Oral and Parenteral vs. Parenteral Antibiotic Prophylaxis for Patients Undergoing Laparoscopic Colorectal Resection: An Intervention Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Types of studiesAll randomized clinical trials (RCTs). Nonrandomized and quasirandomized trials were excluded. Studies where it was not possible to obtain the complete manuscript were excluded.
- Types of participantsPatients undergoing laparoscopic colorectal surgery.
- Types of interventions and comparisonOral and parenteral antibiotic prophylaxis vs. parenteral-only prophylaxis.
- Types of outcome measuresPrimary outcomes: SSIs. Secondary outcomes: other infectious and noninfectious postoperative complications. Studies with no outcome of interest were excluded.
2.2. Search Strategy
2.3. Selection Process
2.4. Data Collection Process
2.5. Risk of Bias Assessment
- Bias arising from the randomization process;
- Bias due to deviations from intended interventions;
- Bias due to missing outcome data;
- Bias in measurement of the outcome;
- Bias in selection of the reported result.
2.6. Statistical Analysis
2.7. Certainty Assessment
3. Results
3.1. Study Selection
3.2. Risk of Bias Assessment
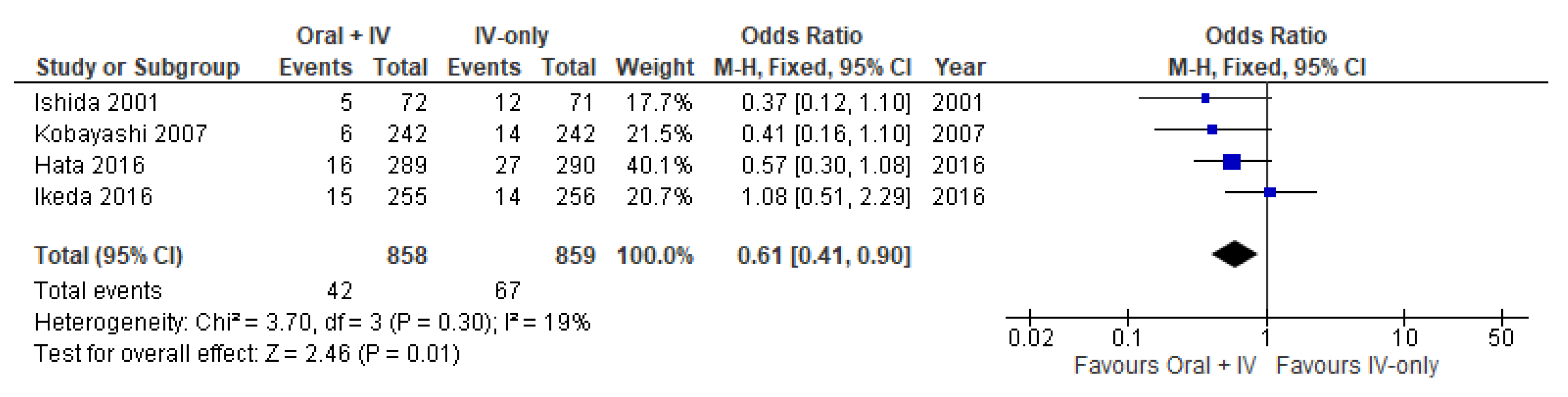
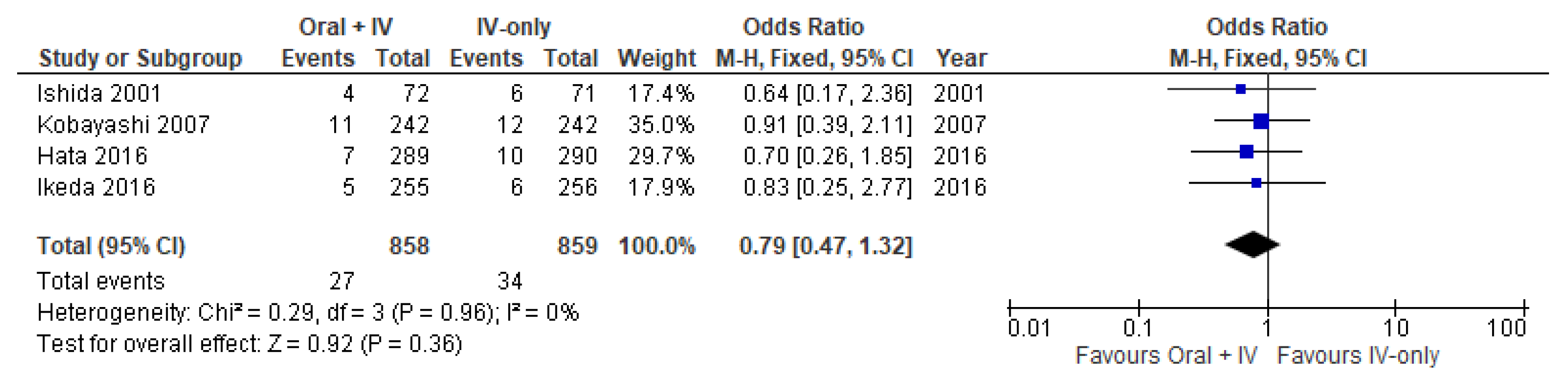
3.3. Surgical Site Infections
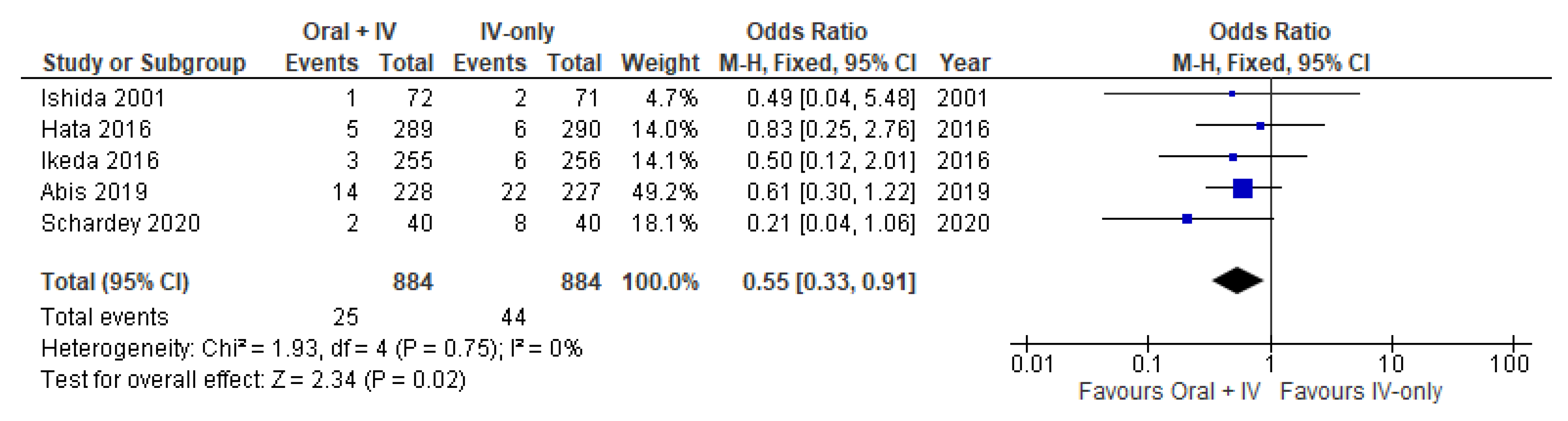
3.4. Anastomotic Leakage
3.5. Enteritis/Colitis
3.6. Pneumonia
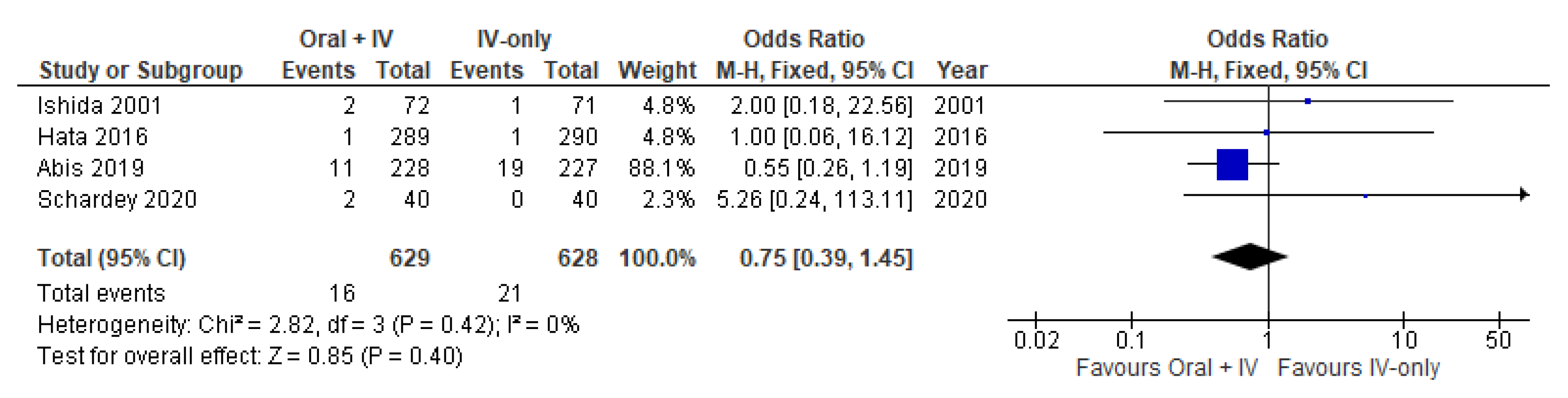
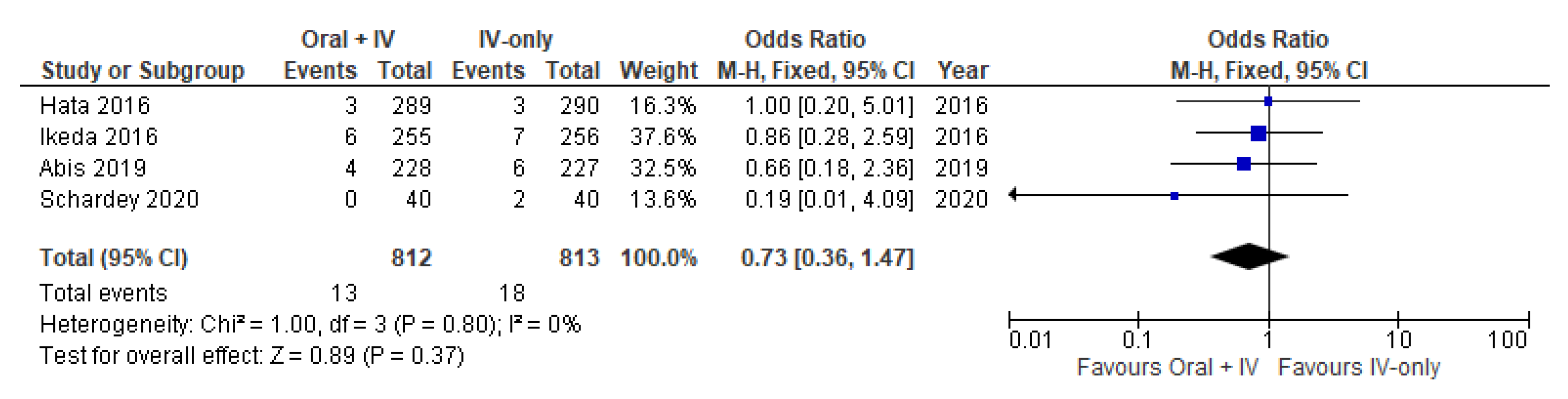
3.7. Urinary Tract Disorder
3.8. Bowel Obstruction
3.9. Certainty Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
Appendix A. Search Strategy
| Database | Date of Search | Search Strategy |
|---|---|---|
| Cochrane Central Register of Controlled Trials (Central) | 1 May 2021, updated 6 December 2021 | #1 MeSH descriptor: [Laparoscopy] explode all trees #2 MeSH descriptor: [Colorectal Neoplasms] explode all trees #3 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees #4 MeSH descriptor: [Surgical Wound Infection] explode all trees #5 Laparoscop * AND Colorectal neoplasm* #6 antibiotic * OR prophyla* #7 #1 AND #2 #8 #5 OR #7 #9 #3 OR #6 #10 surgical wound * OR wound * infection * #11 #4 OR #10 #12 #8 AND #9 #11 |
| PUBMED (Ovid SP) | 1 May 2021, updated 6 December 2021 | (1) “laparoscopie”[All Fields] OR “laparoscopy”[MeSH Terms] OR “laparoscopy”[All Fields] OR “laparoscopies”[All Fields] (2) “colorectal neoplasms”[MeSH Terms] OR (“colorectal”[All Fields] AND “neoplasms”[All Fields]) OR “colorectal neoplasms”[All Fields] OR (“colorectal”[All Fields] AND “neoplasm”[All Fields]) OR “colorectal neoplasm”[All Fields] (3) “antibiotic prophylaxis”[MeSH Terms] OR (“antibiotic”[All Fields] AND “prophylaxis”[All Fields]) OR “antibiotic prophylaxis”[All Fields] (4) “oral”[All Fields] OR “mouth”[MeSH Terms] OR “mouth”[All Fields] (5) “infusions, parenteral”[MeSH Terms] OR (“infusions”[All Fields] AND “parenteral”[All Fields]) OR “parenteral infusions”[All Fields] OR (“infusions”[All Fields] AND “parenteral”[All Fields]) OR “infusions, parenteral”[All Fields] (6) “surgical wound infection”[MeSH Terms] OR (“surgical”[All Fields] AND “wound”[All Fields] AND “infection”[All Fields]) OR “surgical wound infection”[All Fields] (7) 1 AND 2 (8) 3 OR 4 OR 5 (9) 7 AND 8 AND 6 |
