Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results
Abstract
1. Introduction
2. Results
2.1. Disk Diffusion Method
2.2. Microdilution Method
2.3. Checkerboard Method
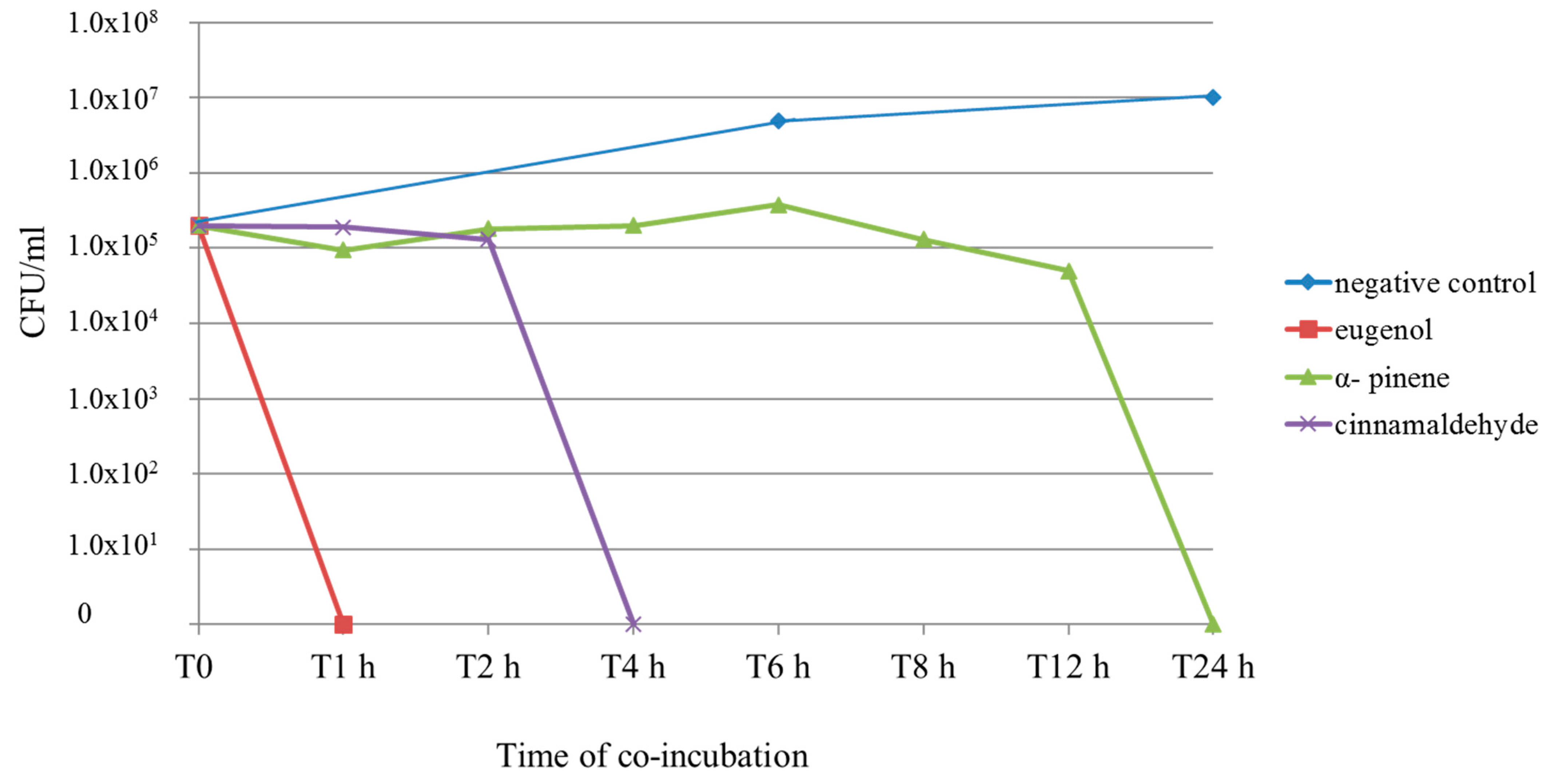
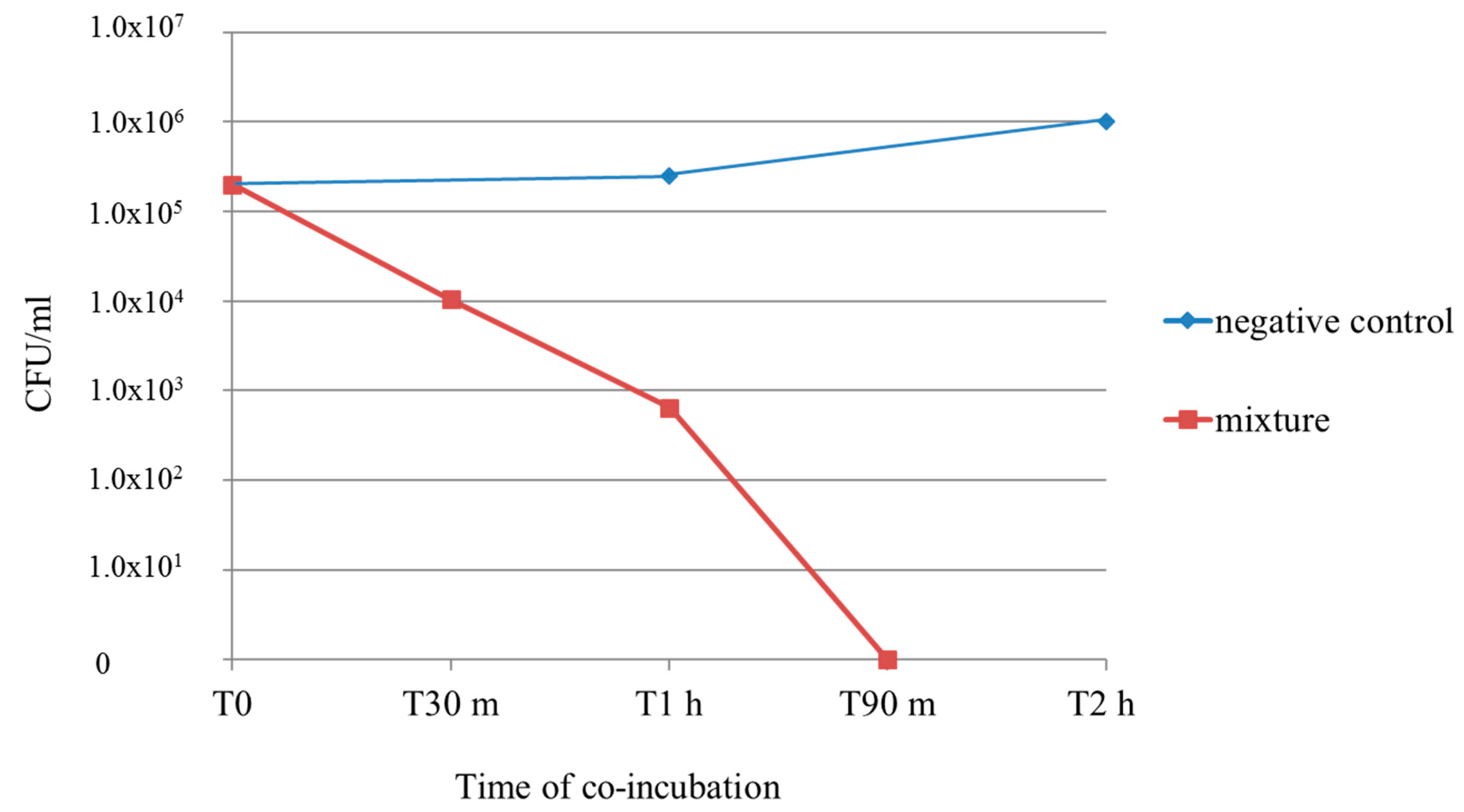
2.4. Time–Kill Curve
2.5. Antimicrobial Activity on Lactobacillus acidophilus
3. Discussion
4. Materials and Methods
4.1. Natural Compounds
4.2. Candida spp. Culture Conditions
4.3. Antifungal Activity—Disk Diffusion Method
4.4. Antifungal Activity—Microdilution Method
4.5. Checkerboard Method
- FICI 1: MIC (cinnamaldehyde in combination with eugenol)/MIC (cinnamaldehyde) + MIC (eugenol in combination with cinnamaldehyde)/MIC (eugenol)
- FICI 2: MIC (cinnamaldehyde in combination with α-pinene)/MIC (cinnamaldehyde) + MIC (α-pinene in combination with cinnamaldehyde)/MIC (α-pinene)
- FICI 3: MIC (eugenol in combination with α-pinene)/MIC (eugenol) + MIC (α-pinene in combination with eugenol)/MIC (α-pinene)
4.6. Time–Kill Curve (Fungicidal Activity)
4.7. Antimicrobial Effects on Lactobacillus acidophilus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Odds, F.C. Candida infections: An overview. Crit. Rev. Microbiol. 1987, 15, 1–5. [Google Scholar] [CrossRef]
- Casadevall, A. Fungi and the rise of mammals. PLoS Pathog. 2012, 8, e1002808. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Whiteway, M.; Bachewich, C. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 2007, 61, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.P. Fungus produces a toxic surprise. Nature 2016, 532, 41–42. [Google Scholar] [CrossRef]
- Williams, D.W.; Kuriyama, T.; Silva, S.; Malic, S.; Lewis, M.O. Candida biofilms and oral candidosis: Treatment and prevention. Periodontology 2000, 55, 250–265. [Google Scholar] [CrossRef]
- Jayatilake, J.A.; Samaranayake, Y.H.; Cheung, L.K.; Samaranayake, L.P. Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non-albicans Candida species in reconstituted human oral epithelium. J. Oral Pathol. Med. 2006, 35, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 2009, 52, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Harrison, L.H.; Farley, M.M.; Hollick, R.; Stein, B.; Chiller, T.M.; Lockhart, S.R.; Park, B.J. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: Results from population based surveillance. PLoS ONE 2015, 10, e0120452. [Google Scholar] [CrossRef]
