The Engineered Antibiotic Peptide PLG0206 Eliminates Biofilms and Is a Potential Treatment for Periprosthetic Joint Infections
Abstract
:1. Introduction
2. Pharmacology
2.1. Preclinical Pharmacology
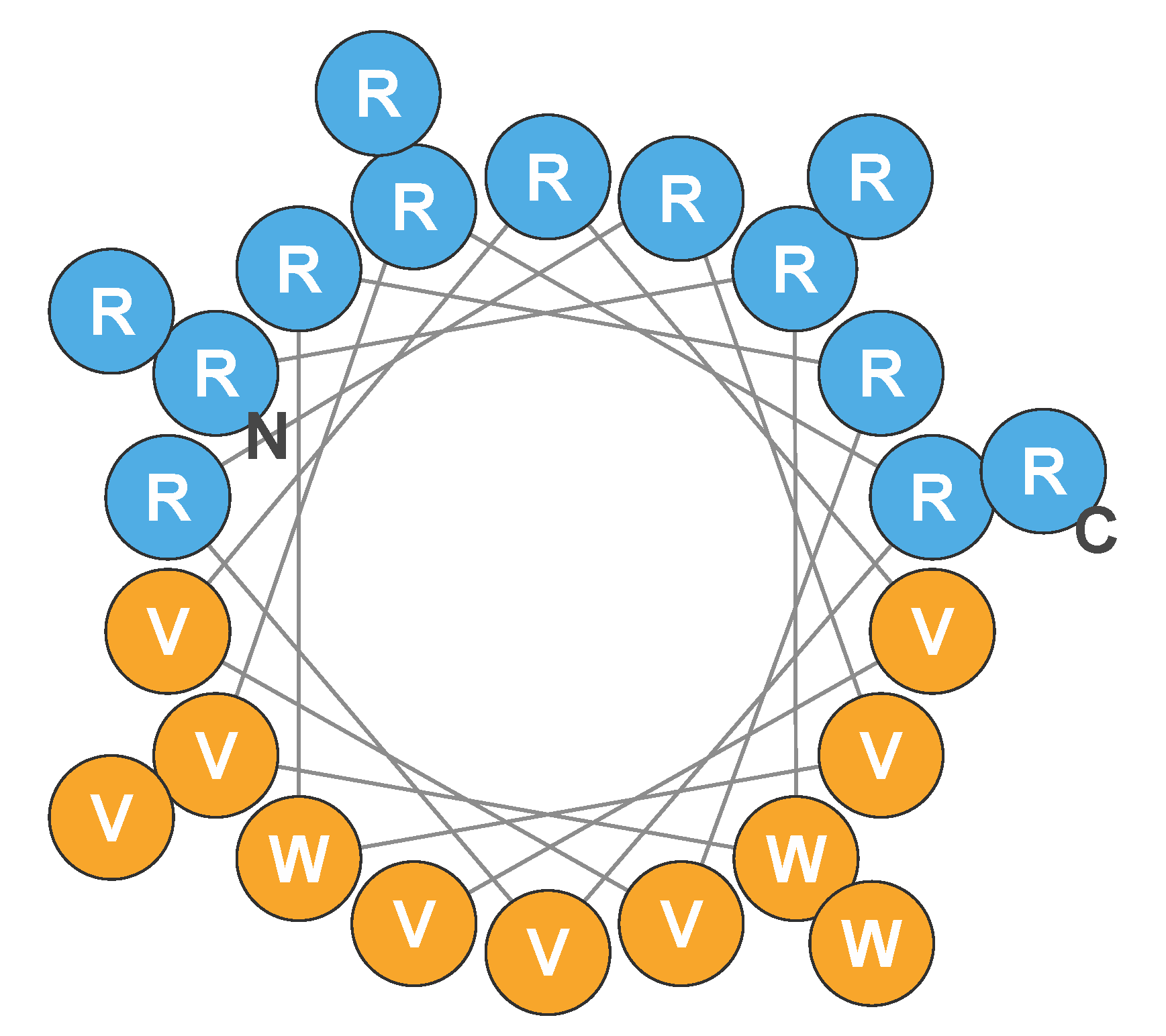
2.1.1. Mechanism of Action
2.1.2. Selectivity for Bacterial Cells