| EMBASE (Ovid SP) | 1 May 2021, updated 6 December 2021 | 1. “laparoscopie”[All Fields] OR “laparoscopy”[MeSH Terms] OR “laparoscopy”[All Fields] OR “laparoscopies”[All Fields] 2. “colorectal neoplasms”[MeSH Terms] OR (“colorectal”[All Fields] AND “neoplasms”[All Fields]) OR “colorectal neoplasms”[All Fields] OR (“colorectal”[All Fields] AND “neoplasm”[All Fields]) OR “colorectal neoplasm”[All Fields] 3. “antibiotic prophylaxis”[MeSH Terms] OR (“antibiotic”[All Fields] AND “prophylaxis”[All Fields]) OR “antibiotic prophylaxis”[All Fields] 4. “oral”[All Fields] OR “mouth”[MeSH Terms] OR “mouth”[All Fields] 5. “infusions, parenteral”[MeSH Terms] OR (“infusions”[All Fields] AND “parenteral”[All Fields]) OR “parenteral infusions”[All Fields] OR (“infusions”[All Fields] AND “parenteral”[All Fields]) OR “infusions, parenteral”[All Fields] 6. “surgical wound infection”[MeSH Terms] OR (“surgical”[All Fields] AND “wound”[All Fields] AND “infection”[All Fields]) OR “surgical wound infection”[All Fields] 7. 1 AND 2 8. 3 OR 4 OR 5 9. 7 AND 8 AND 6 |
| Science Citation Index Expanded | 6 December 2021 | # 5 #4 AND #3 # 4 TS=(surgical wound infection *) # 3 #2 AND #1 # 2 TS=(laparoscop * and colorectal neoplasm *) # 1 TS=(antibiotic * or prophyla * or oral or parenteral) |
| Ishida 2001 | |
| Risk of bias arising from the randomization process | Some concerns |
| Risk of bias due to deviations from the intended interventions | Low |
| Bias due to missing outcome data | Low |
| Risk of bias in measurement of the outcome | High |
| Risk of bias in selection of the reported result | Some concerns |
| Overall risk of bias | High |
| Kobayashi 2007 | |
| Risk of bias arising from the randomization process | Low |
| Risk of bias due to deviations from the intended interventions | Some concerns |
| Bias due to missing outcome data | Low |
| Risk of bias in measurement of the outcome | High |
| Risk of bias in selection of the reported result | High |
| Overall risk of bias | High |
| Ikeda 2016 | |
| Risk of bias arising from the randomization process | Low |
| Risk of bias due to deviations from the intended interventions | Low |
| Bias due to missing outcome data | Low |
| Risk of bias in measurement of the outcome | Low |
| Risk of bias in selection of the reported result | Low |
| Overall risk of bias | Low |
| Hata 2016 | |
| Risk of bias arising from the randomization process | Low |
| Risk of bias due to deviations from the intended interventions | Some concerns |
| Bias due to missing outcome data | Low |
| Risk of bias in measurement of the outcome | Low |
| Risk of bias in selection of the reported result | Low |
| Overall risk of bias | Some concerns |
| Abis 2019 | |
| Risk of bias arising from the randomization process | Low |
| Risk of bias due to deviations from the intended interventions | Some concerns |
| Bias due to missing outcome data | Low |
| Risk of bias in measurement of the outcome | Low |
| Risk of bias in selection of the reported result | Low |
| Overall risk of bias | Some concerns |
| Schardey 2020 | |
