Iso-Partricin, an Aromatic Analogue of Amphotericin B: How Shining Light on Old Drugs Might Help Create New Ones
Abstract
1. Introduction
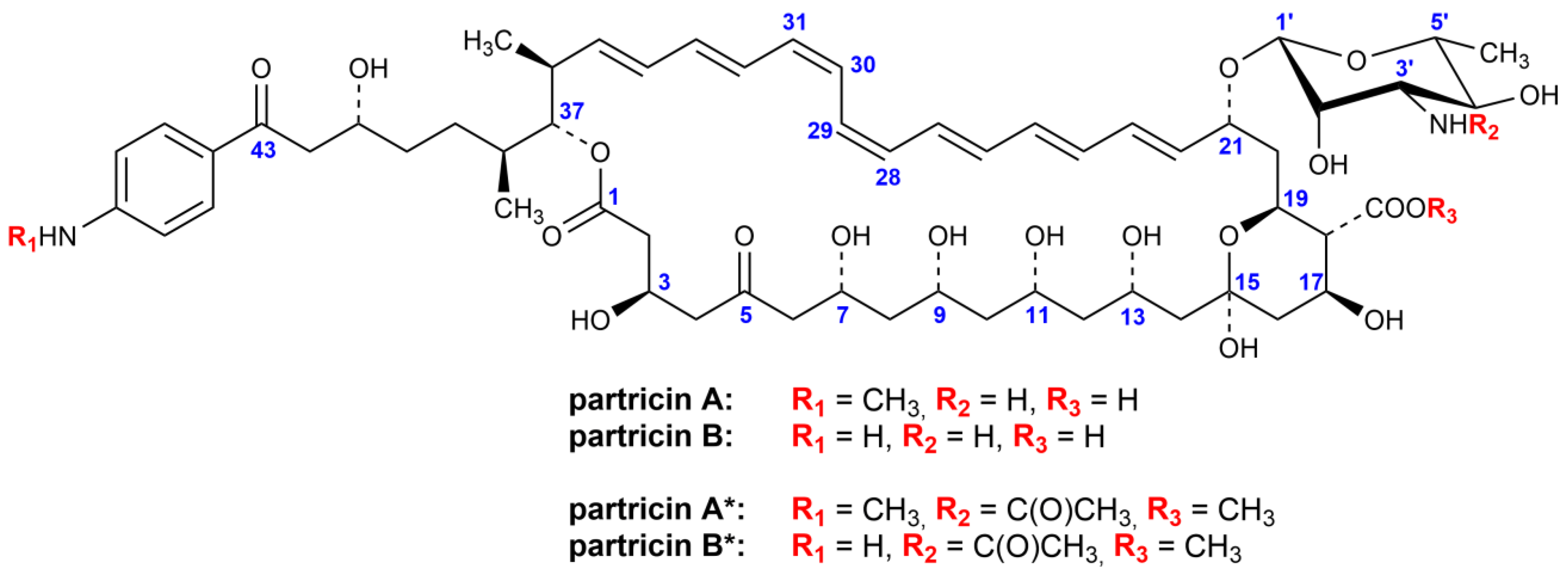
2. Results
2.1. Shedding A New Light to Old Matters
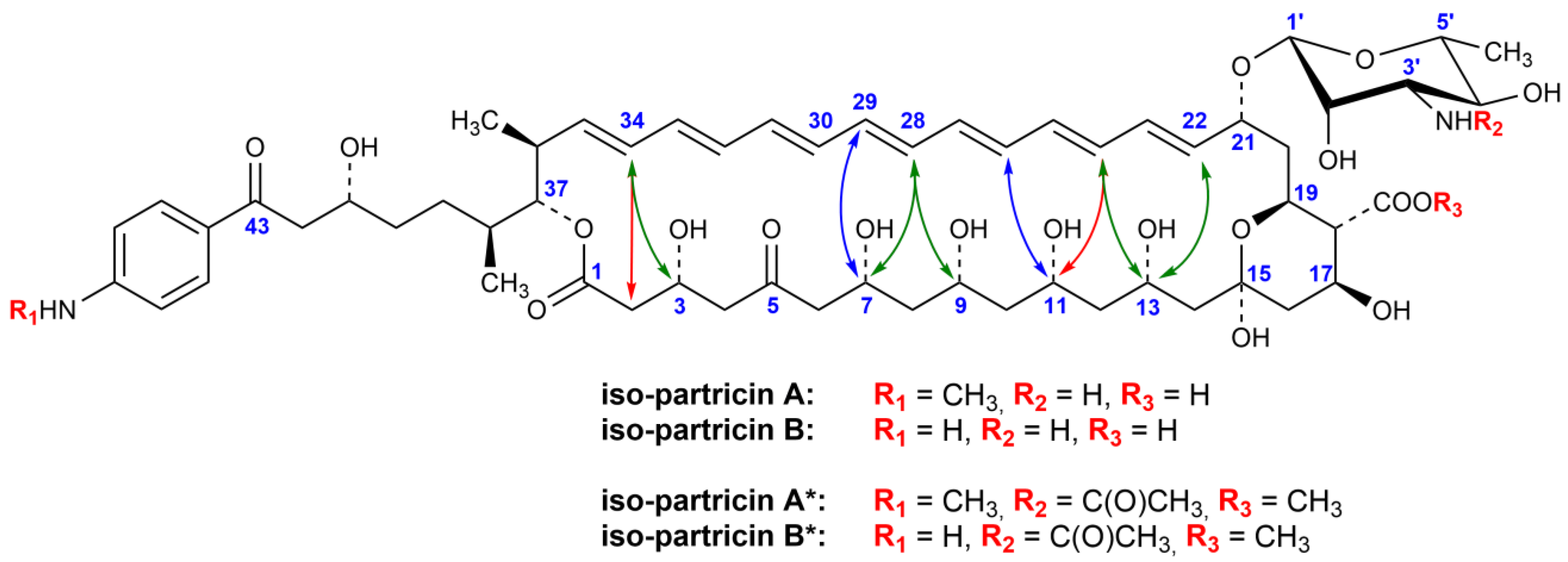
2.2. NMR Studies on Iso-Partricin A and B
3. Discussion
4. Materials and Methods
4.1. Partricin Complex
4.2. Synthesis of Methyl Ester of 3′-N-Acetylpartricin Complex (Partricin*)
4.3. Photochemical Cis−Trans Isomerization of Partricin*
4.4. Isolation of the Methyl Esters of 3′-N-Acetyl-Iso-Partricin A and B (Iso-Partricin A* and Iso-Partricin B*)
4.5. NMR Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Iso-Partricin A* and B* | |||||
|---|---|---|---|---|---|
| Position | δC, Type | δH | JH,H (Hz) | ROE Contacts | |
| Aglycone | |||||
| 1 | 170.85, | C | – | – | – |
| 2ab 1 | 43.48, | CH2 | 2.796 | ? (3) 1 | 3, 4a, 4b |
| 3 | 64.18, | CH | 4.863 | ? (2ab) 1, 8.8 (4a), 4.3 (4b) | 2ab, 4a, 4b, 34, Me38 |
| 4a | 51.05, | CH2 | 2.849 | 8.8 (3), 16.8 (4b) | 2ab, 3, 4b |
| 4b | 2.990 | 4.3 (3), 16.8 (4b) | 2ab, 3, 4a | ||
| 5 | 208.54, | C | – | – | – |
| 6a | 51.55, | CH2 | 2.632 | 16.4 (6b), 1.9 (7) | 4a, 6b, 7 |
| 6b | 2.874 | 16.4 (6a), 10.2 (7) | 4b, 6a, 7 | ||
| 7 | 67.79, | CH | 4.591 | 1.9 (6a), 10.2 (6b), 2.0 (8a), 9.7 (8b) | 6a, 6b, 8a, 8b, 9, 28 |
| 8a | 43.77, | CH2 | 1.529 | 2.0 (7), 13.8 (8b), 1.9 (9) | 6a, 7, 8b, 9, 10a |
| 8b | 1.732 | 9.7 (7), 13.8 (8b), 10.0 (9) | 6b, 7, 8a, 10b | ||
| 9 | 72.79, | CH | 4.269 | 1.9 (8a), 10.0 (8b), 2.2 (10a), 9.8 (10b) | 7, 8a, 10a, 11, 28 |
| 10a | 44.49, | CH2 | 1.465 | 2.2 (9), 14.1 (10b), 1.9 (11) | 8a, 9, 10b, 11, 12a |
| 10b | 1.670 | 9.8 (9), 14.1 (10a), 10.3 (11) | 8b, 10a, 11 | ||
| 11 | 73.02, | CH | 4.283 | 1.9 (10a), 10.3 (10b), 2.2 (12a), 9.4 (12b) | 9, 10a, 10b, 12a, 13, 24 |
