Iriflophenone-3-C-β-d Glucopyranoside from Dryopteris ramosa (Hope) C. Chr. with Promising Future as Natural Antibiotic for Gastrointestinal Tract Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Used
2.2. Formation of Crude Methanolic Plant Extract
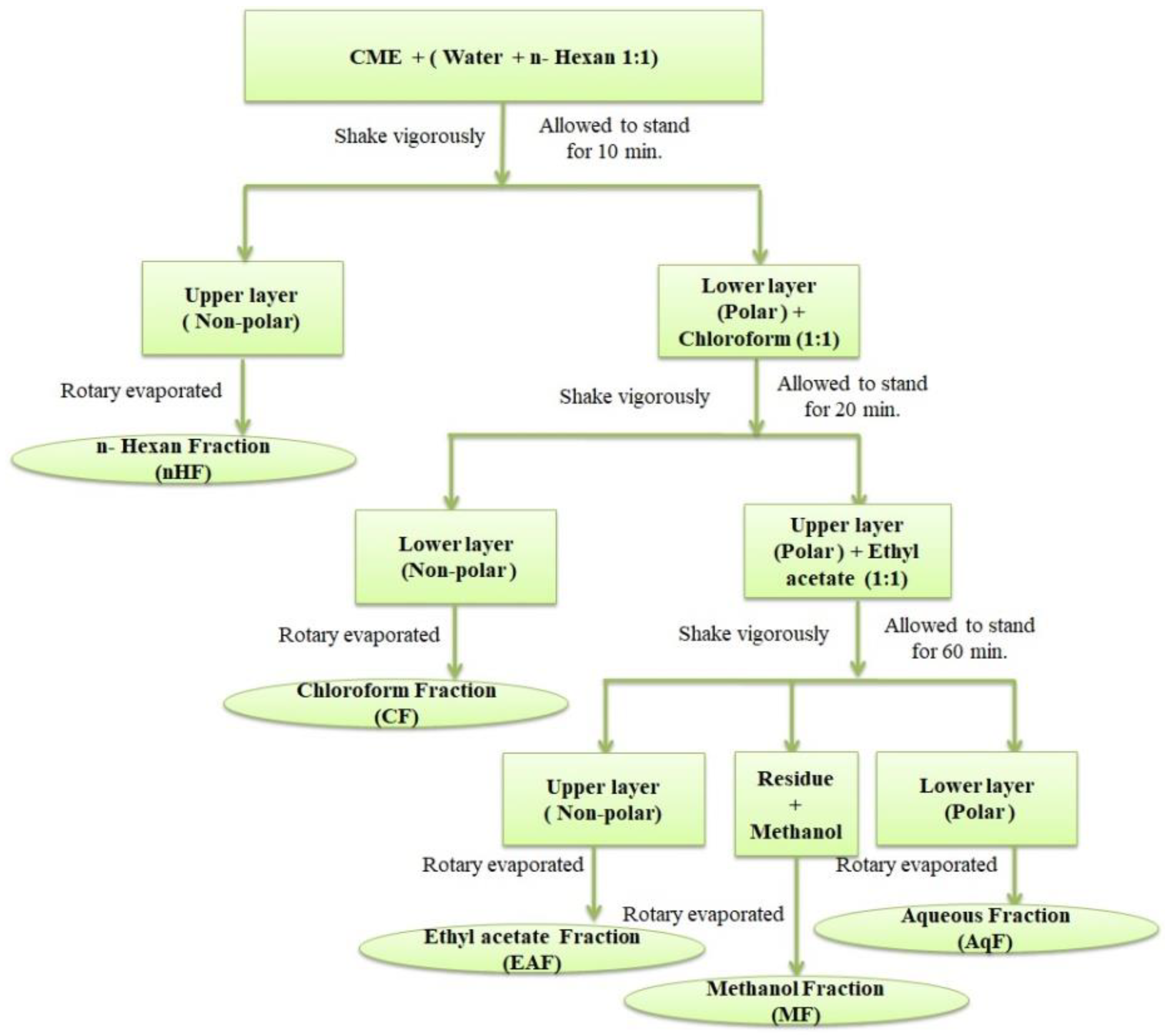
2.3. Formation of Aqueous Fraction of D. ramosa
2.4. Isolation of Bioactive Compounds from AqF of D. ramosa
2.5. Structure Elucidation of Isolated Compound
2.5.1. UV–Vis Spectroscopy
2.5.2. Mass Spectroscopy (MS Analysis): Instrument: HR-TOF Mass Spectrometer (maXis, Bruker Daltonics)
2.5.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.6. Evaluation of Antibacterial Properties
2.6.1. Microorganisms Used
2.6.2. Control Experiments
2.6.3. Preparation of Inoculum
2.6.4. Antibacterial Assay