- Low, C.Y.; Rotstein, C. Emerging fungal infections in immunocompromised patients. F1000 Med. Rep. 2011, 3, 14. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Revisiting the source of candidemia: Skin or gut? Clin. Infect. Dis. 2001, 33, 1959–1967. [Google Scholar] [CrossRef]
- Gunsalus, K.T.W.; Tornberg-Belanger, S.N.; Matthan, N.R.; Lichtenstein, A.H.; Kumamoto, C.A. Manipulation of host diet to reduce gastrointestinal colonization by the opportunistic pathogen Candida albicans. Host Microb. Biol. 2015, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Coker, O.O.; Nakatsu, G.; Dai, R.Z.; Wu, W.K.K.; Wong, S.H.; Ng, S.C.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 2019, 68, 654–662. [Google Scholar] [CrossRef]
- Zi-Qi, G.; Kuo-Yao, T.; Yu-Huan, T. Candida gut commensalism and inflammatory disease. Med. Microecol. 2020, 3, 100008. [Google Scholar]
- Standaert-Vitse, A.; Sendid, B.; Joossens, M.; François, N.; Vandewalle-El Khoury, P.; Branche, J.; Van Kruiningen, H.; Jouault, T.; Rutgeerts, P.; Gower-Rousseau, C.; et al. Candida albicans colonization and ASCA in familial Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 1745–1753. [Google Scholar] [CrossRef]
- Ksiadzyna, D.; Semianow-Wejchert, J.; Nawrot, U.; Wlodarczyk, K.; Paradowski, L. Serum concentration of interleukin 10, anti-mannan Candida antibodies and the fungal colonization of the gastrointestinal tract in patients with ulcerative colitis. Adv. Med. Sci. 2009, 54, 170–176. [Google Scholar] [CrossRef]
- Zwolinska-Wcislo, M.; Brzozowski, T.; Budak, A.; Kwiecien, S.; Sliwowski, Z.; Drozdowicz, D.; Trojanowska, D.; Rudnicka-Sosin, L.; Mach, T.; Konturek, S.J.; et al. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J. Physiol. Pharmacol. 2009, 60, 107–118. [Google Scholar] [PubMed]
