Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
Research Questions
- Is XHP able to obtain significant pre-clinical data in order to justify its clinical use in the management of patients affected by uncomplicated cystitis?
- Is XHP able to obtain a clinical and/or microbiological cure in women affected by uncomplicated cystitis?
2. Results
2.1. Pre-Clinical and Non-Randomized Clinical Studies
2.2. RCTs
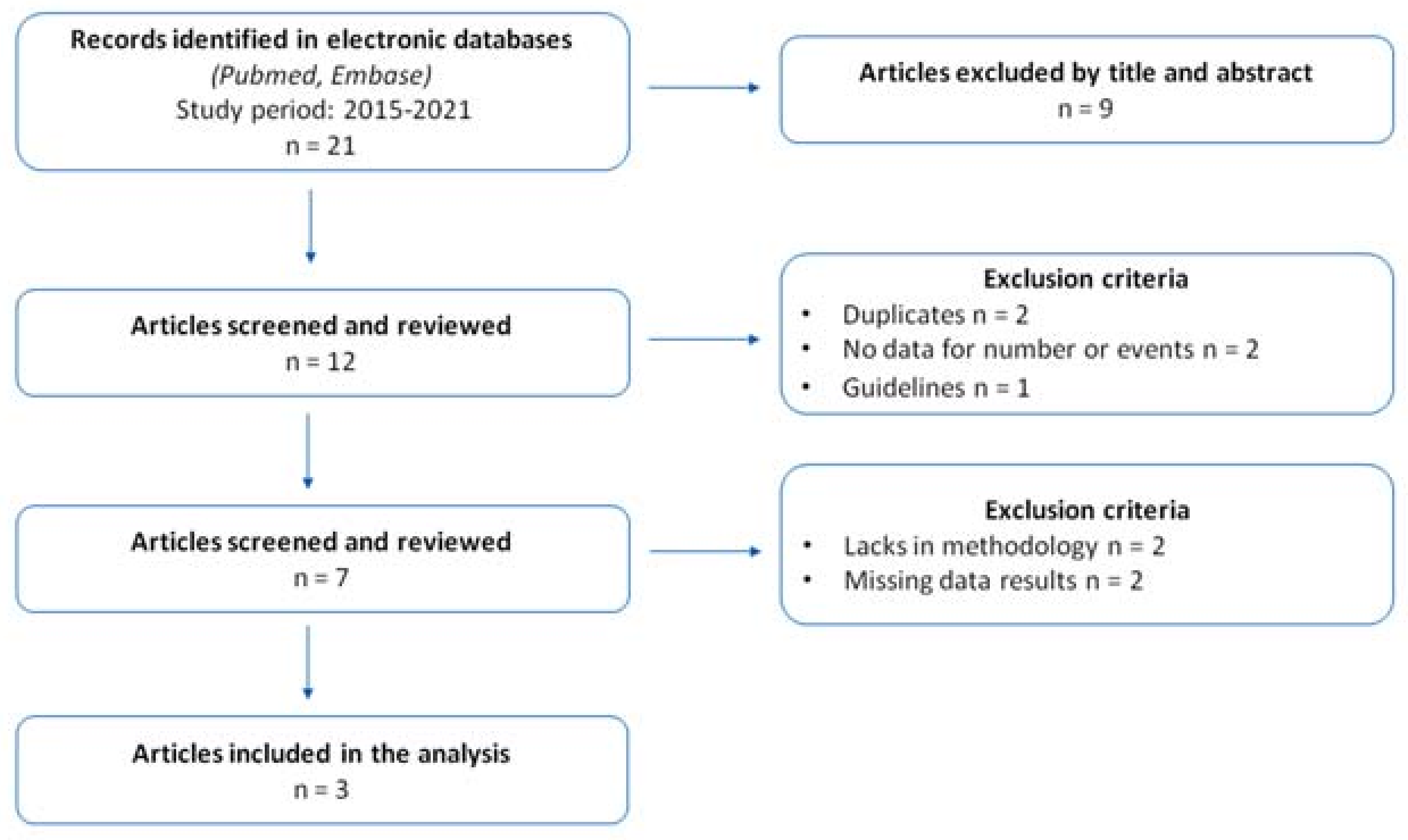
2.2.1. Evidence Synthesis, Study Characteristics and Quality Assessment Results
2.2.2. Characteristics of Studies Included in the Meta-Analysis
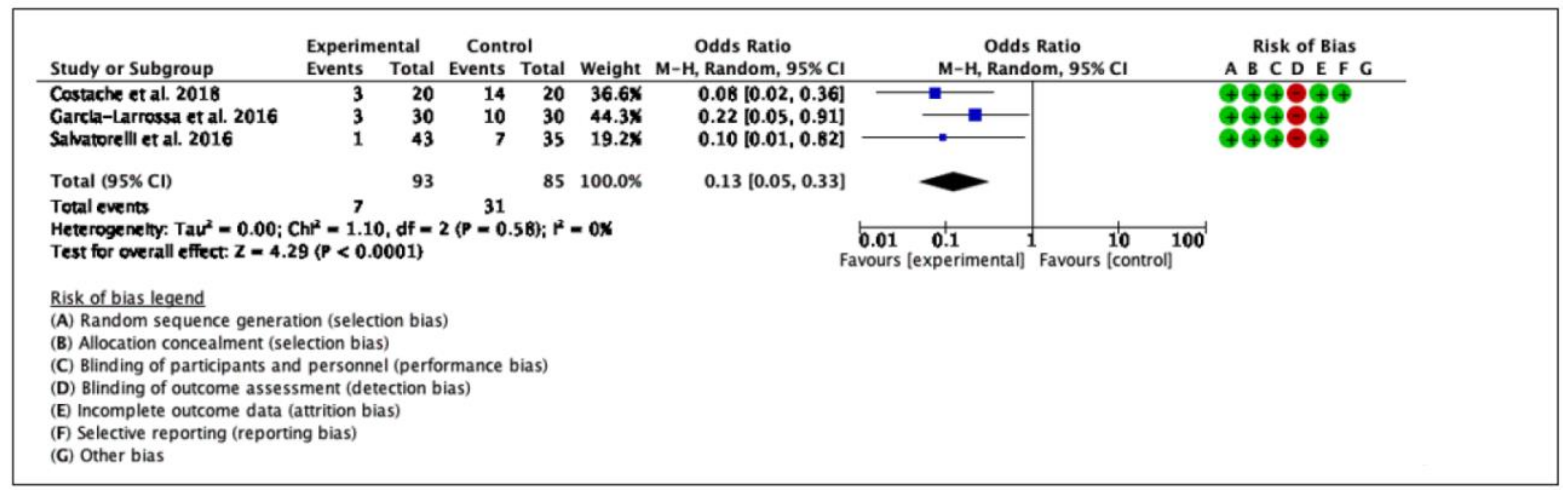