2.6.5. Determination of Minimum Inhibitory Concentration (MIC)
2.7. Determination of Cytotoxic Potential of Isolated Pure Compound from AqF of D. ramosa
2.7.1. Hatching of Brine Shrimps
2.7.2. Bioassay—BSLT Procedure
2.8. Statistical Analysis
3. Results
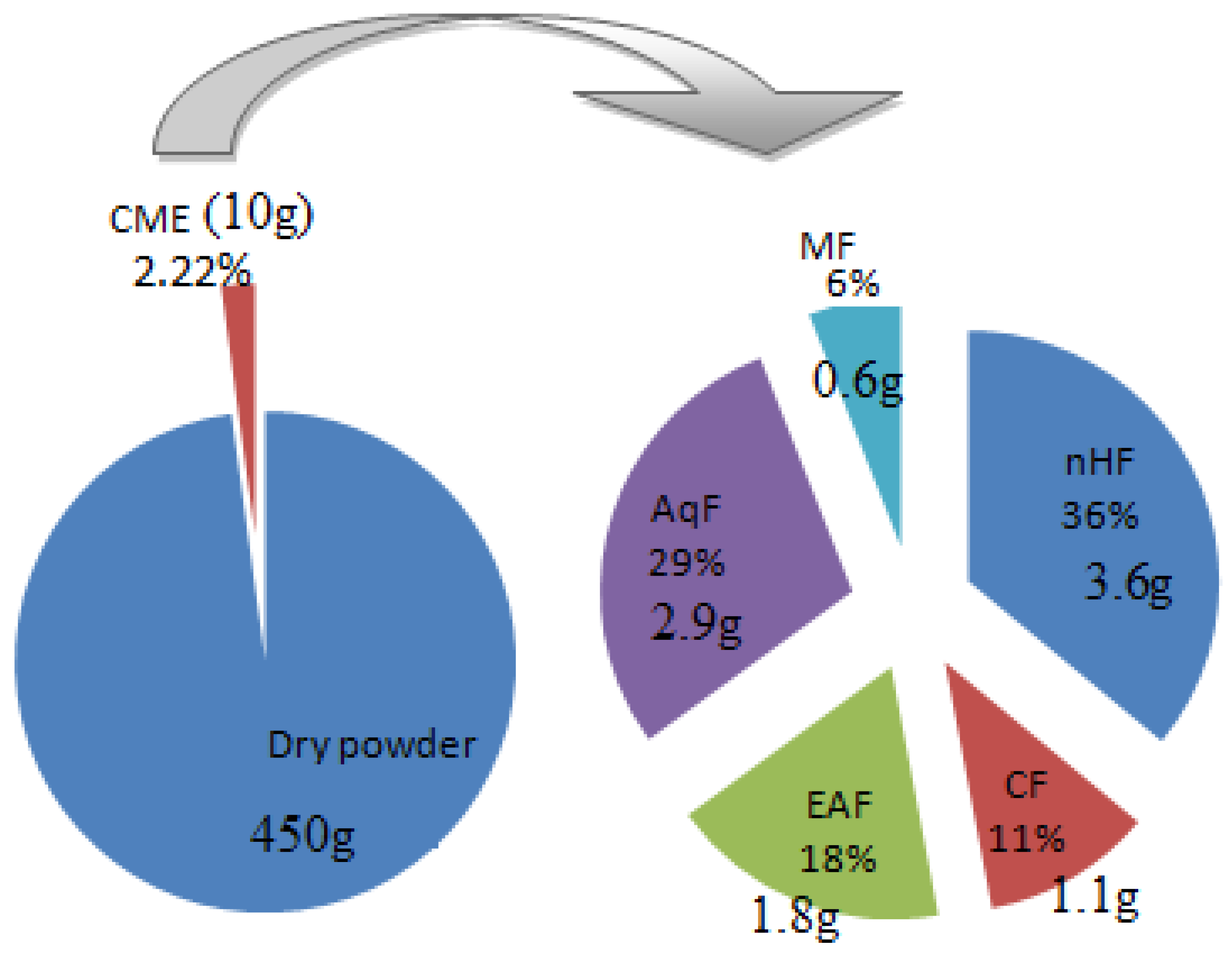
3.1. Extraction and Fractionation
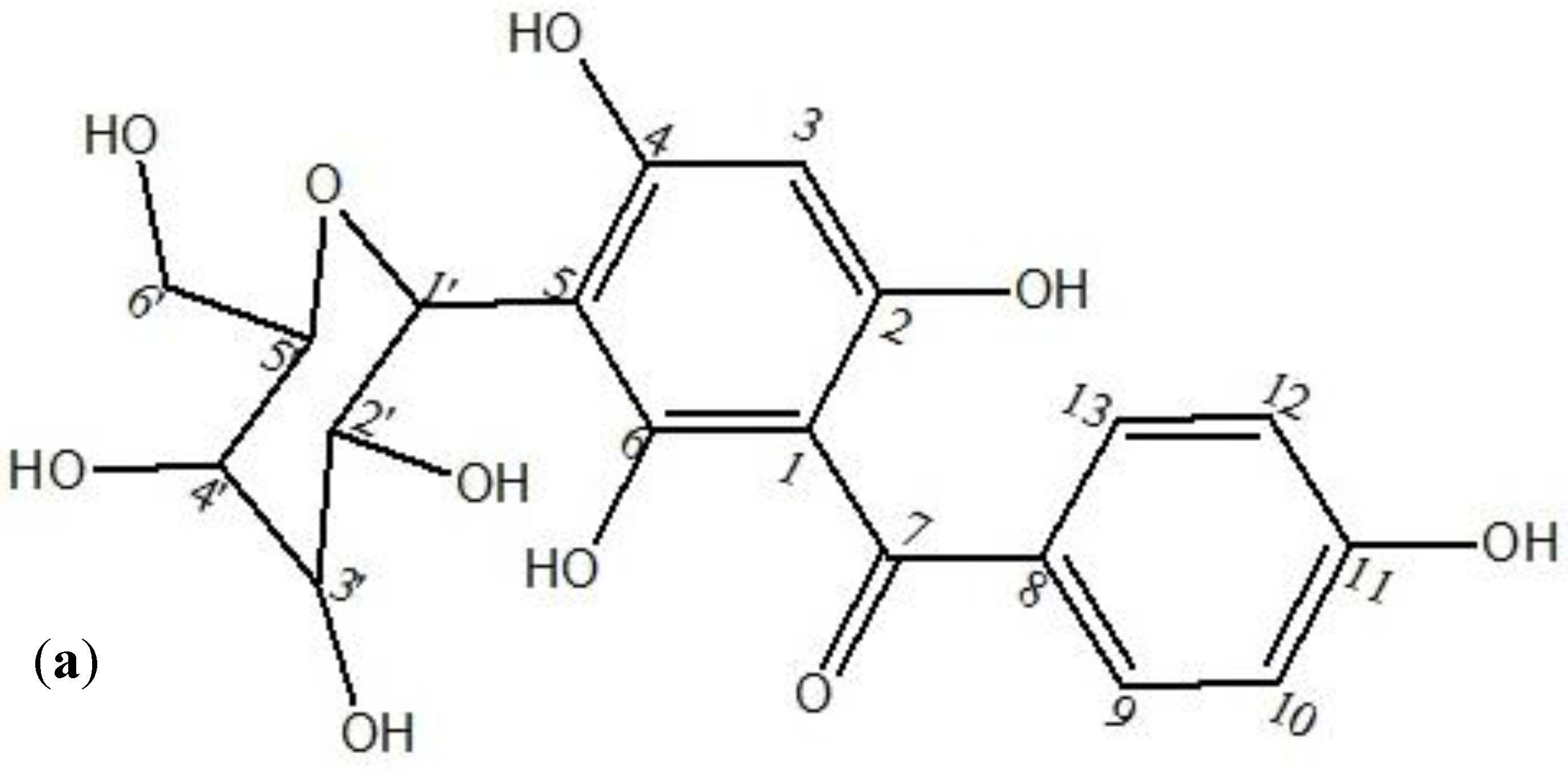
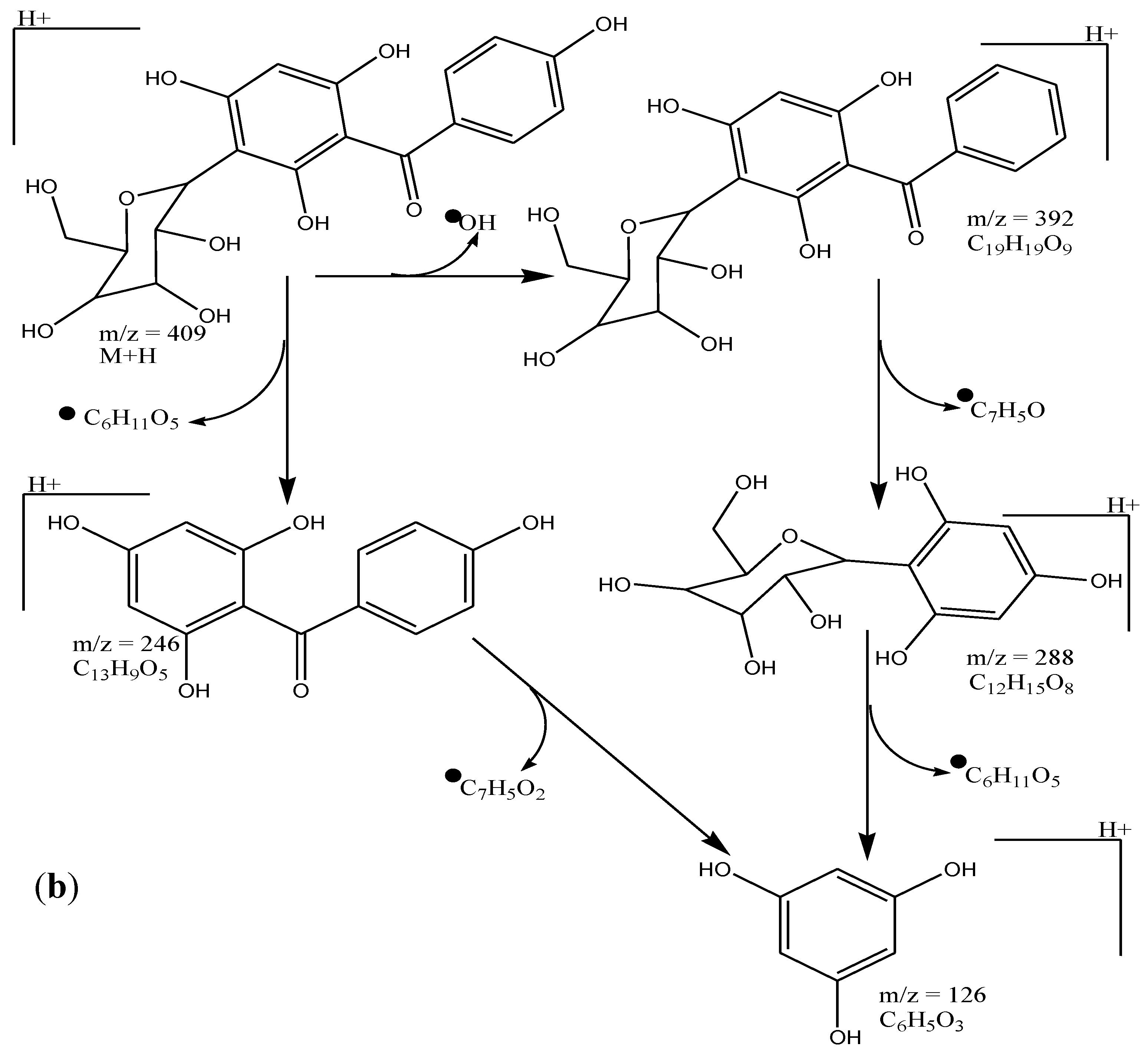
3.2. Spectroscopic Analysis of Isolated Pure Compound from AqF of D. ramosa
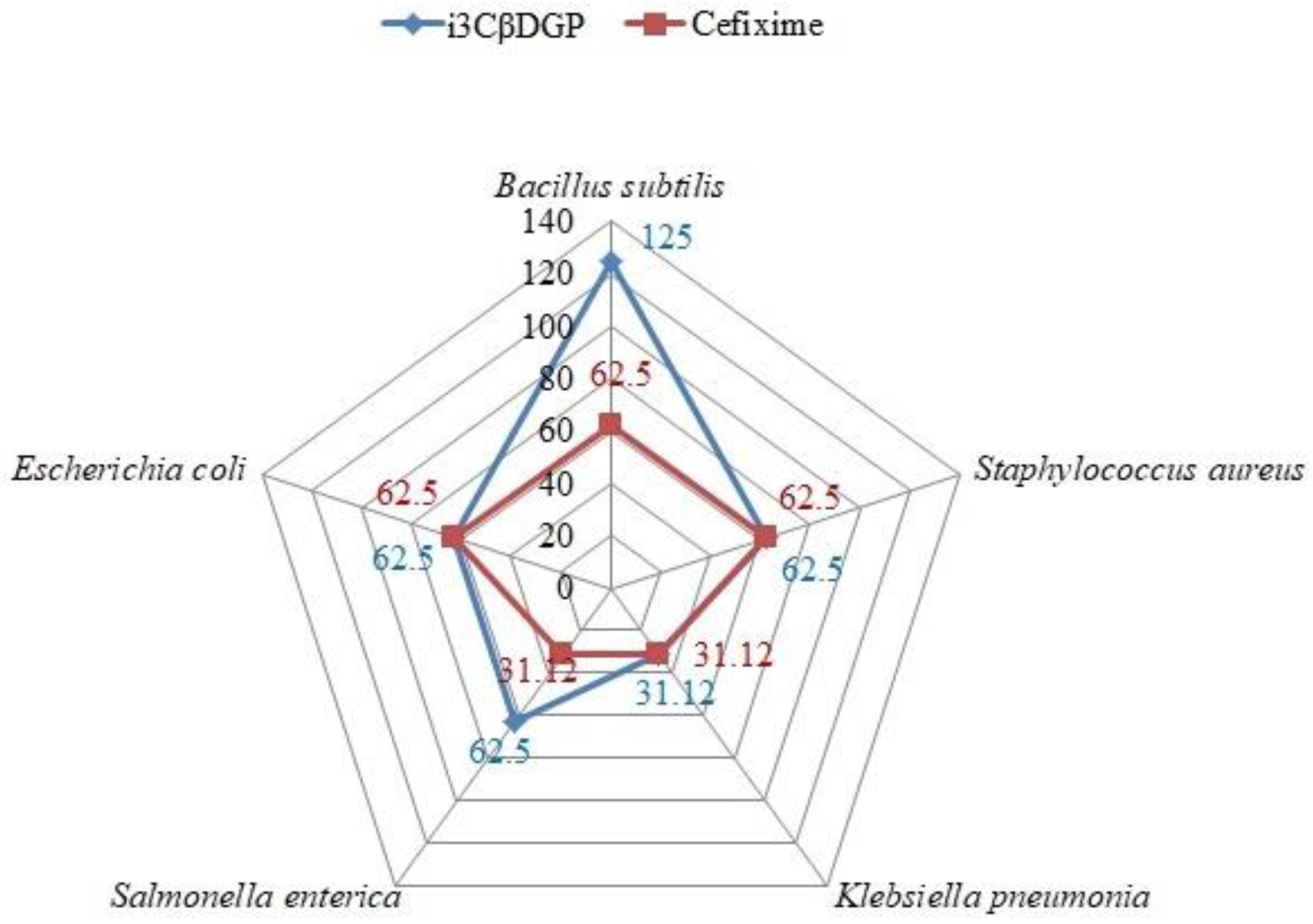
3.3. Antibacterial Potential of “Iriflophenone-3-C-β-D Glucopyranoside”
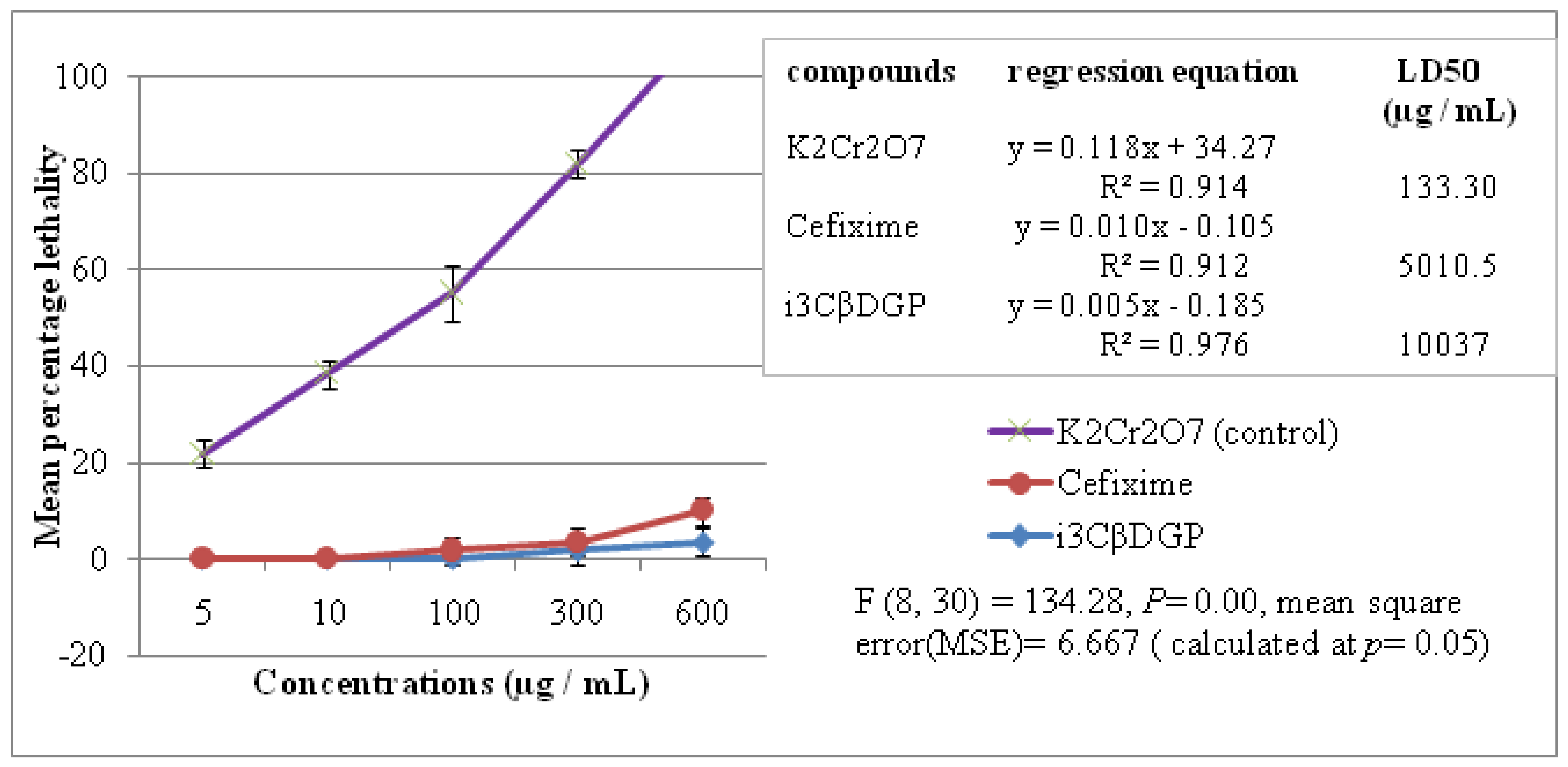
3.4. Cytotoxic Properties of Iriflophenone-3-C-β-D Glucopyranoside
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AqF | Aqueous fraction |
| BSLT | Brine shrimp lethality test |