2.1.3. Degradation
2.1.4. Pharmacokinetics and Absorption, Distribution, Metabolism, and Excretion
2.1.5. Preclinical Safety
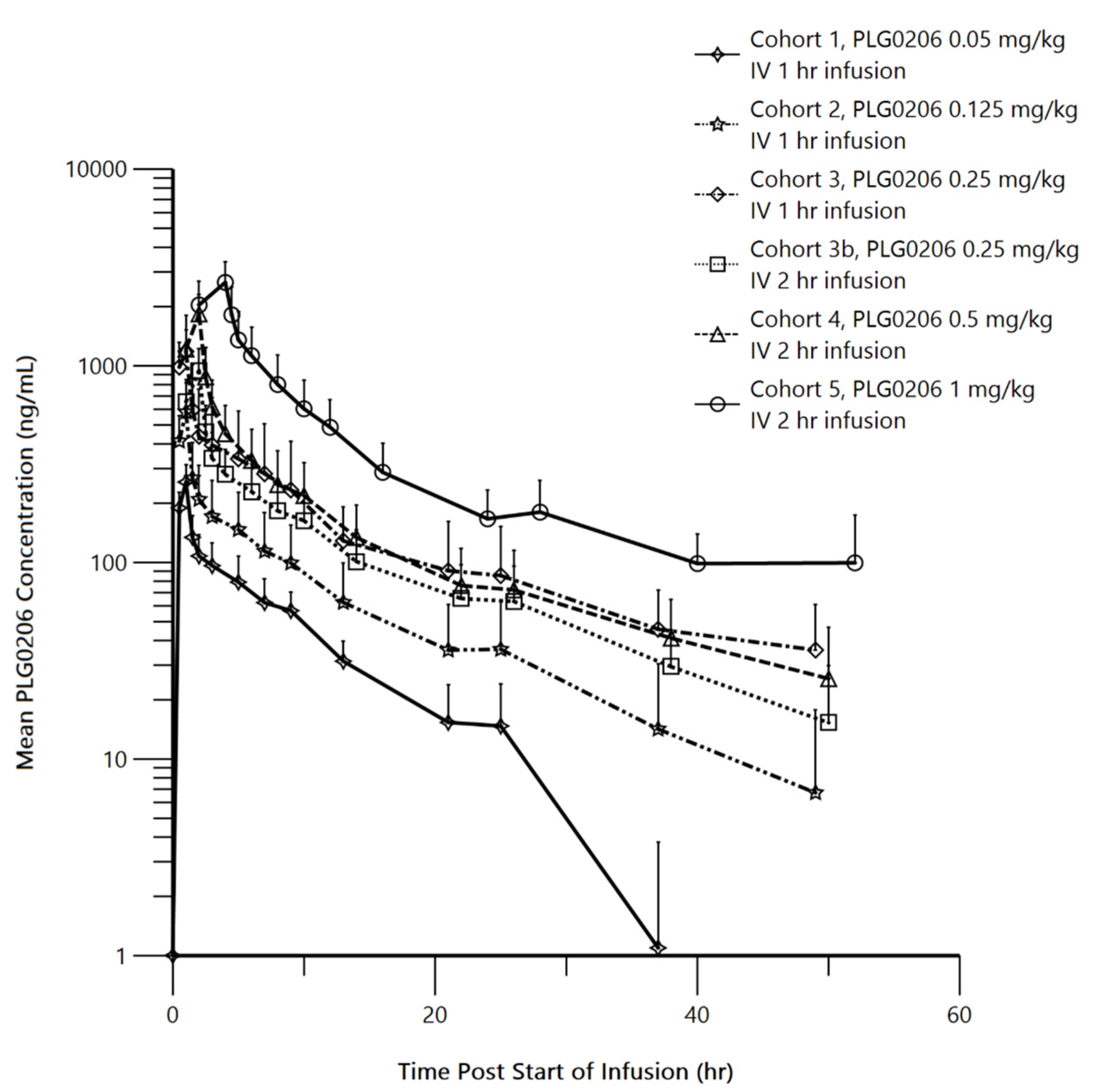
2.2. Human Pharmacokinetics
3. Microbiology
3.1. PLG0206’s Antibiofilm Activity
3.2. Antibacterial Activity of PLG0206 on Implant Surfaces