- Goenka, M.K.; Kochhar, R.; Chakrabarti, A.; Kumar, A.; Gupta, O.; Talwar, P.; Mehta, S.K. Candida overgrowth after treatment of duodenal ulcer. A comparison of cimetidine, famotidine, and omeprazole. J. Clin. Gastroenterol. 1996, 23, 7–10. [Google Scholar] [CrossRef]
- Zwolinska-Wcislo, M.; Brzozowski, T.; Mach, T.; Budak, A.; Trojanowska, D.; Konturek, P.C.; Pajdo, R.; Drozdowicz, D.; Kwiecien, S. Are probiotics effective in the treatment of fungal colonization of the gastrointestinal tract? Experimental and clinical studies. J. Physiol. Pharmacol. 2006, 57, 35–49. [Google Scholar]
- Jin, L.; Yoshida, M.; Nakamura, T.; Ishikawa, H.; Wakabayashi, G.; Tanabe, M.; Kawachi, S.; Shinoda, M.; Saikawa, Y.; Wada, N.; et al. Candida albicans infection delays duodenal ulcer healing in cysteamine-induced duodenal ulcers in rats. Dig. Dis. Sci. 2008, 53, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Yen, D.; Cheung, J.; Scheerens, H.; Poulet, F.; McClanahan, T.; McKenzie, B.; Kleinschek, M.A.; Owyang, A.; Mattson, J.; Blumenschein, W.; et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Investig. 2006, 116, 1310–1316. [Google Scholar] [CrossRef]
- Saunus, J.M.; Wagner, S.A.; Matias, M.A.; Hu, Y.; Zaini, Z.M.; Farah, C.S. Early activation of the interleukin-23-17 axis in a murine model of oropharyngeal candidiasis. Mol. Oral Microbiol. 2010, 25, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Fornari, G.; Vicente, V.A.; Gomes, R.R.; Muro, M.D.; Pinheiro, R.L.; Ferrari, C.; Herkert, P.F.; Takimura, M.; Carvalho, N.S.; Queiroz-Telles, F. Susceptibility and molecular characterization of Candida species from patients with vulvovaginitis. Braz. J. Microbiol. 2016, 47, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Barrenetxea, Z.G. Vulvovaginitis candidiásica. Rev. Iberoam. Micol. 2002, 19, 22–24. [Google Scholar]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Zafarghandi, S.; Abbasabadi, B.; Tavallaee, M. The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 155, 199–203. [Google Scholar]
- Workowski, K.A.; Bolan, G.A. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR Recomm. Rep. 2015, 64, 1. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 1985, 152, 924. [Google Scholar] [CrossRef]