| 12a | 44.31, | CH2 | 1.411 | 2.2 (11), 13.2 (12b), 1.8 (13) | 10a, 11, 12b, 13, 14a |
| 12b | 1.685 | 9.4 (11), 13.2 (12a), 10.1 (13) | 12a, 14b | ||
| 13 | 69.11, | CH | 4.746 | 1.8 (12a), 10.1 (12b), 2.0 (14a), 9.4 (14b) | 11, 12a, 14a, 22, 24 |
| 14a | 46.71, | CH2 | 1.755 | 2.0 (13), 14.6 (14b) | 12a, 13, 14b |
| 14b | 1.953 | 9.4 (13), 14.6 (14a) | 12b, 14a, 16b | ||
| 15 | 98.11, | C | – | – | – |
| 16a | 45.28, | CH2 | 1.743 | 12.4 (16b), 10.3 (17) | 16b, 18 |
| 16b | 2.543 | 12.4 (16a), 4.5 (17) | 14b, 16a, 17 | ||
| 17 | 66.34, | CH | 5.006 | 10.3 (16a), 4.5 (16b), 10.2 (18) | 16b, 18, 19 |
| 18 | 58.39, | CH | 2.853 | 10.2 (17), 10.1 (19) | 16a, 17, 19, 20a, 21 |
| 19 | 66.21, | CH | 5.049 | 10.1 (18), 10.5 (20a) | 17, 18, 20a, 20b, 22, 1′, 2′ |
| 20a | 37.86, | CH2 | 2.013 | 10.5 (19), 15.6 (20b) | 18, 19, 20b, 21 |
| 20b | 2.431 | 15.6 (20a), 9.6 (21) | 19, 20a, 21, 1′ | ||
| 21 | 75.91, | CH | 4.924 | 9.6 (20b), 9.2 (22) | 18, 20a, 20b, 22, 1′ |
| 22 | 136.89, | CH | 6.403 | 9.2 (21), 15.3 (23) | 13, 19, 21, 24 |
| 23 | 132.93, | CH | 6.449 | 15.3 (22), 11.1 (24) | 25 |
| 24 | 133.90, | CH | 6.674 | 11.1 (23), 15.4 (25) | 11, 13, 22, 26 |
| 25 | 130.00, | CH | 6.380 | 15.4 (24), 10.9 (26) | 23, 27 |
| 26 | 133.89, | CH | 6.476 | 10.9 (25), 15.1 (27) | 24, 28 |
| 27 | 132.42, | CH | 6.299 | 15.1(26), 11.0 (28) | 27, 29 |
| 28 | 133.90, | CH | 6.649 | 11.0 (27), 15.6 (29) | 7, 9, 26, 30 |
| 29 | 133.88, | CH | 6.630 | 15.6 (28), 10.9 (30) | 27, 31 |
| 30 | 133.25, | CH | 6.496 | 10.9 (29), 15.4 (31) | 28, 32 |
| 31 | 133.91, | CH | 6.673 | 15.4 (30), 11.3 (32) | 29, 33 |
| 32 | 133.01, | CH | 6.405 | 11.3 (31), 15.1 (33) | 30, 34 |
| 33 | 133.84, | CH | 6.624 | 15.1 (32), 11.0 (34) | 31, 35 |
| 34 | 132.49, | CH | 6.301 | 11.0 (33), 15.5 (35) | 2ab, 3, 32, 36 |
| 35 | 136.99, | CH | 5.598 | 15.5 (34), 9.3 (36) | 33, 36, 37, Me36 |