| CFU | Colony forming unit |
| CME | Crude methanolic extract |
| COSY | Correlation Spectroscopy |
| DMSO | Dimethyl sulfoxide |
| GIT | Gastrointestinal tract |
| HMBC | Heteronuclear Multiple-Bond Correlation |
| I3CβDGP | Iriflophenone-3-C-β D glucopyranoside |
| MIC | Minimum inhibitory concentration |
| MPLC | Medium-pressure liquid chromatography |
| PLA | Pyogenic liver abscess |
References
- Newman, D.J.; Cragg, G.M.; Sander, K.M. Natural products as source of new drugs. J. Nat. Prod. 2003, 6, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Colegate, M.S.; Molyneux, R.J. Bioactive Natural Products, Detection, Isolation and Structural Determination, 2nd ed.; CRC press Taylor & Francis Group: New York, NY, USA, 2008; pp. 11–76. [Google Scholar]
- Abbasi, A.M.; Khan, M.A.; Shah, M.H.; Shah, M.M.; Pervez, A.; Ahmad, A. Ethnobotanical appraisal and cultural values of medicinally important wild edible vegetables of Lesser Himalayas Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Shafique, K.A.; Habib, S. Indigenous Knowledge of Some Medicinal Plants of Himalaya Region, Dawarian Village, Neelum Valley, Azad Jammu and Kashmir, Pakistan. Uni. Versal. J. Plant Sci. 2014, 2, 40–47. [Google Scholar]
- Abbasi, A.M.; Shah, M.H.; Khan, M.A. Wild Edible Vegetables of Lesser Himalaya, Ethnobotanical and Neutraceutical Aspect; Springer: New York, NY, USA, 2015; Volume 1, p. 86. [Google Scholar]
- Amjad, M.S.; Arshad, M.; Qureshi, R. Ethnobotanical inventory and folk uses of indigenous plants from Pir Nasoora National Park, Azad Jammu and Kashmir. Asian Pacific. J. Trop. Biomed. 