- Blostein, F.; Levin-Sparenberg, E.; Wagner, J.; Foxman, B. Recurrent vulvovaginal candidiasis. Ann. Epidemiol. 2017, 27, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Idaomar, M.; Averbeck, S.; Averbeck, D. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Demitri, F. Chemistry of essential oils. In Flavour and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin, Germany, 2007; pp. 43–86. [Google Scholar]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2017, 43, 587–595. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- De Fazio, L.; Spisni, E.; Cavazza, E.; Strillacci, A.; Candela, M.; Centanni, M.; Ricci, C.; Rizzello, F.; Campieri, M.; Valerii, M.C. Dietary Geraniol by Oral or Enema Administration Strongly Reduces Dysbiosis and Systemic Inflammation in Dextran Sulfate Sodium-Treated Mice. Front. Pharmacol. 2016, 7, 38. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol Pharmacokinetics, Bioavailability and Its Multiple Effects on the Liver Antioxidant and Xenobiotic-Metabolizing Enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Rizzello, F.; Ricci, C.; Scandella, M.; Cavazza, E.; Giovanardi, E.; Valerii, M.C.; Campieri, M.; Comparone, A.; De Fazio, L.; Candela, M.; et al. Dietary geraniol ameliorates intestinal dysbiosis and relieves symptoms in irritable bowel syndrome patients: A pilot study. BMC Complement. Altern. Med. 2018, 18, 338. [Google Scholar] [CrossRef]
- Petrocelli, G.; Farabegoli, F.; Valerii, M.C.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885. [Google Scholar] [CrossRef]
- Compound Summary. Cinnamaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cinnamaldehyde (accessed on 20 November 2021).
- Compound Summary. Alpha-Pinene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Pinene (accessed on 20 November 2021).
- Compound Summary. Limonene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Limonene (accessed on 20 November 2021).
- Compound Summary. Eucalyptol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Eucalyptol (accessed on 20 November 2021).
- Compound Summary. Eugenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Eugenol (accessed on 20 November 2021).