2.2.3. Clinical Cure
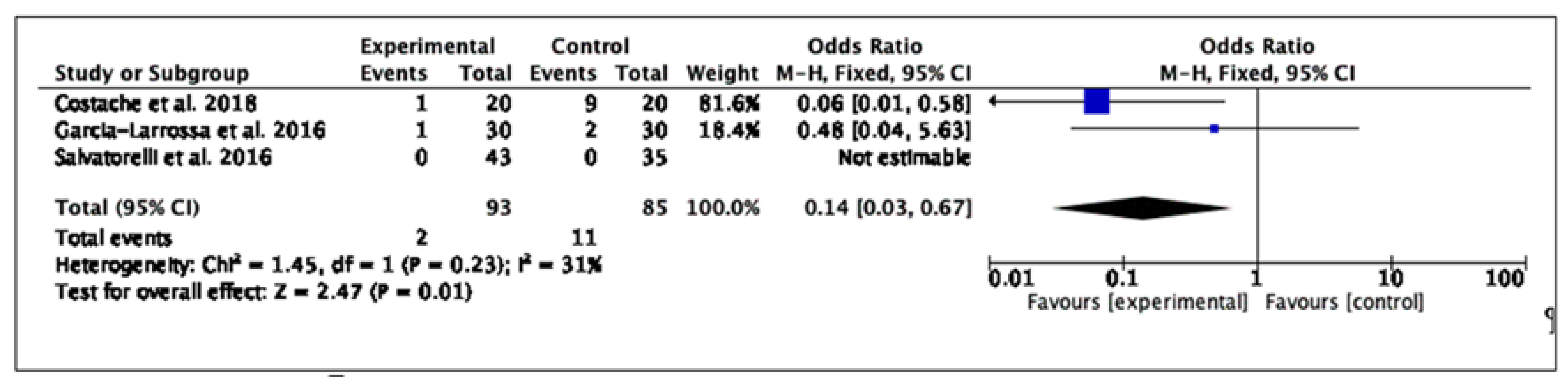
2.2.4. Safety Outcomes
3. Discussion
3.1. Main Findings
3.2. Evidence and Implications for Clinical Practice
- —
- Mechanical barrier protection (mucoprotectant effect) on intestinal cell mucosa.
- —
- Environmental changes in urine (mild decrease of pH) that prevent bacteria proliferation in the urinary tract.
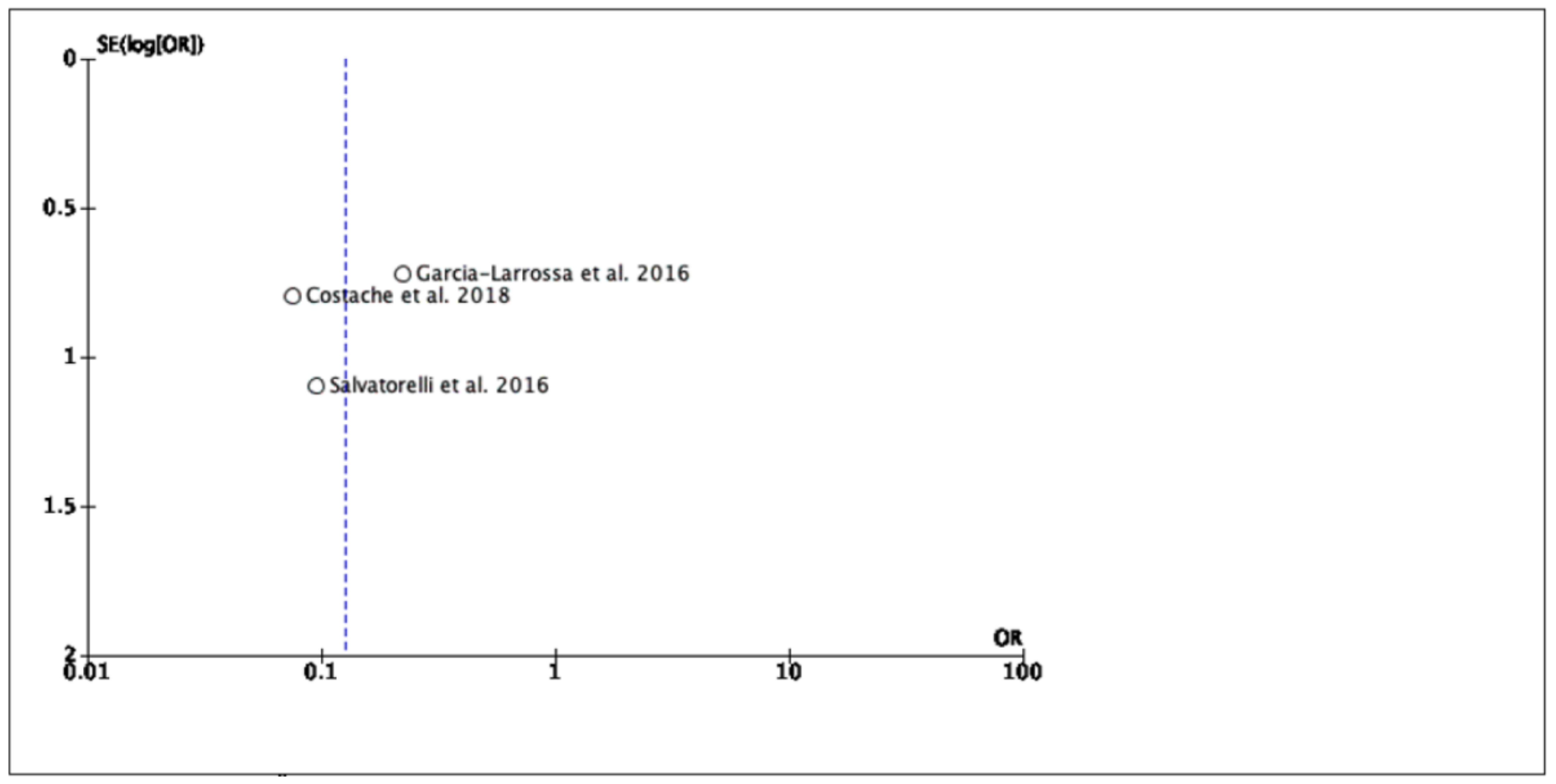
3.3. Study Strengths and Limitations
4. Materials and Methods
4.1. Research Strategy and Literature Search
4.2. Selection Criteria for Study Inclusion in Meta-Analysis and Data Extraction
4.3. Main Outcomes Measures