2015, 5, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Tariq, A.; Adnani, M.; Begum, S. Medicinal plants density along an altitudinal gradient in and around ayubia national park. Pak. J. Bot. 2016, 48, 1537–1546. [Google Scholar]
- Ishaque, M.; Bibi, Y.; Qayyum, A.; Khalid, M.; Rafiq, M.; Arshad, M.; Saqlan Naqvi, S.M.; Nisa, S.; Jenks, M.A. Antioxidant potential, total phenolic and flavonoid contents of three culinary medicinal plant species of Lesser Hamalya, Pakistan. Bulg. Chem. Commun. 2018, 50, 368–373. [Google Scholar]
- Benardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef]
- Rahee, K.J.; Sethupathi, P.; Driks, A.; Lanning, D.K.; Knight, K.L. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 2004, 172, 1118–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loir, L.Y.; Baron, F.; Gautier, M. Staphylococcus aerus and food poisoning. Genet. Mol. Res. 2003, 2, 7–28. [Google Scholar]
- Li, J.; Fu, Y.; Wang, J.Y. Early diagnosis and therapeutic choice of Klebsiella pneumoniae liver abscess. Front. Med. China 2010, 4, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.K.; Fung, C.; Chang, F. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J. Clin. Microbiol. 2011, 49, 3761–3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, C.P.; Lin, Y.T.; Lin, C.J.; Chen, L.T.; Yeh, M.K.C.; Chang, Y.F.; Chuang, C.C.H.; Wu, S.H.; Tseng, P.C.; Siu, K.L. Klebsiella pneumoniae in Gastrointestinal Tract and Pyogenic Liver Abscess. Emerg. Infect Dis. 2012, 18, 1322–1325. [Google Scholar] [CrossRef]
- Giannella, R.A. Salmonella. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8435/ (accessed on 17 July 2021).