- Angebault, C.; Djossou, F.; Abélanet, S.; Permal, E.; Soltana, M.B.; Diancourt, L.; Bouchier, C.; Woerther, P.L.; Catzeflis, F.; Andremont, A.; et al. Candida albicans is not always the preferential yeast colonizing humans: A study in Wayampi Amerindians. J. Infect. Dis. 2013, 208, 1705–1716. [Google Scholar] [CrossRef]
- McKay, S.; Sawant, P.; Fehlberg, J.; Almenar, E. Antimicrobial activity of orange juice processing waste in powder form and its suitability to produce antimicrobial packaging. Waste Manag. 2021, 120, 230–239. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ahd, A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010, 6, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Cantamessa, S.; Pavan, M.; Novello, G.; Massa, N.; Rocchetti, A.; Berta, G.; Gamalero, E. Sensitivity of Candida albicans to essential oils: Are they an alternative to antifungal agents? J. Appl. Microbiol. 2016, 121, 1530–1545. [Google Scholar] [CrossRef]
- Jafri, H.; Banerjee, G.; Khan, M.S.A.; Ahmad, I.; Abulreesh, H.H.; Althubiani, A.S. Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express 2020, 10, 185. [Google Scholar] [CrossRef]
- Lepargneur, J.P.; Rousseau, V. Protective role of Doderlein flora. J. Gynécol. Obs. Biol. Reprod. 2002, 31, 485–494. [Google Scholar]
- Standaert-Vitse, A.; Jouault, T.; Vandewalle, P.; Mille, C.; Seddik, M.; Sendid, B.; Mallet, J.M.; Colombel, J.F.; Poulain, D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology 2006, 130, 1764–1775. [Google Scholar] [CrossRef]
- Brzozowski, T.; Zwolinska-Wcislo, M.; Konturek, P.C.; Kwiecien, S.; Drozdowicz, D.; Konturek, S.J.; Stachura, J.; Budak, A.; Bogdal, J.; Pawlik, W.W.; et al. Influence of gastric colonization with Candida albicans on ulcer healing in rats: Effect of ranitidine, aspirin and probiotic therapy. Scand. J. Gastroenterol. 2005, 40, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/14462 (accessed on 20 November 2021).
- Available online: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/13694 (accessed on 20 November 2021).
- Shreaz, S.; Sheikh, R.A.; Bhatia, R.; Neelofar, K.; Imran, S.; Hashmi, A.A.; Manzoor, N.; Basir, S.F.; Khan, L.A. Antifungal activity of α-methyl trans cinnamaldehyde, its ligand and metal complexes: Promising growth and ergosterol inhibitors. Biometals 2011, 24, 923–933. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, I.; Cameotra, S.S. Phenyl aldehyde and propanoids exert multiple sites of action towards cell membrane and cell wall targeting ergosterol in Candida albicans. AMB Express 2013, 3, 54. [Google Scholar] [CrossRef]
- Kaur, S.; Mishra, P. Dimorphism-associated changes in plasma membrane H(+)-ATPase activity of Candida albicans. Arch. Microbiol. 1991, 156, 412–415. [Google Scholar] [CrossRef]
- Gow, N.A.; Brown, A.J.; Odds, F.C. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 2002, 5, 366–371. [Google Scholar] [CrossRef]
- Pootong, A.; Norrapong, B.; Cowawintaweewat, S. Antifungal activity of cinnamaldehyde against Candida albicans. Southeast Asian J. Trop. Med. Public Health 2017, 48, 150–158. [Google Scholar]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Van Minnebruggen, G.; Francois, I.E.J.A.; Cammue, B.P.A.; Thevissen, K.; Vroome, V.; Borgers, M.; Shroot, G. General overview on past, present and future antimycotics. Open Mycol. J. 2010, 4, 22–32. [Google Scholar] [CrossRef]
- Bennis, S.; Chami, F.; Chami, N.; Bouchikhi, T.; Remmal, A. Surface alteration of Saccharomyces cerevisiae induced by thymol and eugenol. Lett. Appl. Microbiol. 2014, 38, 454–458. [Google Scholar] [CrossRef]
- Latifah-Munirah, B.; Himratul-Aznita, W.H.; Mohd Zain, N. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front. Life Sci. 2015, 8, 231–240. [Google Scholar] [CrossRef]
- Upadhyay, A.; Arsi, K.; Wagle, B.R.; Upadhyaya, I.; Shrestha, S.; Donoghue, A.M.; Donoghue, D.J. Trans-Cinnamaldehyde, Carvacrol, and Eugenol Reduce Campylobacter jejuni Colonization Factors and Expression of Virulence Genes in Vitro. Front. Microbiol. 2017, 8, 713. [Google Scholar] [CrossRef]
- Guinea, J.; Recio, S.; Escribano, P.; Torres-Narbona, M.; Peláez, T.; Sánchez-Carrillo, C.; Rodríguez-Créixems, M.; Bouza, E. Rapid antifungal susceptibility determination for yeast isolates by use of Etest performed directly on blood samples from patients with fungemia. J. Clin. Microbiol. 2010, 48, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, K.; Kumar, H.; Farooqi, J.; Mehboob, R.; Brandt, M.E.; Zafar, A. Agreement of Direct Antifungal Susceptibility Testing from Positive Blood Culture Bottles with the Conventional Method for Candida Species. J. Clin. Microbiol. 2016, 54, 343–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Available online: https://eucast.org/astoffungi/clinicalbreakpointsforantifungals (accessed on 20 November 2021).