4. Clinical
4.1. Safety
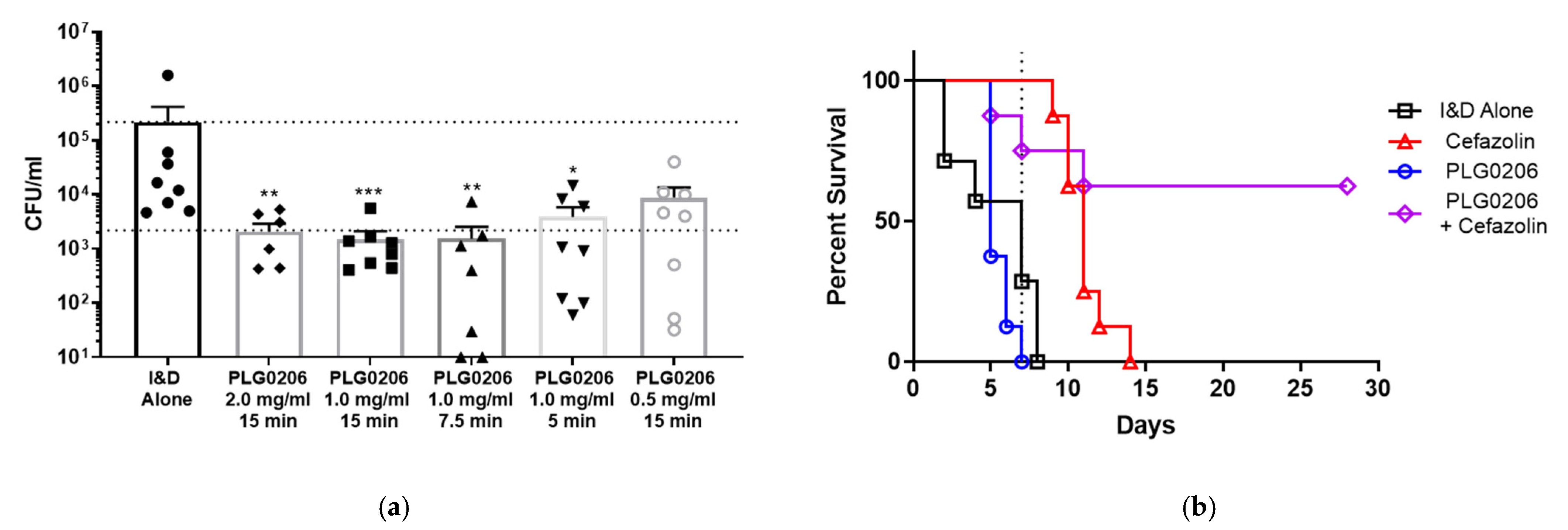
4.2. PLG0206’s Activity in Ex Vivo Model of PJI
5. Regulatory
6. Conclusions and Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Patents
References
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 9 December 2021).