- Rolhion, N.; Darfeuille-Michaud, A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 1277–1283. [Google Scholar] [CrossRef]
- Valgas, C.; de Souza, S.M.; Smânia, F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- De Souza, S.M.; Delle–Monache, F.; Smânia, A., Jr. Antibacterial activity of oumarins. Z. Natur Forsch. 2005, 60, 693–700. [Google Scholar]
- Akinyemi, O.; Oluwa, K.; Omomigbehin, E.O. Antimicrobial activity of crude extracts of three medicinal plants used in south-west nigerian folk medicine on some food borne bacterial pathogens. Afr. J. Trad. Cam. 2006, 3, 13–22. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, L.L.; Rogers, L.L. The use of biological assays to evaluate the botanicals. Drug Inf. 1998, 32, 513–524. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Matu, E.N.; Van Staden, J. Antibacterial and antiinflammatory activities of some plants used for medicinal purposs in Kenya. J. Ethnopharmacol. 2003, 87, 35–41. [Google Scholar] [CrossRef]
- Ahvazi, M. Introduction of medicinal plants species with the most traditional usage in alamut region. Int. J. Pharmacol. Res. 2008, 11, 185–194. [Google Scholar]
- Kokotkiewicz, A.; Luczkiewicz, M.; Sowinski, P.; Glod, D.; Gorynski, K.; Bucinski, A. Isolation and structure elucidation of phenolic compounds from Cyclopia subternata Vogel (honeybush) intact plant and in vitro cultures. Food Chem. 2012, 133, 1373–1382. [Google Scholar] [CrossRef]
- De Beer, D.; Schulze, A.E.; Joubert, E.; De Villiers, A.; Malherbe, C.J.; Stander, M.A. Food Ingredient Extracts of Cyclopia subternata (Honeybush): Variation in Phenolic Composition and Antioxidant Capacity. Molecules 2012, 17, 14602–14624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nessa, F.; Ismailb, Z.; Mohamed, N. Antimicrobial Activities of Extracts and Flavonoid Glycosides of Corn Silk (Zea mays L). Int. J. Biotech. Wellness Ind. 2012, 1, 115–121. [Google Scholar] [CrossRef]
- Xiao, I. Dietary flavonoid aglycones and their glycosides: Which show better biological significance. Crit. Rev. Food Sci. Nut. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Tagousop, C.N.; Tamokou, J.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Putalun, W.; Yusakul, G.; Saensom, P.; Sritularak, B.; Tanaka, H. Determination of iriflophenone 3-C-β-D-glucoside from Aquilaria spp. by an indirect competitive enzyme-linked immunosorbent assay using a specific polyclonal antibody. J. Food. Sci. 2013, 78, 1363–1367. [Google Scholar] [CrossRef]
- Supasuteekul, C.; Nuamnaichati, N.; Mangmool, S.; Likhitwitayawuid, K.; Tengamnuay, P.; Putalun, W.; Sritularak, B. Antioxidant Activity and Upregulation of Antioxidant Enzymes of Phenolic Glycosides from Aquilaria crassna Leaves. Nat. Prod. Commun. 2017, 12, 1691–1694. [Google Scholar] [CrossRef] [Green Version]
- Salih, E.Y.A.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Alam, F.; Khan, S.H.A.; Bin, A.M.H.H. Phytochemical, antimicrobial, antioxidant and enzyme inhibitory potential of medicinal plant Dryopteris ramosa (Hope) C. Chr. BMC Complement. Med. Therap. 2021, 21, 197. [Google Scholar] [CrossRef]
- Phull, A.-R.; Abbas, Q.; Ali, A.; Raza, H.; Kim, S.J.; Zia, M.; Haq, I. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliate. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Nisar, M.; Sheema, S.S.M.; Mukarram, S.; Abdul, S.; Khan, I.; Khan, S.M.H.S. Larvicidal, insecticidal, brine shrimp cytotoxicity and anti-oxidant activities of Diospyros kaki (L.) reported from Pakistan. J. Pharm. Sci. 2015, 28, 1239–1243. [Google Scholar]


| Microorganism | Importance in GIT (Human) |
|---|---|