| Risk of bias arising from the randomization process | Low |
| Risk of bias due to deviations from the intended interventions | Low |
| Bias due to missing outcome data | Low |
| Risk of bias in measurement of the outcome | Some concerns |
| Risk of bias in selection of the reported result | High |
| Overall risk of bias | High |
References
- Hansen, S.; Sohr, D.; Geffers, C.; Astagneau, P.; Blacky, A.; Koller, W.; Morales, I.; Moro, M.L.; Palomar, M.; Szilagyi, E.; et al. Concordance between European and US Case Definitions of Healthcare-Associated Infections. Antimicrob. Resist. Infect. Control 2012, 1, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eagye, K.J.; Nicolau, D.P. Deep and Organ/Space Infections in Patients Undergoing Elective Colorectal Surgery: Incidence and Impact on Hospital Length of Stay and Costs. Am. J. Surg. 2009, 198, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.; Long, J.; Hynan, L.S.; Sarosi, G.A.; Nwariaku, F.; Huth, J.; Jones, C.; Parker, B.J.N.; Rege, R. Surgical Complications Exert a Lasting Effect on Disease-Specific Health-Related Quality of Life for Patients with Colorectal Cancer. Surgery 2003, 134, 119–125. [Google Scholar] [CrossRef]
- Kashimura, N.; Kusachi, S.; Konishi, T.; Shimizu, J.; Kusunoki, M.; Oka, M.; Wakatsuki, T.; Sumiyama, Y. Impact of Surgical Site Infection after Colorectal Surgery on Hospital Stay and Medical Expenditure in Japan. Surg. Today 2012, 42, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Peri-Operative Antimicrobial Prophylaxis. Available online: https://www.ecdc.europa.eu/en/publications-data/directory-guidance-prevention-and-control/prudent-use-antibiotics/peri-operative (accessed on 28 November 2021).
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect. Control. Hosp. Epidemiol. 1999, 20, 247–280. [Google Scholar] [CrossRef]
- Espin-Basany, E.; Sanchez-Garcia, J.L.; Lopez-Cano, M.; Lozoya-Trujillo, R.; Medarde-Ferrer, M.; Armadans-Gil, L.; Alemany-Vilches, L.; Armengol-Carrasco, M. Prospective, Randomised Study on Antibiotic Prophylaxis in Colorectal Surgery. Is It Really Necessary to Use Oral Antibiotics? Int. J. Colorectal Dis. 2005, 20, 542–546. [Google Scholar] [CrossRef]
- Young Tabusso, F.; Celis Zapata, J.; Berrospi Espinoza, F.; Payet Meza, E.; Ruiz Figueroa, E. Mechanical preparation in elective colorectal surgery, a usual practice or a necessity? Rev. Gastroenterol. Peru 2002, 22, 152–158. [Google Scholar]
- Nelson, R.L.; Gladman, E.; Barbateskovic, M. Antimicrobial Prophylaxis for Colorectal Surgery. Cochrane Database Syst. Rev. 2014, CD001181. [Google Scholar] [CrossRef] [Green Version]
- Huscher, C.G.S.; Bretagnol, F.; Corcione, F. Laparoscopic Colorectal Cancer Resection in High-Volume Surgical Centers: Long-Term Outcomes from the LAPCOLON Group Trial. World J. Surg. 2015, 39, 2045–2051. [Google Scholar] [CrossRef]