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslouches, B.; Di, Y.P. Antimicrobial Peptides: A Potential Therapeutic Option for Surgical Site Infections. Clin. Surg. 2017, 2, 1740. [Google Scholar] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The Human Antimicrobial Peptide LL-37 is a Multifunctional Modulator of Innate Immune Responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaara, M. New Approaches in Peptide Antibiotics. Curr. Opin. Pharmacol. 2009, 9, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Islam, K.; Craigo, J.K.; Paranjape, S.M.; Montelaro, R.C.; Mietzner, T.A. Activity of the De Novo Engineered Antimicrobial Peptide WLBU2 against Pseudomonas aeruginosa in Human Serum and Whole Blood: Implications for Systemic Applications. Antimicrob. Agents Chemother. 2005, 49, 3208–3216. [Google Scholar] [CrossRef] [Green Version]
- Ryder, M.P.; Wu, X.; McKelvey, G.R.; McGuire, J.; Schilke, K.F. Binding Interactions of Bacterial Lipopolysaccharide and the Cationic Amphiphilic Peptides Polymyxin B and WLBU2. Colloids Surf. B Biointerfaces 2014, 120, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslouches, B.; Phadke, S.M.; Lazarevic, V.; Cascio, M.; Islam, K.; Montelaro, R.C.; Mietzner, T.A. De Novo Generation of Cationic Antimicrobial Peptides: Influence of Length and Tryptophan Substitution on Antimicrobial Activity. Antimicrob. Agents Chemother. 2005, 49, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslouches, B.; Montelaro, R.C.; Urish, K.L.; Di, Y.P. Engineered Cationic Antimicrobial Peptides (eCAPs) to Combat Multidrug-Resistant Bacteria. Pharmaceutics 2020, 12, 501. [Google Scholar] [CrossRef]
- Deslouches, B.; Steckbeck, J.D.; Craigo, J.K.; Doi, Y.; Burns, J.L.; Montelaro, R.C. Engineered Cationic Antimicrobial Peptides to Overcome Multidrug Resistance by ESKAPE Pathogens. Antimicrob. Agents Chemother. 2015, 59, 1329–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urish, K.L.; Bullock, A.G.; Kreger, A.M.; Shah, N.B.; Jeong, K.; Rothenberger, S.D.; Infected Implant, C. A Multicenter Study of Irrigation and Debridement in Total Knee Arthroplasty Periprosthetic Joint Infection: Treatment Failure Is High. J. Arthroplast. 2018, 33, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.E3. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Cheng, A.C.; Buising, K.L.; Choong, P.F. Microbiological Aetiology, Epidemiology, and Clinical Profile of Prosthetic Joint Infections: Are Current Antibiotic Prophylaxis Guidelines Effective? Antimicrob. Agents Chemother. 2012, 56, 2386–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.L.; Hou, Y.F.; Zhang, B.Q.; Chen, Y.F.; Wang, Q.; Wang, K.; Chen, Z.Y.; Li, X.W.; Lin, J.H. Identifying Common Pathogens in Periprosthetic Joint Infection and Testing Drug-Resistance Rate for Different Antibiotics: A Prospective, Single Center Study in Beijing. Orthop. Surg. 2018, 10, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [Green Version]