4.4. Statistical Considerations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silverman, J.A.; Schreiber, H.L.; Hooton, T.M.; Hultgren, S.J. From physiology to pharmacy: Developments in the pathogenesis and treatment of recurrent urinary tract infections. Curr. Urol. Rep. 2013, 14, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, T.; Tamanini, I.; Kulchavenya, E.; Perepanova, T.; Köves, B.; Wagenlehner, F.M.E.; Tandogdu, Z.; Bonkat, G.; Bartoletti, R.; Bjerklund Johansen, T.E. The role of nutraceuticals and phytotherapy in the management of urinary tract infections: What we need to know? Arch. Ital. Urol. Androl. 2017, 89, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, T.; Cocci, A.; Tiscione, D.; Puglisi, M.; Di Maida, F.; Malossini, G.; Verze, P.; Palmieri, A.; Mirone, V.; Bjerklund Johansen, T.E. L-Methionine associated with Hibiscus sabdariffa and Boswellia serrata extracts are not inferior to antibiotic treatment for symptoms relief in patients affected by recurrent uncomplicated urinary tract infections: Focus on antibiotic-sparing approach. Arch. Ital. Urol. Androl. 2018, 90, 97–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F. EAU Guidelines on Urological Infections. Available online: https://uroweb.org/guideline/urological-infections/ (accessed on 14 September 2021).
