R.M.; Pollett, S.; Sakoulas, G. A current perspective on daptomycin for the clinical microbiologist. Clin. Microbiol. Rev. 2013, 26, 759–780. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Performance Standards for Antimicrobial Susceptibility Testing-26th Edition: CLSI Supplement M100S; NCCLS: Wayne, PA, USA, 2016. [Google Scholar]
- Gallo, S.W.; Ferreira, C.A.S.; de Oliveira, S.D. Combination of polymyxin B and meropenem eradicates persister cells from Acinetobacter baumannii strains in exponential growth. J. Med. Microbiol. 2017, 66, 1257–1260. [Google Scholar] [CrossRef]
- Carpenter, C.F.; Chambers, H.F. Daptomycin: Another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 2004, 38, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug Combinations against Borrelia burgdorferi Persisters In Vitro: Eradication Achieved by Using Daptomycin, Cefoperazone and Doxycycline. PLoS ONE 2015, 10, e0117207. [Google Scholar] [CrossRef] [PubMed]
- Pogliano, J.; Pogliano, N.; Silverman, J.A. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J. Bacteriol. 2012, 194, 4494–4504. [Google Scholar] [CrossRef]
- Cue, D.; Junecko, J.M.; Lei, M.G.; Blevins, J.S.; Smeltzer, M.S.; Lee, C.Y. SaeRS-Dependent Inhibition of Biofilm Formation in Staphylococcus aureus Newman. PLoS ONE 2015, 10, e0123027. [Google Scholar] [CrossRef]
- Barrett, M.S.; Jones, R.N.; Erwin, M.E.; Johnson, D.M.; Briggs, B.M. Antimicrobial activity evaluations of two new quinolones, PD127391 (CI-960 and AM-1091) and PD131628. Diagn. Microbiol. Infect. Dis. 1991, 14, 389–401. [Google Scholar] [CrossRef]
- Cohen, M.A.; Yoder, S.L.; Huband, M.D.; Roland, G.E.; Courtney, C.L. In vitro and in vivo activities of clinafloxacin, CI-990 (PD 131112), and PD 138312 versus enterococci. Antimicrob. Agents Chemother. 1995, 39, 2123–2127. [Google Scholar] [CrossRef]
- Balwan, A.; Nicolau, D.P.; Wungwattana, M.; Zuckerman, J.B.; Waters, V. Clinafloxacin for Treatment of Burkholderia cenocepacia Infection in a Cystic Fibrosis Patient. Antimicrob. Agents Chemother. 2016, 60, 1–5. [Google Scholar] [CrossRef]
- Levine, D.P.; Holley, H.P.; Eiseman, I.; Willcox, P.; Tack, K. Clinafloxacin for the treatment of bacterial endocarditis. Clin. Infect. Dis. 2004, 38, 620–631. [Google Scholar] [CrossRef]
- Yuan, R.Y.; Gour, N.; Dong, X.Z.; Jie, F.; Shi, W.L.; Zhang, Y. Ranking of Major Classes of Antibiotics for Activity against Stationary Phase Pseudomonas aeruginosa and Identification of Clinafloxacin + Cefuroxime + Gentamicin Drug Combination that Eradicates Persistent P. aeruginosa Infection in a Murine Cystic Fibrosis Model. bioRxiv, 2019. [Google Scholar] [CrossRef]

| Drug | Dosage | Route | Times Treated | Cmax Tested (Clinically Achievable Concentrations) * |
|---|---|---|---|---|
| Vancomycin | 110 mg/kg | Intraperitoneally | Twice/daily | 20 µg/mL |
| Daptomycin | 50 mg/kg | Intraperitoneally | Once/daily | 80 µg/mL |
| Meropenem | 50 mg/kg | Intraperitoneally | Once/daily | 20 µg/mL |
| Clinafloxacin | 50 mg/kg | Intraperitoneally | Once/daily | 2 µg/mL |
| Doxycycline | 100 mg/kg | Oral | Twice/daily | 5 µg/mL |
| Rifampin | 10 mg/kg | Oral | Twice/daily | 5 µg/mL |
| Moxifloxacin | 100 mg/kg | Oral | Once/daily | 4 µg/mL |
| ADEP4 | 25 mg/kg and 35 mg/kg | Intraperitoneally | Twice/daily | |
| Rifampin (for ADEP4 combination) | 30 mg/kg | Intraperitoneally | Once/daily | |
| Gentamicin | 20 mg/kg | Intraperitoneally | Once/daily | |
| Fructose | 1.5 g/kg | Intraperitoneally | Once/daily | |
| Oritavancin | --- | --- | --- | 5 µg/mL |
| Ciprofloxacin | --- | --- | --- | 10 µg/mL |
| Levofloxacin | --- | --- | --- | 10 µg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, R.; Yuan, Y.; Tarff, A.; Brayton, C.; Gour, N.; Feng, J.; Zhang, Y. Eradication of Staphylococcus aureus Biofilm Infection by Persister Drug Combination. Antibiotics 2022, 11, 1278. https://doi.org/10.3390/antibiotics11101278
Yee R, Yuan Y, Tarff A, Brayton C, Gour N, Feng J, Zhang Y. Eradication of Staphylococcus aureus Biofilm Infection by Persister Drug Combination. Antibiotics. 2022; 11(10):1278. https://doi.org/10.3390/antibiotics11101278
Chicago/Turabian StyleYee, Rebecca, Yuting Yuan, Andreina Tarff, Cory Brayton, Naina Gour, Jie Feng, and Ying Zhang. 2022. "Eradication of Staphylococcus aureus Biofilm Infection by Persister Drug Combination" Antibiotics 11, no. 10: 1278. https://doi.org/10.3390/antibiotics11101278
APA StyleYee, R., Yuan, Y., Tarff, A., Brayton, C., Gour, N., Feng, J., & Zhang, Y. (2022). Eradication of Staphylococcus aureus Biofilm Infection by Persister Drug Combination. Antibiotics, 11(10), 1278. https://doi.org/10.3390/antibiotics11101278