- Zmistowski, B.; Karam, J.A.; Durinka, J.B.; Casper, D.S.; Parvizi, J. Periprosthetic Joint Infection Increases the Risk of One-Year Mortality. J. Bone Jt. Surg. Am. 2013, 95, 2177–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urish, K.L.; DeMuth, P.W.; Kwan, B.W.; Craft, D.W.; Ma, D.; Haider, H.; Tuan, R.S.; Wood, T.K.; Davis, C.M., 3rd. Antibiotic-Tolerant Staphylococcus aureus Biofilm Persists on Arthroplasty Materials. Clin. Orthop Relat Res. 2016, 474, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Svensson Malchau, K.; Tillander, J.; Zaborowska, M.; Hoffman, M.; Lasa, I.; Thomsen, P.; Malchau, H.; Rolfson, O.; Trobos, M. Biofilm Properties in Relation to Treatment Outcome in Patients with First-time Periprosthetic Hip or Knee Joint Infection. J. Orthop. Translat. 2021, 30, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, F.; Salyapongse, A.; Kumagai, A.; Dupuy, F.G.; Shukla, K.; Penk, A.; Huster, D.; Ernst, R.K.; Pavlova, A.; Gumbart, J.C.; et al. Synergistic Biophysical Techniques Reveal Structural Mechanisms of Engineered Cationic Antimicrobial Peptides in Lipid Model Membranes. Chemistry 2020, 26, 6247–6256. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.; Dupuy, F.G.; Arsov, Z.; Elhady, Y.; Moody, D.; Ernst, R.K.; Deslouches, B.; Montelaro, R.C.; Peter Di, Y.; Tristram-Nagle, S. Elastic Behavior of Model Membranes with Antimicrobial Peptides Depends on Lipid Specificity and D-Enantiomers. Soft Matter 2019, 15, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Steckbeck, J.D.; Craigo, J.K.; Doi, Y.; Mietzner, T.A.; Montelaro, R.C. Rational Design of Engineered Cationic Antimicrobial Peptides Consisting Exclusively of Arginine and Tryptophan, and Their Activity against Multidrug-Resistant Pathogens. Antimicrob. Agents Chemother. 2013, 57, 2511–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starr, C.G.; Wimley, W.C. Antimicrobial Peptides Are Degraded by the Cytosolic Proteases of Human Erythrocytes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Dobbins, D.; Ghahramani, P.; Friedland, I.; Steckbeck, J. A Phase 1 Study of Safety, Tolerability and Pharmacokinetics of Single Ascending Doses of a First in Human Engineered Cationic Peptide, PLG0206, Intravenously Administered in Healthy Subjects. Antimicrob. Agents Chemother. 2021, AAC0144121. [Google Scholar] [CrossRef]
- Huang, D.P.; (Peptilogics, Pittsburgh, PA, USA); Pachuda, N.; (Peptilogics, Pittsburgh, PA, USA); Sauer, J.M.; (Peptilogics, Pittsburgh, PA, USA). Unpublished work. 2016.
- Beumer, J.H.; Guo, J.; Ray, E.C.; Scemama, J.; Parise, R.A.; Deslouches, B.; Steckbeck, J.D.; Montelaro, R.C.; Eiseman, J.L. Mass Balance Study of the Engineered Cationic Antimicrobial Peptide, WLBU2, Following a Single Intravenous Dose of 14C-WLBU2 in Mice. Curr Rev. Clin. Exp. Pharmacol 2021, 16, 263–272. [Google Scholar] [CrossRef]
- Investigator’s Brochure for PLG0206; (Peptilogics, Pittsburgh, PA, USA). Unpublished work. 2021.
- Murray, B.P.; (Peptilogics, Pittsburgh, PA, USA); Hufnagel, D.; (Peptilogics, Pittsburgh, PA, USA); Pillar, C.M.; (Peptilogics, Pittsburgh, PA, USA). In Vitro Activity of PLG0206 against a Representative Panel of Staphylococcus epidermidis and Other Coagulase-Negative Staphylococci, and Resistant Gram-Negative Pathogens. Unpublished work. 2020. [Google Scholar]