| 36 | 40.14, | CH | 2.542 | 9.3 (35), 9.2 (37), 6.8 (Me36) | 34, 35, 37, 39a, 39b, Me36, Me38 |
| 37 | 78.63, | CH | 5.080 | 9.2 (36), 2.9 (38) | 35, 36, 38, 39a, Me36, Me38 |
| 38 | 33.81, | CH | 1.927 | 2.9 (37), ? (39a, 39b) 2, 6.7 (Me38) | 37, 39a, 39b, 40ab, 41, Me36, Me38 |
| 39a | 30.84, | CH2 | A: 1.719 B: 1.714 | ? (38, 39b) 2, ? (40ab) 1 | 36, 37, 38, 39b, 40ab, 41, Me38 |
| 39b | A: 1.761 B: 1.757 | ? (38, 39a) 2, ? (40ab) 1 | 36, 37, 38, 39a, 40ab, 41, Me38 | ||
| 40ab 1 | A: 35.64, B: 35.66, | CH2 | A: 1.895 B: 1.877 | ? (39a, 39b, 41) 1 | 38, 39a, 39b, 41, 42a, 42b, Me38 |
| 41 | A: 68.35, B: 68.28, | CH | A: 4.609 B: 4.592 | ? (40ab)1, 3.5 (42a), 9.1 (42b) | 38, 39a, 39b, 40ab, 42a, 42b, 45/45′ |
| 42a | A: 46.11, B: 46.04, | CH2 | A: 3.233 B: 3.211 | 3.5 (41), 15.2 (42b) | 40ab, 41, 42b, 45/45′ |
| 42b | A: 3.435 B: 3.410 | 9.1 (41), 15.2 (42a) | 40ab, 41, 42a, 45/45′ | ||
| 43 | A: 197.76, B: 197.67, | C | – | – | – |
| Me36 | 16.48, | CH3 | 0.972 | 6.8 (36) | 35, 36, 37, 38 |
| Me38 | 12.91, | CH3 | 1.040 | 6.7 (38) | 36, 37, 38, 39a, 39b |
| COOMe | 173.91, | C | – | – | – |
| COOMe | 51.66, | CH3 | 3.818 | – | 2′, 3′ |
| NHMe | A: 29.36, B: –, | CH3 | A: 2.811 B: – | – | 46/46′ |
| Aromatic moiety | |||||
| 45/45′ | A: 131.05, B: 131.23, | CH | 8.161 | 8.6 (46/46′) | 41, 42a, 42b, 46/46′ |
| 46/46′ | A: 110.94, B: 110.24, | CH | 6.757 | 8.6 (45/45′) | 45/45′, NHMe |
| C*CO | A: 154.38, B: 154.25, | C | – | – | – |
| C*NH | A: 126.12, B: 126.64, | C | – | – | – |
| Mycosamine moiety | |||||
| 1′ | 98.16, | CH | 4.974 | 1.9 (2′) | 2′, 3′, 5′, 19, 20b, 21 |
| 2′ | 70.79, | CH | 4.444 | 1.9 (1′), 3.5 (3′) | 1′, 3′, 19, COOMe |
| 3′ | 56.09, | CH | 4.677 | 3.5 (2′), 9.4 (4′), 4.5 (NHCOMe) | 1′, 2′, 5′, COOMe, NHCOMe |
| 4′ | 74.62, | CH | 4.034 | 9.4 (3′), 9.5 (5′) | 6′, NHCOMe |
| 5′ | 74.69, | CH | 3.790 | 9.5 (4)’, 6.3 (6′) | 1′, 3′, 6′ |
| 6′ | 18.41, | CH3 | 1.591 | 6.3 (5′) | 4′, 5′ |
