The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Sample Collection
2.3. Isolation and Identification of Bacterial Isolates
2.4. Antibiotic Susceptibility Testing (AST)
2.5. Statistical Analysis
3. Results
3.1. Distribution of Clinical Bacterial Isolates among Coronavirus Disease 2019 (COVID-19) Patients
3.2. Clinical Isolates from Various Infections
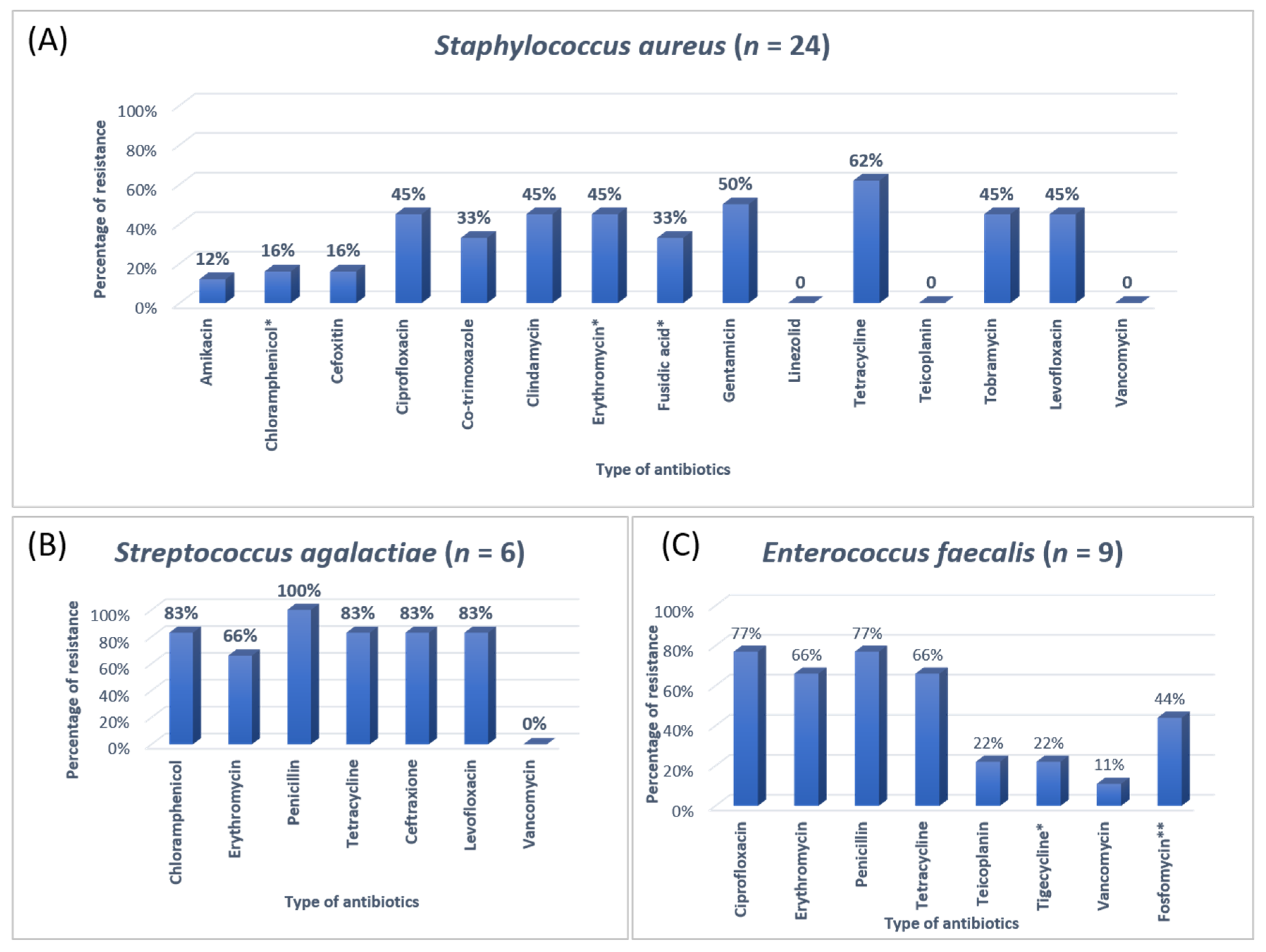
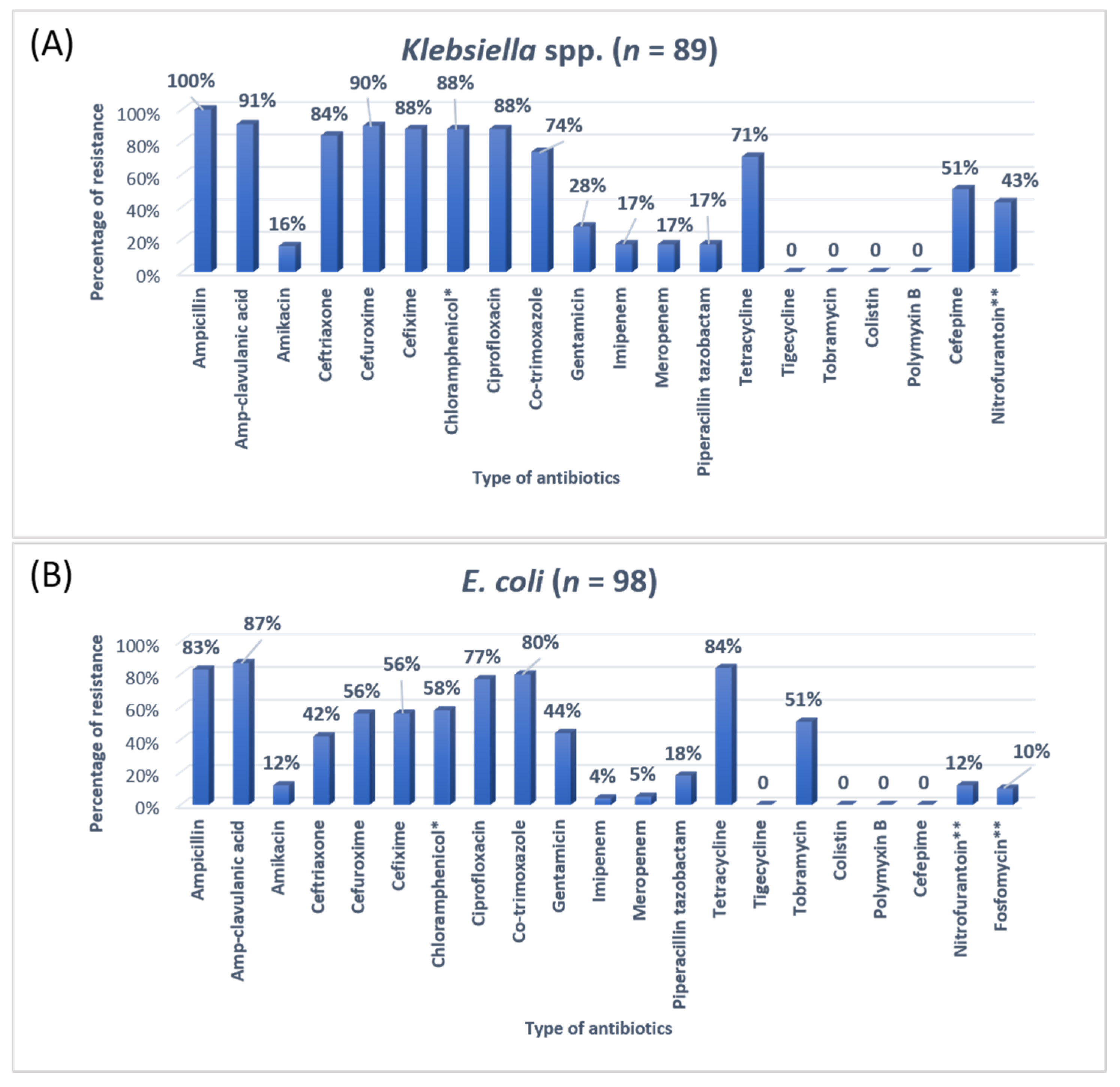
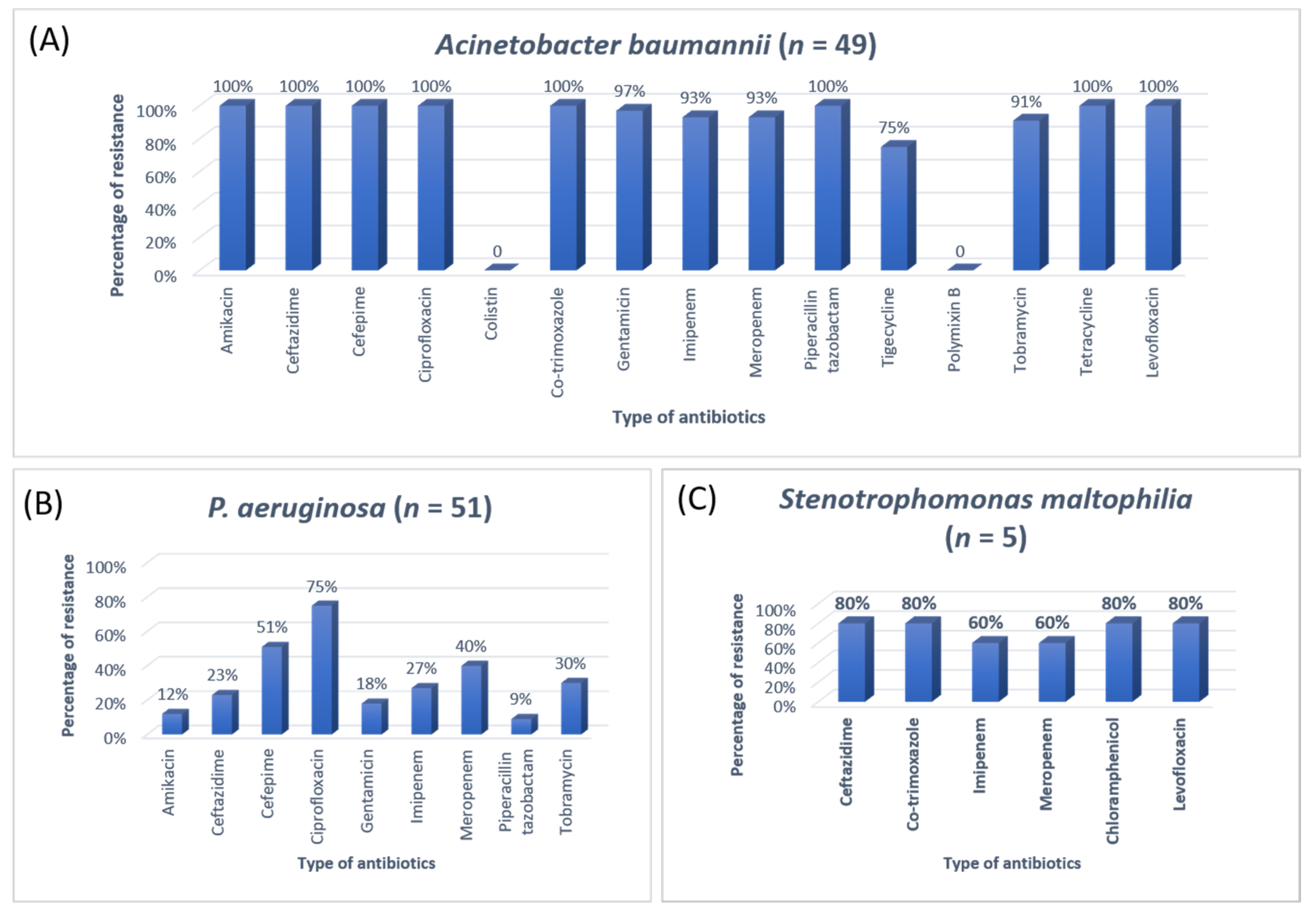
3.3. Antibiotic Susceptibility Patterns of Clinical Isolates
3.4. Distribution of COVID-19 Patients with Major Comorbidities
3.5. Mortality and Recovery Rate of the Patient
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clancy, C.J.; Buehrle, D.J.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC-Antimicrob. Resist. 2020, 2, dlaa049. [Google Scholar] [CrossRef]
- Mondal, M.K.; Roy, B.R.; Yeasmeen, S.; Haque, F.; Huda, A.Q.; Banik, D. Prevalence of microorganism and emergence of bacterial resistance in ICU of Bangabandhu Sheikh Mujib Medical University of Bangladesh. J. Bangladesh Soc. Anaesthesiol. 2013, 26, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Parveen, S.; Saqib, S.; Ahmed, A.; Shahzad, A.; Ahmed, N. Prevalence of MRSA colonisation among healthcare-workers and effectiveness of decolonisation regimen in ICU of a Tertiary care Hospital, Lahore, Pakistan. Adv. Life Sci. 2020, 8, 38–41. [Google Scholar]
- Ventola, C.L. The antibiotic resistance crisis: Part 2: Management strategies and new agents. Pharm. Ther. 2015, 40, 344. [Google Scholar]