- Lin, Q.; Deslouches, B.; Montelaro, R.C.; Di, Y.P. Prevention of ESKAPE pathogen biofilm formation by antimicrobial peptides WLBU2 and LL37. Int. J. Antimicrob. Agents 2018, 52, 667–672. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Hola, V.; Imbert, C.; Kirketerp-Moller, K.; et al. ESCMID Guideline for the Diagnosis and Treatment of Biofilm Infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. 1), S1–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lashua, L.P.; Melvin, J.A.; Deslouches, B.; Pilewski, J.M.; Montelaro, R.C.; Bomberger, J.M. Engineered Cationic Antimicrobial Peptide (eCAP) Prevents Pseudomonas aeruginosa Biofilm Growth on Airway Epithelial Cells. J. Antimicrob. Chemother. 2016, 71, 2200–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melvin, J.A.; Lashua, L.P.; Kiedrowski, M.R.; Yang, G.; Deslouches, B.; Montelaro, R.C.; Bomberger, J.M. Simultaneous Antibiofilm and Antiviral Activities of an Engineered Antimicrobial Peptide during Virus-Bacterium Coinfection. mSphere 2016, 1, e00083-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandell, J.B.; Koch, J.A.; Deslouches, B.; Urish, K.L. Direct Antimicrobial Activity of Cationic Amphipathic Peptide WLBU2 Against Staphylococcus aureus Biofilms is Enhanced in Physiologic Buffered Saline. J. Orthop. Res. 2020, 38, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.B.; Deslouches, B.; Montelaro, R.C.; Shanks, R.M.Q.; Doi, Y.; Urish, K.L. Elimination of Antibiotic Resistant Surgical Implant Biofilms Using an Engineered Cationic Amphipathic Peptide WLBU2. Sci. Rep. 2017, 7, 18098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagi, H.; Kihara, S.; Mittwede, P.N.; Maher, P.L.; Rothenberg, A.C.; Falcione, A.; Chen, A.; Urish, K.L.; Tuan, R.S.; Alexander, P.G. Development of a Large Animal Rabbit Model for Chronic Periprosthetic Joint Infection. Bone Jt. Res. 2021, 10, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Brothers, K.M.; Mandell, J.B.; Taguchi, M.; Parker, D.M.; Alexander, P.G.; Dobbins, D.; Huang, D.B.; Pachuda, N.; Urish, K.L. PLG0206, an Engineered Antimicrobial Peptide, has Potent Activity against Biofilm and Results in Disease Free Survival in a Rabbit Model of Staphylococcus aureus Periprosthetic Joint Infection. In Proceedings of the 2022 Annual Meeting of the Orthopaedic Research Society, Tampa, FL, USA, 4–8 February 2022. accepted. [Google Scholar]
- Mandell, J.B.; Brothers, K.; Shinaberger, D.; Pillar, C.; Huang, D.; Steckbeck, J.; Urish, K.L. The Engineered Antimicrobial Peptide PLG0206 has High Biofilm and Planktonic Activity against Multi-Drug Resistant ESKAPE Organisms. In Proceedings of the Annual Meeting of the American Academy of Orthopaedic Surgeons, Chicago, IL, USA, 22–26 March 2022. submitted. [Google Scholar]
- Parker, D.M.; Brothers, K.M.; Mandell, J.B.; Koch, J.A.; Pachuda, N.; Dobbins, D.; Steckbeck, J.; Huang, D.B.; Urish, K.L. Prospective Ex Vivo Activity of PLG0206, an Engineered Antimicrobial Peptide, on Chronic Periprosthetic Joint Infection Total Knee Arthroplasty Components: The Knee Explant Analysis (KnEA) Study. In Proceedings of the 2022 Annual Meeting of the Orthopaedic Research Society, Tampa, FL, USA, 4–8 February 2022. accepted. [Google Scholar]
| Organism | Drug | MIC Range, µg/mL |
|---|---|---|
| All Staphylococcus epidermidis (104) | PLG0206 CAMHB 1 | 0.25 to 4 |
| PLG0206 RPMI 1 | 0.03 to 0.12 | |
| Imipenem | <0.008 to >8 | |
| Levofloxacin | 0.12 to >4 | |
| Tigecycline | 0.12 to 2 | |
| Vancomycin | 1 to 4 | |
| Linezolid | 0.5 to >8 | |
| Oxacillin | 0.03 to >16 | |