- Cai, T.; Tamanini, I.; Cocci, A.; Di Maida, F.; Caciagli, P.; Migno, S.; Mereu, L.; Tateo, S.; Malossini, G.; Palmieri, A.; et al. Xyloglucan, hibiscus and propolis to reduce symptoms and antibiotics use in recurrent UTIs: A prospective study. Future Microbiol. 2019, 14, 1013–1021. [Google Scholar] [CrossRef]
- Costache, R.C.; Novac, B.; Bardan, T.R.; Agapie, D.N.; Edu, A. Xyloglucan + gelose combination versus placebo as adjuvant therapy to first-line antimicrobials for uncomplicated urinary tract infection in adults. Urol. Int. 2019, 102, 468–475. [Google Scholar] [CrossRef]
- Garcia-Larrosa, A.; Alexe, O. Efficacy and Safety of a Medical Device versus Placebo in the Early Treatment of Patients with Symptoms of Urinary Tract Infection: A Randomized Controlled Trial. Clin. Microbiol. 2016, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Salvatorelli, N.; Garcia-Larrosa, A.; Allegrini, A.; Pavone, D. A New Approach to the Treatment of Uncomplicated Cystitis: Results of a Randomized Placebo-Controlled Clinical Trial. Urol. Int. 2016, 97, 347–351. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef]
- De Servi, B.; Ranzini, F.; Piqué, N. Effect of Utipro(®) (containing gelatin-xyloglucan) against Escherichia coli invasion of intestinal epithelial cells: Results of an in vitro study. Future Microbiol. 2016, 11, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Olier, M.; Sekkal, S.; Harkat, C.; Eutamene, H.; Theodorou, V. Evaluation of reticulated gelatin-hibiscus-propolis against intestinal commensal species commonly associated with urinary tract infections. Future Microbiol. 2017, 12, 505–513. [Google Scholar] [CrossRef]
- Fraile, B.; Alcover, J.; Royuela, M.; Rodríguez, D.; Chaves, C.; Palacios, R.; Piqué, N. Xyloglucan, hibiscus and propolis for the prevention of urinary tract infections: Results of in vitro studies. Future Microbiol. 2017, 12, 721–731. [Google Scholar] [CrossRef]
- Beerepoot, M.; Geerlings, S. Non-antibiotic prophylaxis for urinary tract infections. Pathogens 2016, 5, 36. [Google Scholar] [CrossRef]
- Alshami, I.; Alharbi, A.E. Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections. Asian Pac. J. Trop. Biomed. 2014, 4, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Lavigne, J.P.; Vitrac, X.; Bernard, L.; Bruyère, F.; Sotto, A. Propolis can potentialise the anti-adhesion activity of proanthocyanidins on uropathogenic Escherichia coli in the prevention of recurrent urinary tract infections. BMC Res. Notes 2011, 4, 522. [Google Scholar] [CrossRef] [Green Version]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective effects of xyloglucan in association with the polysaccharide gelose in an experimental model of gastroenteritis and urinary tract infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef] [Green Version]
- Betschart, C.; Albrich, W.C.; Brandner, S.; Faltin, D.; Kuhn, A.; Surbek, D.; Geissbuehler, V. Guideline of the Swiss Society of Gynaecology and Obstetrics (SSGO) on acute and recurrent urinary tract infections in women, including pregnancy. Swiss Med. Wkly. 2020, 150, w20236. [Google Scholar] [CrossRef]
- Cai, T.; Konstantinidis, C.; Ward, S. A non-pharmacological approach to the treatment of urinary tract infections: Case reports with Utipro® Plus. Drugs Context. 2021, 10, 2021-2-2. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Köves, B.; Cai, T.; Veeratterapillay, R.; Pickard, R.; Seisen, T.; Lam, T.B.; Yuan, C.Y.; Bruyere, F.; Wagenlehner, F.; Bartoletti, R.; et al. Benefits and Harms of Treatment of Asymptomatic Bacteriuria: A Systematic Review and Meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur. Urol. 2017, 72, 865–868. [Google Scholar] [CrossRef]
- Available online: https://www.erim.eur.nl/fileadmin/erim_content/images/metaessentials/Metaanalyze_dichotomous_data.pdf (accessed on 14 September 2021).