- Jayne, D.G.; Thorpe, H.C.; Copeland, J.; Quirke, P.; Brown, J.M.; Guillou, P.J. Five-Year Follow-up of the Medical Research Council CLASICC Trial of Laparoscopically Assisted vs. Open Surgery for Colorectal Cancer. Br. J. Surg. 2010, 97, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Fujita, S.; Ishiguro, S.; Akasu, T.; Moriya, Y. Wound Infection after a Laparoscopic Resection for Colorectal Cancer. Surg. Today 2008, 38, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, R.; Kuhry, E.; Hop, W.C.J.; Jeekel, J.; Kazemier, G.; Bonjer, H.J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Laparoscopic Surgery vs. Open Surgery for Colon Cancer: Short-Term Outcomes of a Randomised Trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group; Nelson, H.; Sargent, D.J.; Wieand, H.S.; Fleshman, J.; Anvari, M.; Stryker, S.J.; Beart, R.W.; Hellinger, M.; Flanagan, R.; et al. A Comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer. N. Engl. J. Med. 2004, 350, 2050–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; Cuesta, M.A.; van der Pas, M.H.G.M.; de Lange-de Klerk, E.S.M.; Lacy, A.M.; Bemelman, W.A.; Andersson, J.; Angenete, E.; et al. A Randomized Trial of Laparoscopic vs. Open Surgery for Rectal Cancer. N. Engl. J. Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, M.H.; Hwang, G.S.; Phelan, M.J.; Bui, T.-L.; Carmichael, J.C.; Mills, S.D.; Stamos, M.J.; Pigazzi, A. Laparoscopic right hemicolectomy: Short- and long-term outcomes of intracorporeal vs. extracorporeal anastomosis. Surg. Endosc. 2016, 30, 3933–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, J.; Naiken, S.; Christou, N.; Liot, E.; Toso, C.; Buchs, N.C.; Ris, F. Reducing anastomotic leak in colorectal surgery: The old dogmas and the new challenges. World J. Gastroenterol. 2019, 25, 5017–5025. [Google Scholar] [CrossRef]
- Nikolian, V.C.; Kamdar, N.S.; Regenbogen, S.E.; Morris, A.M.; Byrn, J.C.; Suwanabol, P.A.; Campbell, D.A.; Hendren, S. Anastomotic leak after colorectal resection: A population-based study of risk factors and hospital variation. Surgery 2017, 161, 1619–1627. [Google Scholar] [CrossRef]
- Kryzauskas, M.; Bausys, A.; Degutyte, A.E.; Abeciunas, V.; Poskus, E.; Bausys, R.; Dulskas, A.; Strupas, K.; Poskus, T. Risk factors for anastomotic leakage and its impact on long-term survival in left-sided colorectal cancer surgery. World J. Surg. Oncol. 2020, 18, 205. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Systematic Review and Evidence-Based Guidance on Perioperative Antibiotic Prophylaxis; ECDC: Stockholm, Sweden, 2013; ISBN 978-92-9193-484-3. [CrossRef]
- Walming, S.; Angenete, E.; Block, M.; Bock, D.; Gessler, B.; Haglind, E. Retrospective review of risk factors for surgical wound dehiscence and incisional hernia. BMC Surg. 2017, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Tevis, S.E.; Kennedy, G.D. Postoperative Complications: Looking Forward to a Safer Future. Clin. Colon. Rectal. Surg. 2016, 29, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, H.; Maghsoudi, L.H.; Soltanian, A.; Gholami, F. Surgical complications in colorectal cancer patients. Ann. Med. Surg. 2020, 55, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Purba, A.K.R.; Setiawan, D.; Bathoorn, E.; Postma, M.J.; Dik, J.H.; Friedrich, A.W. Prevention of Surgical Site Infections: A Systematic Review of Cost Analyses in the Use of Prophylactic Antibiotics. Front. Pharmacol. 2018, 9, 776. [Google Scholar] [CrossRef]