- Pickens, C.I.; Wunderink, R.G. Principles and practice of antibiotic stewardship in the ICU. Chest 2019, 156, 163–171. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Coast, J. The true cost of antimicrobial resistance. BMJ 2013, 346, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dyar, O.J.; Castro-Sánchez, E.; Holmes, A.H. What makes people talk about antibiotics on social media? A retrospective analysis of Twitter use. J. Antimicrob. Chemother. 2014, 69, 2568–2572. [Google Scholar] [CrossRef] [Green Version]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. Elife 2021, 10, e64139. [Google Scholar] [CrossRef] [PubMed]
- Monnet, D.L.; Harbarth, S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Eurosurveillance 2020, 25, 2001886. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Jatoi, M.A.; Al-Wraikat, M.; Ahmed, N.; Li, J. Time to Enhance Immunity via Functional Foods and Supplements: Hope for SARS-CoV-2 Outbreak. Altern. Ther. Health Med. 2020, 27, 30–44. [Google Scholar]
- Adiga, M.S.; Alwar, M.; Pai, M.; Adiga, U.S. Pattern of antimicrobial agents use in hospital deliveries: A prospective comparative study. Online J. Health Allied Sci. 2010, 8, 10. [Google Scholar]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [Green Version]
- Zahra, N.; Zeshan, B.; Qadri, M.M.A.; Ishaq, M.; Afzal, M.; Ahmed, N. Phenotypic and Genotypic Evaluation of Antibiotic Resistance of Acinetobacter baumannii Bacteria Isolated from Surgical Intensive Care Unit Patients in Pakistan. Jundishapur J. Microbiol. 2021, 14, e113008. [Google Scholar] [CrossRef]
- Bataineh, H.A.; Alrashed, K.M. Resistant gram-negative bacilli and antibiotic consumption in Zarqa, Jordan. Pak. J. Med. Sci. 2007, 23, 59–63. [Google Scholar]