- Cai, T.; Tamanini, I.; Tascini, C.; Köves, B.; Bonkat, G.; Gacci, M.; Novelli, A.; Horcajada, J.P.; Bjerklund Johansen, T.E.; Zanel, G. Fosfomycin Trometamol versus Comparator Antibiotics for the Treatment of Acute Uncomplicated Urinary Tract Infections in Women: A Systematic Review and Meta-Analysis. J. Urol. 2020, 203, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses, 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 14 September 2021).
| Variable | De Servi et al., (2016) | Esposito et al., (2020) | Fraile et al., (2020) | Olier et al., (2017) | Cai et al., (2019) |
|---|---|---|---|---|---|
| Study design | Evaluation of EC adherence/intracellular invasion in intestinal epithelial cells in vitro | Efficacy of xyloglucan and XG in an in vivo rat model on extraintestinal UTIs | To assess the properties of a medical device containing xyloglucan, propolis and hibiscus to create a bioprotective barrier to avoid the contact of uropathogenic Escherichia coli strains on cell walls in models of intestinal (CacoGoblet) and uroepithelial (RWPE1) cells (derived from normal human prostate epithelium) | Efficacy of RGHP, RG and vehicle on intestinal commensals commonly involved in UTIs in pretreatment animal model (streptomycin) | Single-center, observational, prospective study |
| Study endpoints | Cytotoxicity, preservation of tight junctions, preservation of paracellular flux, cell invasion, anti-adherence effects | Bacterial growth, intestinal damage and neutrophil infiltration, tight junction permeability, urine volume and pH, effect on bacterial infection of the UT, effect on bladder | Assays of bacterial adhesion, bacterial quantification and antibacterial activity | Bacterial analysis performed in samples recovered from feces | Capability to reduce the number of symptomatic recurrences, efficacy in improving QoL |
| Study duration | 21 days | 7 days | - | 7–11 days | 6 months |
| Agent | Utipro® (Xyloglucan, Hibiscus sabdariffa, propolis, silicon dioxide, magnesium stearate and corn) | Xyloglucan and xyloglucan plus gelose (oral) | Xyloglucan (Tamarindus indica) and extracts of HSP | RGHP or RG adult human posology adapted to rat metabolism | Monurelle Plus® (Oral XHP) |
| Inclusion criteria | Caco-2 cells as intestinal mucosa model | Rats randomly divided into 6 groups
|
|
| Adult women with rUTIs, defined as ≥2 infections in 6 months or ≥3 infections in 1 year |
| Study protocol/Endpoint |
|
|
| Fecal samples (days 7–11). EC and other bacteria monitored by selective chromogenic agar plates. | After antibiotic treatment: one capsule a day for 15 days (one cycle every month for 6 months). Clinical and microbiological analyses at baseline (T0) and 1, 3 and 6 months after enrollment. SF-36 validated questionnaire for QoL assessment. |
| Assessment | Descriptive analysis of quantitative data | Descriptive analysis of quantitative data | Descriptive analysis of quantitative data | Comparison | Descriptive analysis; AEs |
| Results |
| -Presence of xyloglucan at all tested concentrations did not significantly affect bacterial growth -Xyloglucan did not induce bacterial lysis in the bacterial strain assessed or cause any irregularities in bacterial cell membranes -Xyloglucan markedly reduced the degree of tissue injury whereas no decrease in E. coli-induced histopathological changes was observed with xyloglucan + gelose -A trend toward decrease in MPO activity was observed in the intestines from rats treated with xyloglucan or XG -XG significantly increased occluding- positive staining-Neither xyloglucan nor XG modified pH values -Bacterial load per gram of urethra (CFU/g) was reduced by 47 and 58% after xyloglucan and XG treatments, respectively -Morphological histological improvement of bladders treated with xyloglucan or XG after EC inoculation |
|
| Significant reduction in antibiotic use (31.2%) Improvement in urinary symptoms (67.2%) |