- Bellows, C.F.; Mills, K.T.; Kelly, T.N.; Gagliardi, G. Combination of oral non-absorbable and intravenous antibiotics vs. intravenous antibiotics alone in the prevention of surgical site infections after colorectal surgery: A meta-analysis of randomized controlled trials. Tech. Coloproctol. 2011, 15, 385–395. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021; Available online: www.Training.Cochrane.Org/Handbook (accessed on 1 May 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- The Cochrane Collaboration. 2020 Review Manager (RevMan) [Computer Program], Version 5.4; The Cochrane Collaboration: London, UK, 2020; Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 1 May 2021).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]; McMaster University: 2020 (Developed by Evidence Prime, Inc.). Available online: Gradepro.Org (accessed on 1 May 2021).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group: 2013. Available online: Guidelinedevelopment.Org/Handbook (accessed on 1 May 2021).
- Ikeda, A.; Konishi, T.; Ueno, M.; Fukunaga, Y.; Nagayama, S.; Fujimoto, Y.; Akiyoshi, T.; Yamaguchi, T. Randomized Clinical Trial of Oral and Intravenous vs. Intravenous Antibiotic Prophylaxis for Laparoscopic Colorectal Resection. Br. J. Surg. 2016, 103, 1608–1615. [Google Scholar] [CrossRef]
- Hata, H.; Yamaguchi, T.; Hasegawa, S.; Nomura, A.; Hida, K.; Nishitai, R.; Yamanokuchi, S.; Yamanaka, T.; Sakai, Y. Oral and Parenteral Versus Parenteral Antibiotic Prophylaxis in Elective Laparoscopic Colorectal Surgery (JMTO PREV 07-01): A Phase 3, Multicenter, Open-Label, Randomized Trial. Ann. Surg. 2016, 263, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Yokoyama, M.; Nakada, H.; Inokuma, S.; Hashimoto, D. Impact of Oral Antimicrobial Prophylaxis on Surgical Site Infection and Methicillin-Resistant Staphylococcus Aureus Infection after Elective Colorectal Surgery. Results of a Prospective Randomized Trial. Surg. Today 2001, 31, 979–983. [Google Scholar] [CrossRef]
- Abis, G.S.A.; Stockmann, H.B.A.C.; Bonjer, H.J.; van Veenendaal, N.; van Doorn-Schepens, M.L.M.; Budding, A.E.; Wilschut, J.A.; van Egmond, M.; Oosterling, S.J.; SELECT Trial Study Group. Randomized Clinical Trial of Selective Decontamination of the Digestive Tract in Elective Colorectal Cancer Surgery (SELECT Trial). Br. J. Surg. 2019, 106, 355–363. [Google Scholar] [CrossRef]
- Schardey, H.M.; Wirth, U.; Strauss, T.; Kasparek, M.S.; Schneider, D.; Jauch, K.W. Prevention of Anastomotic Leak in Rectal Cancer Surgery with Local Antibiotic Decontamination: A Prospective, Randomized, Double-Blind, Placebo-Controlled Single Center Trial. Int. J. Colorectal. Dis. 2020, 35, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Mohri, Y.; Tonouchi, H.; Miki, C.; Nakai, K.; Kusunoki, M.; Mie Surgical Infection Research Group. Randomized Clinical Trial Comparing Intravenous Antimicrobial Prophylaxis Alone with Oral and Intravenous Antimicrobial Prophylaxis for the Prevention of a Surgical Site Infection in Colorectal Cancer Surgery. Surg. Today 2007, 37, 383–388. [Google Scholar] [CrossRef]