- Vuuren, S.V.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1, 8-cineole alone and in combination. Flavour Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Stanojevic, D.; Comic, L.; Stefanovic, O.; Solujic-Sukdolak, S. In vitro synergistic antibacterial activity of Salvia officinalis L. and some preservatives. Arch. Biol. Sci. Belgrade 2010, 62, 175–183. [Google Scholar] [CrossRef]

| IZ Diameter (mm) | % of Inhibited Strains | |
|---|---|---|
| AmphotericinB | ||
| mean | 15.4 | |
| range | 10.0 to 20.5 | |
| standard deviation | 2.7 | |
| Cinnamaldehyde | 100% (18/18) | |
| mean | 69.0 | |
| range | 55.0 to 75.0 | |
| standard deviation | 6 | |
| α-Pinene | 88.9% (16/18) | |
| mean | 21.2 | |
| range | 14.0 to 3.01 | |
| standard deviation | 5.7 | |
| Limonene | 33.3 (6/18) | |
| mean | 13.9 | |
| range | 10.0 to 21.5 | |
| standard deviation | 3.2 | |
| Eucalyptol | // | // |
| Eugenol | 100% (18/18) | |
| mean | 35.2 | |
| range | 30.5 to 41.0 | |
| standard deviation | 3.5 | |
| Neg. control(DMSO) | // |
| IZ Means (mm) | ||||||
|---|---|---|---|---|---|---|
| Amphotericin B | Cinnamaldehyde | α-Pinene | Limonene | Eucalyptol | Eugenol | |
| C. albicans 1 | 13.0 | 65.0 | 27.0 | 14.5 | // | 35.0 |
| C. albicans 2 | 15.5 | 70.0 | 17.5 | 10.5 | // | 35.0 |
| C. albicans 3 | 16.0 | 67.0 | 23.5 | 12.0 | // | 33.0 |
| C. albicans 4 | 19.5 | 66.0 | 25.0 | 12.0 | // | 35.0 |
| C. albicans 5 | 16.0 | 71.0 | 23.0 | 17.5 | // | 35.0 |
| C. albicans 6 | 16.0 | 67.0 | 24.0 | 12.0 | // | 36.0 |
| C. albicans 7 | 16.0 | 75.0 | 30.0 | 21.5 | // | 44.0 |
| C. albicans 8 | 20.5 | 67.0 | 31.0 | 14.0 | // | 41.0 |
| C. albicans 9 | 12.0 | 70.5 | 14.5 | 14.0 | // | 34.0 |
| C. albicans 10 | 15.0 | 72.5 | 19.5 | 14.5 | // | 34.5 |
| C. albicans 11 | 14.5 | 75.0 | 15.0 | 12.0 | // | 34.0 |
| C. albicans 12 | 16.5 | 75.0 | 15.5 | 13.5 | // | 34.0 |
| C. albicans 13 | 16.0 | 71.0 | 20.0 | 12.0 | // | 35.0 |
| C. albicans 14 | 19.0 | 75.0 | 14.0 | 13.5 | // | 34.0 |
| C. albicans 15 | 15.5 | 70.0 | 15.5 | 14.0 | // | 34.0 |
| C. glabrata 1 | 15.0 | 55.0 | 20.0 | 10.0 | // | 35.0 |
| C. glabrata 2 | 11.0 | 55.0 | 16.5 | 12.0 | // | 30.5 |
| C. lusitaniae 1 | 10.0 | 75.0 | 30.5 | 21.5 | // | 43.0 |
| MIC Mean | MIC SD | MIC Range | MFC Mean | MFC SD | MFC Range | MFC/MIC | |
|---|---|---|---|---|---|---|---|
| Amphotericin B | 0.052 | 0.04 | ≤0.016–0.125 | 0.11 | 0.08 | ≤0.016–0.25 | |
| Cinnamaldehyde | 50.05 | 36.78 | ≤20.48–163.80 | 109.26 | 102.50 | ≤21.00–656.25 | 2.18 |
| α-Pinene | 195.41 | 127.01 | ≤16.73–536.25 | 251.27 | 158.20 | 33.46–536.25 | 1.28 |
| Eugenol | 455.42 | 327.11 | 82.68–1325.00 | 690.09 | 424.10 | 165.36–1325.00 | 1.51 |