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement M100; Clinical Laboratory Standards Institute (CLSI) Guidelines; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Ahmed, N.; Ali, Z.; Riaz, M.; Zeshan, B.; Wattoo, J.I.; Aslam, M.N. Evaluation of Antibiotic Resistance and Virulence Genes among Clinical Isolates of Pseudomonas aeruginosa from Cancer Patients. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1333–1338. [Google Scholar] [CrossRef]
- Mehta, T.; Chauhan, B.; Rathod, S.; Pethani, J.; Shah, P.D. Bacterilogical profile and drug resistance pattern of isolates of the patients admitted in medical intensive care unit of a tertiary care hospital in Ahmedabad. Med. Sci. 2015, 4, 222–225. [Google Scholar]
- Barai, L.; Fatema, K.; Haq, J.A.; Faruq, M.O.; Ahsan, A.A.; Morshed, M.A.H.G.; Hossain, M.B. Bacterial profile and their antimicrobial resistance pattern in an intensive care unit of a tertiary care hospital of Dhaka. Ibrahim Med. Coll. J. 2010, 4, 66–69. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Abramson, M.A.; Beekmann, S.E.; Gallagher, G.; Riedel, S.; Diekema, D.J.; Quinn, J.P.; Doern, G.V. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J. Clin. Microbiol. 2007, 45, 3352–3359. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.Y.; Kumar, A. Empiric antimicrobial therapy in severe sepsis and septic shock: Optimising pathogen clearance. Curr. Infect. Dis. Rep. 2015, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Zeshan, B.; Naveed, M.; Afzal, M.; Mohamed, M. Antibiotic resistance profile in relation to virulence genes fimH, hlyA and usp of uropathogenic E. coli isolates in Lahore, Pakistan. Trop. Biomed. 2019, 36, 559–568. [Google Scholar]