| Conclusions | Utipro® increases the resistance of cell tight junctions and protects cells against the adherence of EC | Barrier effect of xyloglucan on the epithelial intestine reduces the colonization of EC reservoirs, preventing UTI development |
| RGHP reduces fecal colonization by EC and Enterococci or by a dominant human EC strain; RGHP reduces the risk of contamination of the urinary tract by opportunistic bacteria | XHP device improves quality of life in women with rUTIs, reduce recurrences and antibiotic use. |
| Variable | Costache et al., (2019) | Garcia-Larrosa et al., (2016) | Salvatorelli et al., (2016) |
|---|---|---|---|
| Study design | Multi-center, randomized, placebo-controlled, parallel group, double blind, phase IV study | Double-blind, placebo-controlled trial | Prospective, randomized, double-blind, placebo-controlled, parallel-group clinical trial |
| Study endpoints | Frequency of symptomatic rUTI recurrence, AEs | Safety, efficacy of reticulated RGHP in improving urinary symptoms and reducing the need of antibiotics for UTIs | % of symptomatic cystitis recurrences over 6 months, number of micturitions as registered in the diary and dysuria based on a quantitative scale |
| Study duration | 40–90 days | 5–11 days | 6 months |
| Agent | XGHP (oral) | RG with hibiscus and propolis | RG Hibiscus sabdariffa calyx extract and propolis. |
| Inclusion criteria | Adult (men and women) ≥ 18 yrs with uncomplicated UTI |
| Adult women ≥ 18 years with acute cystitis symptoms and a ciprofloxacin-susceptible isolate in urine culture |
| Study protocol/Endpoint | Antimicrobial + 2 daily capsules/ XGHP for 5 days, then 1 capsule of XGHP for 5 days. Follow-up: 1 capsule of XGHP or placebo for 15 consecutive days/month for 2 months | 1:1 MD vs placebo for 5 days
| Ciprofloxacin 500 mg/day and 2 MD capsules/day vs. ciprofloxacin 500 mg/day and matched placebo for 5 days + two additional cycles of MD for 2 weeks at months 1 and 2 after initial treatment. |
| Assessment | Physical examination, Likert scale, urine testing and culture (≥103 CFU/mL = positive) | Comparison | Descriptive analysis; AEs |
| Results | AEs: 5% unrelated to study products; Efficacy analysis: significant reduction of main urinary symptoms (each p < 0.004) |
| No recurrence after 1 month (MD-treated) SRR: -19.4% among patients treated reduced by 19.4% Dysuria scores improvement after 20 days of treatment No severe AEs were observed |
| Conclusions | XGHP as adjuvant therapy to first-line antimicrobials for treatment of uncomplicated UTIs in adults | RGHP reduced the risk of antibiotic treatment and improves UTI symptoms | New MDs prevent the recurrence of uncomplicated cystitis MDs reduce antibiotic use in management of UTIs in women |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, T.; Anceschi, U.; Tamanini, I.; Migno, S.; Rizzo, M.; Liguori, G.; Garcia-Larrosa, A.; Palmieri, A.; Verze, P.; Mirone, V.; et al. Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 14. https://doi.org/10.3390/antibiotics11010014
Cai T, Anceschi U, Tamanini I, Migno S, Rizzo M, Liguori G, Garcia-Larrosa A, Palmieri A, Verze P, Mirone V, et al. Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics. 2022; 11(1):14. https://doi.org/10.3390/antibiotics11010014
Chicago/Turabian StyleCai, Tommaso, Umberto Anceschi, Irene Tamanini, Serena Migno, Michele Rizzo, Giovanni Liguori, Alejandro Garcia-Larrosa, Alessandro Palmieri, Paolo Verze, Vincenzo Mirone, and et al. 2022. "Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis" Antibiotics 11, no. 1: 14. https://doi.org/10.3390/antibiotics11010014
APA StyleCai, T., Anceschi, U., Tamanini, I., Migno, S., Rizzo, M., Liguori, G., Garcia-Larrosa, A., Palmieri, A., Verze, P., Mirone, V., & Bjerklund Johansen, T. E. (2022). Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics, 11(1), 14. https://doi.org/10.3390/antibiotics11010014