| MSSE (46) | PLG0206 CAMHB 1 | 0.25 to 4 |
| PLG0206 RPMI 1 | 0.03 to 0.12 | |
| Imipenem | <0.008 to 0.06 | |
| Levofloxacin | 0.12 to >4 | |
| Tigecycline | 0.12 to 2 | |
| Vancomycin | 1 to 4 | |
| Linezolid | 0.5 to 4 | |
| Oxacillin | 0.06 to 0.25 | |
| MRSE (58) 2 | PLG0206 CAMHB 1 | 0.25 to 4 |
| PLG0206 RPMI 1 | 0.03 to 0.12 | |
| Imipenem | <0.008 to >8 | |
| Levofloxacin | 0.12 to >4 | |
| Tigecycline | 0.12 to 1 | |
| Vancomycin | 1 to 4 | |
| Linezolid | 1 to >8 | |
| Oxacillin | 0.5 to >16 | |
| CoNS, non-epidermidis (53) 3 | PLG0206 CAMHB 1 | <0.12 to 4 |
| PLG0206 RPMI 1 | 0.015 to 2 | |
| Imipenem | <0.008 to >8 | |
| Levofloxacin | 0.12 to >4 | |
| Tigecycline | 0.25 to 2 | |
| Vancomycin | 0.5 to 2 | |
| Linezolid | 1 to 4 | |
| Oxacillin | 0.12 to >16 | |
| Enterobacterales (22) 4 | PLG0206 CAMHB 1 | 1 to >8 |
| PLG0206 RPMI 1 | <0.12 to >128 | |
| Imipenem | 2 to >8 | |
| Levofloxacin | 0.06 to >4 | |
| Tigecycline | 0.25 to 4 | |
| Ceftazidime | 0.12 to >32 | |
| Colistin | 0.06 to >16 | |
| Amikacin | 1 to >64 | |
| Pseudomonas aeruginosa (20) | PLG0206 CAMHB 1 | 8 to >8 |
| PLG0206 RPMI 1 | 0.5 to 4 | |
| Imipenem | 4 to >8 | |
| Levofloxacin | 0.5 to >4 | |
| Tigecycline | 8 to >16 | |
| Ceftazidime | 4 to >32 | |
| Colistin | 0.12 to 2 | |
| Amikacin | 0.5 to >64 | |
| Acinetobacter baumannii (24) | PLG0206 CAMHB 1 | 2 to 8 |
| PLG0206 RPMI 1 | 0.25 to 0.5 | |
| Imipenem | 1 to >8 | |
| Levofloxacin | 4 to >4 | |
| Tigecycline | 1 to 16 | |
| Ceftazidime | >32 to >32 | |
| Colistin | 0.12 to >16 | |
| Amikacin | 2 to >64 |
| PLG0206 Dose, μg/mL | dPBS: pH 7.0, CFU/mL |
|---|---|
| 0 | 460,000 (141,774) |
| 62 | 1257 (1510) |
| 125 | 450 (522) |
| 250 | 280 (226) |
| 500 | 233 (188) |
| 1000 | 417 (492) |
| PJI Implant No. | No Drug, CFU/mL | pH 6.5, CFU/mL | pH 7.0, CFU/mL | pH 7.2, CFU/mL | pH 7.4, CFU/mL |
|---|---|---|---|---|---|
| 1 | 287,000 | 40,000 | 5000 | 7000 | 560 |
| 2 | 933,000 | 183,000 | 53,000 | 33,000 | 133 |
| 3 | 466,000 | 1,233,000 | 3000 | 6900 | 5660 |
| 4 | 540,000 | 266,000 | 80,000 | 12,600 | 8300 |
| 5 | 1,200,000 | 500,000 | 30,000 | 40,000 | 33,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Pachuda, N.; Sauer, J.M.; Dobbins, D.; Steckbeck, J. The Engineered Antibiotic Peptide PLG0206 Eliminates Biofilms and Is a Potential Treatment for Periprosthetic Joint Infections. Antibiotics 2022, 11, 41. https://doi.org/10.3390/antibiotics11010041
Huang D, Pachuda N, Sauer JM, Dobbins D, Steckbeck J. The Engineered Antibiotic Peptide PLG0206 Eliminates Biofilms and Is a Potential Treatment for Periprosthetic Joint Infections. Antibiotics. 2022; 11(1):41. https://doi.org/10.3390/antibiotics11010041
Chicago/Turabian StyleHuang, David, Nicholas Pachuda, John Michael Sauer, Dessie Dobbins, and Jonathan Steckbeck. 2022. "The Engineered Antibiotic Peptide PLG0206 Eliminates Biofilms and Is a Potential Treatment for Periprosthetic Joint Infections" Antibiotics 11, no. 1: 41. https://doi.org/10.3390/antibiotics11010041
APA StyleHuang, D., Pachuda, N., Sauer, J. M., Dobbins, D., & Steckbeck, J. (2022). The Engineered Antibiotic Peptide PLG0206 Eliminates Biofilms and Is a Potential Treatment for Periprosthetic Joint Infections. Antibiotics, 11(1), 41. https://doi.org/10.3390/antibiotics11010041