- Sanjana, R.; Shah, R.; Chaudhary, N.; Singh, Y. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) in CMS-teaching hospital: A preliminary report. J. Coll. Med. Sci.-Nepal 2010, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Radji, M.; Fauziah, S.; Aribinuko, N. Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of Fatmawati Hospital, Indonesia. Asian Pac. J. Trop. Biomed. 2011, 1, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Tuem, K.B.; Desta, R.; Bitew, H.; Ibrahim, S.; Hishe, H.Z. Antimicrobial resistance patterns of uropathogens isolated between 2012 and 2017 from a tertiary hospital in Northern Ethiopia. J. Glob. Antimicrob. Resist. 2019, 18, 109–114. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Jusoh, N.A.; Htike, Z.Z.; Win, S.L. Bacteria identification from microscopic morphology: A survey. Int. J. Soft Comput. Artif. Intell. Appl. (IJSCAI) 2014, 3, 1–12. [Google Scholar] [CrossRef]
- Phillips-Jones, M.K.; Harding, S.E. Antimicrobial resistance (AMR) nanomachines—Mechanisms for fluoroquinolone and glycopeptide recognition, efflux and/or deactivation. Biophys. Rev. 2018, 10, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 10, 91–95. [Google Scholar] [CrossRef]

| Age Group (Years) | Gender | Total | |
|---|---|---|---|
| Male | Female | ||
| 01–20 | 72 | 74 | 146 |
| 21–40 | 99 | 102 | 201 |
| 41–60 | 132 | 76 | 208 |
| 61–80 | 181 | 86 | 267 |
| >80 | 22 | 12 | 34 |
| Total | 506 | 350 | 856 |
| Bacterial Isolates | n | % | |
|---|---|---|---|
| Gram-Positive bacteria (n = 39, 11.40%) | |||
| Staphylococcus aureus | MRSA | 4 | 1.16 |
| MSSA | 20 | 5.84 | |