| Amphotericin B | Cinnamaldehyde | α-Pinene | Eugenol | |||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| C. albicans 1 | 0.032 | 0.064 | 40.95 | 81.90 | 133.85 | 133.85 | 331.25 | 331.25 |
| C. albicans 2 | 0.032 | 0.064 | 40.95 | 81.90 | 133.85 | 133.85 | 331.25 | 331.25 |
| C. albicans 3 | 0.032 | 0.064 | 40.95 | 40.95 | 133.85 | 133.85 | 165.36 | 331.25 |
| C. albicans 4 | 0.032 | 0.125 | ≤20.48 | 81.90 | 268.13 | 268.13 | 662.50 | 662.50 |
| C. albicans 5 | 0.016 | 0.250 | 40.95 | 40.95 | 66.92 | 133.85 | 82.68 | 331.25 |
| C. albicans 6 | 0.032 | 0.064 | 40.95 | 81.90 | 133.85 | 133.85 | 331.25 | 331.25 |
| C. albicans 7 | 0.032 | 0.064 | ≤20.48 | 81.90 | 66.92 | 133.85 | 331.25 | 662.50 |
| C. albicans 8 | 0.016 | 0.032 | 40.95 | 81.90 | 133.85 | 133.85 | 331.25 | 331.25 |
| C. albicans 9 | 0.125 | 0.250 | 163.80 | 656.25 | 536.25 | 536.25 | 1325.00 | 1325.00 |
| C. albicans 10 | 0.032 | 0.032 | ≤20.48 | ≤20.48 | ≤16.73 | 33.46 | 165.36 | 165.36 |
| C. albicans 11 | 0.125 | 0.250 | ≤20.48 | ≤20.48 | 133.85 | 268.13 | 662.50 | 1325.00 |
| C. albicans 12 | ≤0.016 | 0.016 | ≤20.48 | ≤20.48 | ≤16.73 | 66.92 | 165.36 | 662.50 |
| C. albicans 13 | 0.064 | 0.125 | 81.90 | 163.80 | 268.13 | 268.13 | 662.50 | 1325.00 |
| C. albicans 14 | 0.032 | 0.125 | 40.95 | 163.80 | 268.13 | 536.25 | 331.25 | 1325.00 |
| C. albicans 15 | 0.032 | 0.064 | 40.95 | 81.90 | 268.13 | 536.25 | 331.25 | 662.50 |
| C. glabrata 1 | 0.032 | 0.064 | 40.95 | 81.90 | 268.13 | 268.13 | 331.25 | 331.25 |
| C. glabrata 2 | 0.125 | 0.125 | 163.80 | 163.80 | 536.25 | 536.25 | 1325.00 | 1325.00 |
| C. lusitaniae 1 | 0.125 | 0.250 | ≤20.48 | ≤20.48 | 133.85 | 268.13 | 331.25 | 662.50 |
| IZ Mean (mm) | MIC Mean (mg/L) | |
|---|---|---|
| Cinnamaldehyde | 10 | 327.60 |
| α-Pinene | // | 1072.50 |
| Eugenol | // | 2650.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics 2022, 11, 73. https://doi.org/10.3390/antibiotics11010073
Saracino IM, Foschi C, Pavoni M, Spigarelli R, Valerii MC, Spisni E. Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics. 2022; 11(1):73. https://doi.org/10.3390/antibiotics11010073
Chicago/Turabian StyleSaracino, Ilaria Maria, Claudio Foschi, Matteo Pavoni, Renato Spigarelli, Maria Chiara Valerii, and Enzo Spisni. 2022. "Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results" Antibiotics 11, no. 1: 73. https://doi.org/10.3390/antibiotics11010073
APA StyleSaracino, I. M., Foschi, C., Pavoni, M., Spigarelli, R., Valerii, M. C., & Spisni, E. (2022). Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics, 11(1), 73. https://doi.org/10.3390/antibiotics11010073