| Bacillus subtilis | Useful bacteria, in many animal species, feed supplementation with Bacillus subtilis used as prebiotics and probiotics [10]. Induce development of the gut-associated lymphoid tissue (GALT) and the pre-immune antibody repertoire [11]. |
| Staphylococcus aureus | Produce staphylococcal enterotoxins (SEs), the causative agents of staphylococcal food poisoning [12]. |
| Klebsiella pneumoniae | In Asia, it is the leading cause of pyogenic liver abscess (PLA) [13,14] The colonization of virulent type K. pneumoniae in intestine causes PLA [15] |
| Salmonella enterica | Gastroenteritis (diarrhea, abdominal cramps and fever) Enteric fevers, including typhoid fever [16] |
| Escherichia coli | Among the E. coli strains that can cause intestinal disease in humans, there are at least 6 well-characterized classes or pathotypes, as follows: enteropathogenic E. pneumoniae (EPEC), enterohemorrhagic E.coli (EHEC), enterotoxinogenic E.coli (ETEC), enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), and diffusely adherent E. coli (DAEC) [17] |
| Conc. (µg/mL) | Bacillus subtilis ATCC 6633 | Staphylococcus aureus ATCC 6538 | Klebsiella pneumoniae ATCC 700603 | Salmonella enterica. subsp. enterica Serovar Setubal ATCC 19196 | Escherichia coli ATCC 25922 | |
|---|---|---|---|---|---|---|
| Inhibition zone (in millimeters) | ||||||
| CME | 10 | 11 ± 0.7 | 12 ± 0.7 | 12 ± 0.3 | 11 ± 0.7 | 10 ± 0.7 |
| 100 | 19 ± 0.3 | 24 ± 0.3 | 31 ± 0.3 | 22 ± 0.7 | 24 ± 0.7 | |
| 500 | 31 ± 0.7 | 38 ± 0.3 | 45 ± 0.7 | 43 ± 0.7 | 38 ± 0.7 | |
| 1000 | 43 ± 0.3 | 66 ± 0.7 | 65 ± 0.3 | 61 ± 0.3 | 54 ± 0.3 | |
| MIC (µg/mL) | 250 ± 3.9 | 125 ± 7.2 | 62.5 ± 7.2 | 125 ± 3.9 | 125 ± 7.2 | |
| i3βDGP | 10 | 18 ± 0.3 | 23 ± 0.3 | 24 ± 0.7 | 19 ± 0.7 | 19 ± 0.7 |
| 100 | 26 ± 0.7 | 32 ± 0.3 | 49 ± 0.3 | 32 ± 0.7 | 29 ± 0.7 | |
| 500 | 35 ± 0.3 | 45 ± 0.3 | 68 ± 0.3 | 55 ± 0.3 | 42 ± 0.7 | |
| 1000 | 48 ± 0.7 | 71 ± 0.7 | 85 ± 0.3 | 76 ± 0.7 | 55 ± 0.3 | |
| MIC (µg/mL) | 125 ± 7.2 | 62.5 ± 7.2 | 31.1 ± 7.2 | 62.5 ± 14.4 | 62.5 ± 7.2 | |
| Cefixime * | 10 | 19 ± 0.3 | 23 ± 0.3 | 25 ± 0.7 | 26 ± 0.3 | 21 ± 0.3 |
| 100 | 27 ± 0.7 | 31 ± 0.3 | 49 ± 0.3 | 36 ± 0.3 | 32 ± 0.3 | |
| 500 | 39 ± 0.7 | 44 ± 0.7 | 70 ± 0.3 | 59 ± 0.3 | 43 ± 0.7 | |
| 1000 | 51 ± 0.7 | 67 ± 0.3 | 94 ± 0.3 | 78 ± 0.3 | 56 ± 0.3 | |
| MIC (µg/mL) | 62.5 ± 7.2 | 62.5 ± 7.2 | 31.1 ± 7.2 | 31.1 ± 7.2 | 62.5 ± 7.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishaque, M.; Bibi, Y.; Ayoubi, S.A.; Masood, S.; Nisa, S.; Qayyum, A. Iriflophenone-3-C-β-d Glucopyranoside from Dryopteris ramosa (Hope) C. Chr. with Promising Future as Natural Antibiotic for Gastrointestinal Tract Infections. Antibiotics 2021, 10, 1128. https://doi.org/10.3390/antibiotics10091128
Ishaque M, Bibi Y, Ayoubi SA, Masood S, Nisa S, Qayyum A. Iriflophenone-3-C-β-d Glucopyranoside from Dryopteris ramosa (Hope) C. Chr. with Promising Future as Natural Antibiotic for Gastrointestinal Tract Infections. Antibiotics. 2021; 10(9):1128. https://doi.org/10.3390/antibiotics10091128
Chicago/Turabian StyleIshaque, Muhammad, Yamin Bibi, Samha Al Ayoubi, Saadia Masood, Sobia Nisa, and Abdul Qayyum. 2021. "Iriflophenone-3-C-β-d Glucopyranoside from Dryopteris ramosa (Hope) C. Chr. with Promising Future as Natural Antibiotic for Gastrointestinal Tract Infections" Antibiotics 10, no. 9: 1128. https://doi.org/10.3390/antibiotics10091128
APA StyleIshaque, M., Bibi, Y., Ayoubi, S. A., Masood, S., Nisa, S., & Qayyum, A. (2021). Iriflophenone-3-C-β-d Glucopyranoside from Dryopteris ramosa (Hope) C. Chr. with Promising Future as Natural Antibiotic for Gastrointestinal Tract Infections. Antibiotics, 10(9), 1128. https://doi.org/10.3390/antibiotics10091128