| Enterococcus faecalis | Non-VRE | 8 | 2.33 |
| VRE | 1 | 0.29 | |
| Streptococcus agalactiae | 6 | 1.75 | |
| Gram-Negative bacteria (n = 303, 88.59%) | |||
| Escherichia coli | Non-CRE | 93 | 27.19 |
| CRE | 5 | 1.46 | |
| Klebsiella pneumoniae | Non-CRE | 68 | 19.88 |
| CRE | 16 | 4.67 | |
| Pseudomonas aeruginosa | 51 | 14.91 | |
| Acinetobacter baumannii | 49 | 14.32 | |
| Stenotrophomonas maltophilia | 5 | 1.46 | |
| Klebsiella oxytoca | 5 | 1.46 | |
| Citrobacter freundii | 4 | 1.16 | |
| Serratia liquefaciens | 4 | 1.16 | |
| Proteus vulgaris | 3 | 0.87 | |
| Variables | Drug Administration | Number (n) | Percentage (%) | |
|---|---|---|---|---|
| Type of antibiotic used | Vancomycin | IV | 23 | 6.72 |
| Teicoplanin | IV | 2 | 0.58 | |
| Linezolid | IV | 4 | 1.16 | |
| Imipenem | IV | 29 | 8.47 | |
| Meropenem | IV | 27 | 7.89 | |
| Amikacin | IV | 54 | 15.78 | |
| Gentamicin | IV/Oral | 36 | 10.52 | |
| Tobramycin | IV/Oral | 23 | 6.72 | |
| Nitrofurantoin | IV/Oral | 76 | 22.22 | |
| Fosfomycin | IV/Oral | 84 | 24.56 | |
| Piperacillin-tazobactam | IV | 62 | 18.12 | |
| Colistin | IV | 21 | 6.14 | |
| Polymyxin B | IV | 16 | 1.75 | |
| Ciprofloxacin | IV/Oral | 73 | 21.34 | |
| Ceftriaxone | IV | 34 | 9.94 | |
| Cefepime | IV | 23 | 6.72 | |
| Combination of antibiotics | One | 229 | 66.95 | |
| Multiple (two or more) | 113 | 33.04 | ||
| Days of antibiotic treatment | 1 to 7 days | 204 | 59.64 | |
| 8 to 14 days | 91 | 26.60 | ||
| >15 days | 47 | 18.71 | ||
| Sr. No | Comorbidities | Total Number of Patients | Outcome | p-Value |
|---|---|---|---|---|
| 1. | Pneumonia, Aspiration, DM, HTN, IHD, COPD, CLD | 135 | 64 Recovered 71 Died | <0.001 |