- Kow, L.; Toouli, J.; Brookman, J.; McDonald, P.J. Comparison of cefotaxime plus metronidazole vs. cefoxitin for prevention of wound infection after abdominal surgery. World J. Surg. 1995, 19, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.; Mazzei, T.; Novelli, A.; Mazzoni, P.; Ficari, F. Amoxicillin/clavulanic acid vs. cefotaxime for antimicrobial prophylaxis in abdominal surgery: A randomized trial. J. Chemother. 2002, 14, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, J.C.; Van Rij, A.M.; Pettigrew, R.A.; van der Linden, A.J.; Solomon, C.; Bolt, D. A comparison of the prophylactic efficacy of ceftriaxone and cefotaxime in abdominal surgery. Am. J. Surg. 2003, 185, 45–49. [Google Scholar] [CrossRef]
- Chen, M.; Song, X.; Chen, L.Z.; Lin, Z.D.; Zhang, X.L. Comparing Mechanical Bowel Preparation With Both Oral and Systemic Antibiotics Versus Mechanical Bowel Preparation and Systemic Antibiotics Alone for the Prevention of Surgical Site Infection After Elective Colorectal Surgery: A Meta-Analysis of Randomized Controlled Clinical Trials. Dis. Colon. Rectum. 2016, 59, 70–78. [Google Scholar] [CrossRef]
- Morris, M.S.; Graham, L.A.; Chu, D.I.; Cannon, J.A.; Hawn, M.T. Oral Antibiotic Bowel Preparation Significantly Reduces Surgical Site Infection Rates and Readmission Rates in Elective Colorectal Surgery. Ann. Surg. 2015, 261, 1034–1040. [Google Scholar] [CrossRef]
- Uchino, M.; Ikeuchi, H.; Bando, T.; Chohno, T.; Sasaki, H.; Horio, Y.; Nakajima, K.; Takesue, Y. Efficacy of Preoperative Oral Antibiotic Prophylaxis for the Prevention of Surgical Site Infections in Patients With Crohn Disease: A Randomized Controlled Trial. Ann. Surg. 2019, 269, 420–426. [Google Scholar] [CrossRef]
- Joshi, G.P.; Kehlet, H. Enhanced Recovery Pathways: Looking Into the Future. Anesth. Analg. 2019, 128, 5–7. [Google Scholar] [CrossRef]
- Andersen, P.; Andersen, L.M.; Iversen, L.H. Iatrogenic ureteral injury in colorectal cancer surgery: A nationwide study comparing laparoscopic and open approaches. Surg. Endosc. 2015, 29, 1406–1412. [Google Scholar] [CrossRef]
- Sheka, A.C.; Tevis, S.; Kennedy, G.D. Urinary tract infection after surgery for colorectal malignancy: Risk factors and complications. Am. J. Surg. 2016, 211, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Smolarek, S.; Shalaby, M.; Angelucci, G.P.; Missori, G.; Capuano, I.; Franceschilli, L.; Quaresima, S.; Di Lorenzo, N.; Sileri, P. Small-Bowel Obstruction Secondary to Adhesions After Open or Laparoscopic Colorectal Surgery. JSLS 2016, 20, e2016.00073. [Google Scholar] [CrossRef] [Green Version]
- Eto, K.; Kosuge, M.; Ohkuma, M.; Noaki, R.; Neki, K.; Ito, D.; Sugano, H.; Takeda, Y.; Yanaga, K. Defunctioning Ileostomy Is a Key Risk Factor for Small Bowel Obstruction After Colorectal Cancer Resection. Anticancer Res. 2018, 38, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Nathens, A.B.; Marshall, J.C. Selective decontamination of the digestive tract in surgical patients: A systematic 597 review of the evidence. Arch. Surg. 1999, 134, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.K.; Hassanein, K.M.; Hassanein, R.S.; Kim, H.M. Quasi-empirical Bayes methodology for improving meta-analysis. J. Biopharm. Stat. 2006, 16, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.J.; Dwan, K.M.; Altman, D.G.; Gamble, C.; Dodd, S.; Smyth, R.; Williamson, P.R. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010, 15, c365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, E.C.; Vogel, J.D.; Church, J.M.; Remzi, F.; Fazio, V.W. Surgical Site Infections in a “High Outlier” Institution: Are Colorectal Surgeons to Blame? Dis. Colon. Rectum. 2009, 52, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.W.; Huerta, S.; Dineen, S.; Anthony, T. Surgical Site Infection in Colorectal Surgery: A Review of the Nonpharmacologic Tools of Prevention. J. Am. Coll. Surg. 2010, 211, 812–822. [Google Scholar] [CrossRef]
- Konishi, T.; Watanabe, T.; Kishimoto, J.; Nagawa, H. Elective Colon and Rectal Surgery Differ in Risk Factors for Wound Infection: Results of Prospective Surveillance. Ann. Surg. 2006, 244, 758–763. [Google Scholar] [CrossRef]
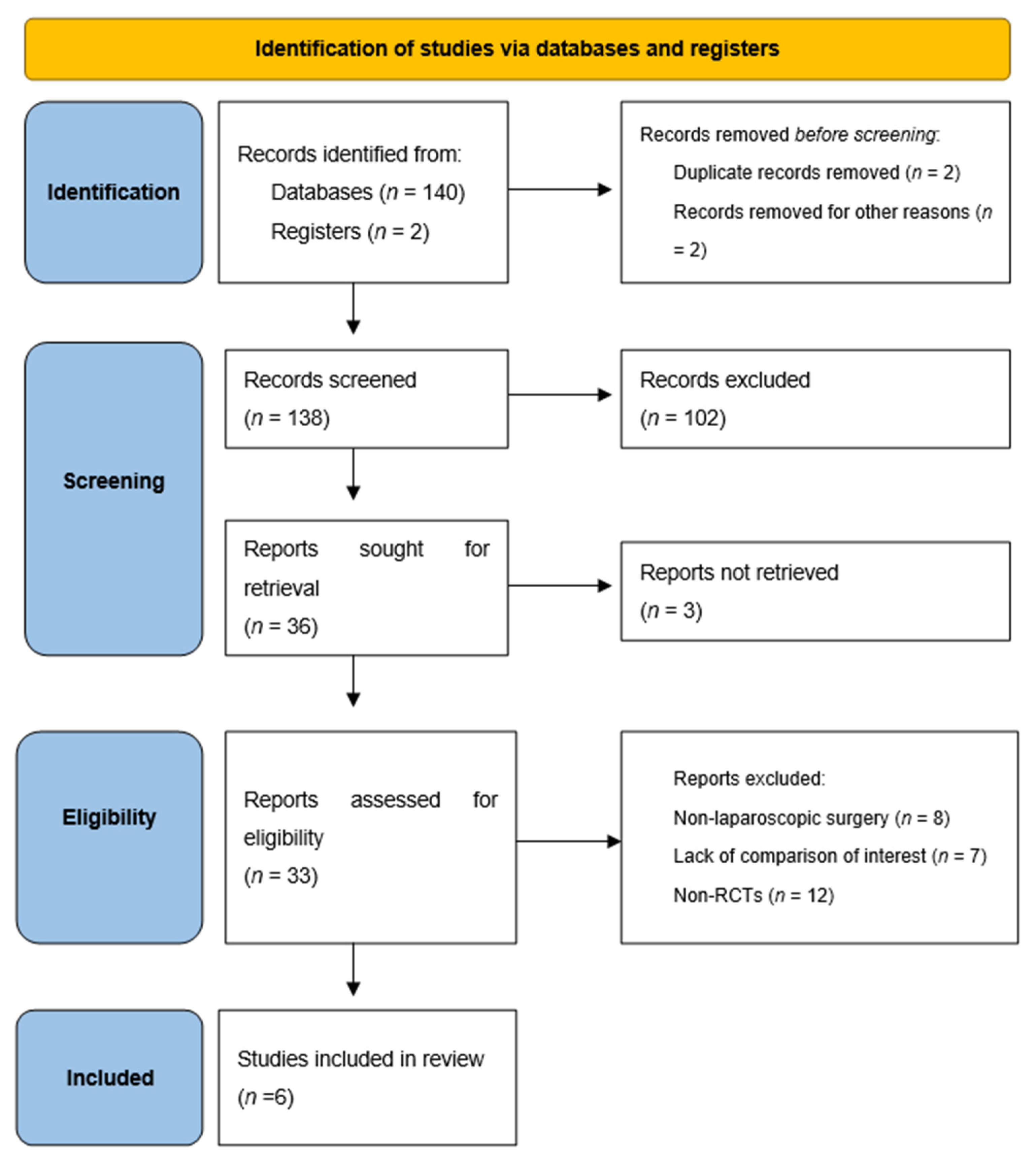



| Study | Design | Total N | Intervention (Oral + IV) N | Control (IV-Only) N | Primary Outcome | Secondary Outcome | Oral Antibiotics | IV Antibiotics | Type of Resection |
|---|---|---|---|---|---|---|---|---|---|
| Ishida 2001 [36] | RCT | 143 | 72 | 71 | SSI | Anastomotic leak, Enteritis/colitis, Pneumonia | Kanamycin 500 mg + Erythromycin 400 mg in 4 daily doses, started 2 days preoperatively + control group treatment | Cefotiam 1 g in 2 daily doses for 48 h |
Colectomy—76 Anterior resection—47 APR—9 Total proctectomy with J pouch—3 Total pelvic exenteration—4 Other—4 |
| Kobayashi 2007 [39] | RCT | 484 | 242 | 242 | SSI | / | Kanamycin 1 g + Erythromycin 400 mg at 14:00, 15:00, and 23:00 + control group treatment |
Cefmetazole 1 g after the induction of anesthesia, additional dose if the operation was prolonged beyond 3 h. Again twice daily for 3 |
Surgical procedure: Colon—241 Rectum—243 |
| Ikeda 2016 [34] | RCT | 511 | 255 | 256 | SSI | Anastomotic leak, Enteritis/colitis, Urinary tract disorder, Bowel obstruction | Kanamycin 1000 mg 2 doses + Metronidazole 750 mg, started 1 day preoperative + control group treatment | Cefmetazole 1 g 3 doses in 24 h |
Colonic surgery—309 Anterior resection—177 APR—25 |
| Hata 2016 [35] | RCT | 579 | 289 | 290 | SSI | Anastomotic leak, Enteritis/colitis; Pneumonia, Urinary tract disorder, Bowel obstruction | Kanamycin 1 g + Metronidazole 750 mg at 13 h and 9 h before the surgery + control group treatment | Cefmetazole 1 g was administered intravenously 30 min before the skin incision, additional dose was given every 3 h during the surgery |
Colectomy—376 Anterior resection—183 APR—20 |
| Abis 2019 [37] | RCT | 455 | 228 | 227 | SSI | Anastomotic leak, Pneumonia, Urinary tract disorder, Bowel obstruction | SDD 3 days prior to surgery until 3 days after surgery or when normal bowel motion occurred + control group treatment | Cefazoline 1 g + Metronidazole 500 mg, intravenously, 30 min prior to skin incision |
Right hemicolectomy—162 Transverse colectomy—17 Left hemicolectomy—41 Sigmoid resection—124 Low anterior resection—103 Other—8 |
| Schardey 2020 [38] | RCT | 80 | 40 | 40 | SSI | Anastomotic leak, Pneumonia, Enteritis/colitis, Urinary tract disorder | Polymyxin B sulphate 100 mg + Tobramycin 80 mg + Vancomycin 125 mg + Amphotericin B 500 mg 4 daily doses, started 1 day preoperatively until day 7 postoperatively. | Amphotericin B 500 mg + Lactulose 305 mg | Low anterior resection with TME—80 |