| 2. | UTIs, Dementia, DM, HTN, CKD | 140 | 121 Recovered 19 Died | |
| 3. | Meningoencephalitis, Parkinsonism, HTN, Stroke | 2 | 2 Recovered | |
| 4. | Sepsis, DM, GI disorders | 32 | 29 Recovered 3 Died |
| Co-Infections | COVID-19 Severity | p-Value | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Upper respiratory tract infections | 4 | 5 | 15 | 0.028 |
| Lower respiratory tract infections | 13 | 21 | 67 | |
| Bacteremia | 3 | 8 | 21 | |
| Gastrointestinal infections | 4 | 23 | 18 | |
| Urinary tract infections | 11 | 41 | 88 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.A.; Ahmed, N.; et al. The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics 2022, 11, 35. https://doi.org/10.3390/antibiotics11010035
Zeshan B, Karobari MI, Afzal N, Siddiq A, Basha S, Basheer SN, Peeran SW, Mustafa M, Daud NHA, Ahmed N, et al. The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics. 2022; 11(1):35. https://doi.org/10.3390/antibiotics11010035
Chicago/Turabian StyleZeshan, Basit, Mohmed Isaqali Karobari, Nadia Afzal, Amer Siddiq, Sakeenabi Basha, Syed Nahid Basheer, Syed Wali Peeran, Mohammed Mustafa, Nur Hardy A. Daud, Naveed Ahmed, and et al. 2022. "The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance" Antibiotics 11, no. 1: 35. https://doi.org/10.3390/antibiotics11010035
APA StyleZeshan, B., Karobari, M. I., Afzal, N., Siddiq, A., Basha, S., Basheer, S. N., Peeran, S. W., Mustafa, M., Daud, N. H. A., Ahmed, N., Yean, C. Y., & Noorani, T. Y. (2022). The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics, 11(1), 35. https://doi.org/10.3390/antibiotics11010035