| Oral and Parenteral vs. Parenteral Antibiotic Prophylaxis for Patients Undergoing Laparoscopic Colorectal Resection | |||||
|---|---|---|---|---|---|
| Patient or population: Patients undergoing laparoscopic colorectal resection Setting: Multicentered study Intervention: Oral and parenteral antibiotic prophylaxis Comparison: Parenteral antibiotic prophylaxis only | |||||
| Outcomes | № of Participants (Studies) Follow-Up | Certainty of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | |
| Risk with Parenteral Antibiotic Prophylaxis | Risk Difference with Oral and Parenteral Antibiotic Prophylaxis | ||||
| Overall Surgical Site Infections | 2252 (6 RCTs) | ⨁⨁⨁◯ Moderate | OR 0.54 (0.40 to 0.72) | 113 per 1000 | 49 fewer per 1000 (64 fewer to 29 fewer) |
| Incisional Surgical Site Infections | 1717 (4 RCTs) | ⨁⨁⨁◯ Moderate | OR 0.61 (0.41 to 0.90) | 78 per 1000 | 29 fewer per 1000 (44 fewer to 7 fewer) |
| Organ/space Surgical Site Infections | 1717 (4 RCTs) | ⨁⨁⨁◯ Moderate | OR 0.79 (0.47 to 1.32) | 40 per 1000 | 8 fewer per 1000 21 fewer to 12 more) |
| Anastomotic Leakage | 1768 (5 RCTs) | ⨁⨁⨁◯ Moderate | OR 0.55 (0.33 to 0.91) | 50 per 1000 | 22 fewer per 1000 (33 fewer to 4 fewer) |
| Enteritis/colitis | 1313 (4 RCTs) | ⨁⨁⨁◯ Moderate | OR 0.67 (0.30 to 1.48) | 23 per 1000 | 7 fewer per 1000 (16 fewer to 11 more) |
| Pneumonia | 1257 (4 RCTs) | ⨁⨁⨁◯ Moderate | OR 0.75 (0.39 to 1.45) | 33 per 1000 | 8 fewer per 1000 (20 fewer to 14 more) |
| Urinary Tract Disorder | 1625 (4 RCTs) | ⨁⨁⨁◯ Moderate | OR 0.73 (0.36 to 1.47) | 22 per 1000 | 6 fewer per 1000 (14 fewer to 10 more) |
| Bowel Obstruction | 1545 (3 RCTs) | ⨁⨁◯ ◯ Low | OR 0.76 (0.44 to 1.32) | 39 per 1000 | 9 fewer per 1000 (21 fewer to 12 more) |
| The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangiorgio, G.; Vacante, M.; Basile, F.; Biondi, A. Oral and Parenteral vs. Parenteral Antibiotic Prophylaxis for Patients Undergoing Laparoscopic Colorectal Resection: An Intervention Review with Meta-Analysis. Antibiotics 2022, 11, 21. https://doi.org/10.3390/antibiotics11010021
Sangiorgio G, Vacante M, Basile F, Biondi A. Oral and Parenteral vs. Parenteral Antibiotic Prophylaxis for Patients Undergoing Laparoscopic Colorectal Resection: An Intervention Review with Meta-Analysis. Antibiotics. 2022; 11(1):21. https://doi.org/10.3390/antibiotics11010021
Chicago/Turabian StyleSangiorgio, Giuseppe, Marco Vacante, Francesco Basile, and Antonio Biondi. 2022. "Oral and Parenteral vs. Parenteral Antibiotic Prophylaxis for Patients Undergoing Laparoscopic Colorectal Resection: An Intervention Review with Meta-Analysis" Antibiotics 11, no. 1: 21. https://doi.org/10.3390/antibiotics11010021
APA StyleSangiorgio, G., Vacante, M., Basile, F., & Biondi, A. (2022). Oral and Parenteral vs. Parenteral Antibiotic Prophylaxis for Patients Undergoing Laparoscopic Colorectal Resection: An Intervention Review with Meta-Analysis. Antibiotics, 11(1), 21. https://doi.org/10.3390/antibiotics11010021