| NHCOMe | 22.83, | CH3 | 2.113 | – | 3′, NHCOMe |
| NHCOMe | 170.90, | C | – | – | – |
| NHCOMe | – | – | 8.850 | 4.5 (3′) | 4′, NHCOMe |
References
- Omura, S.; Tanaka, H. Macrolide Antibiotics. In Chemistry, Biology, Practice, 1st ed.; Omura, S., Ed.; Academic Press: London, UK, 1984. [Google Scholar]
- Borowski, E.; Schaffner, C.P. Structural Relationships among the Heptaene Macrolide Antibiotics. In Proceedings of the V International Congress of Biochemistry, Moscow, Russia, 10–16 August 1961; p. 3. [Google Scholar]
- Oroshnik, W.; Mebane, A.D. The Polyene Antifungal Antibiotics. Prog. Chem. Org. Nat. Prog. 1963, 21, 17–79. [Google Scholar] [CrossRef]
- Cybulska, B.; Ziminski, T.; Borowski, E.; Gary-Bobo, C.M. The influence of electric charge of aromatic heptaene macrolide antibiotics on their activity on biological and lipidic model membranes. Mol. Pharmacol. 1983, 24, 270–276. [Google Scholar] [PubMed]
- Szczeblewski, P.; Laskowski, T.; Kubacki, B.; Dziergowska, M.; Liczmańska, M.; Grynda, J.; Kubica, P.; Kot-Wasik, A.; Borowski, E. Analytical studies on ascosin, candicidin and levorin multicomponent antifungal antibiotic complexes. The stereostructure of ascosin A2. Sci. Rep. 2017, 7, 40158. [Google Scholar] [CrossRef] [PubMed]
- Golik, J.; Zielinski, J.; Borowski, E. The structure of mepartricin A and mepartricin B. J. Antibiot. 1980, 33, 904–907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tweit, R.C.; Pandey, R.C.; Rinehart, K.L., Jr. Characterization of the Antifungal and Antiprotozoal Antibiotic Partricin and Structural Studies on Partricins A and B. J. Antibiot. 1982, 35, 997–1012. [Google Scholar] [CrossRef]
- Sowinski, P.; Gariboldi, P.; Czerwinski, A.; Borowski, E. The structure of vacidin A, an aromatic heptaene macrolide antibiotic. I. Complete assignment of the 1H NMR spectrum and geometry of the polyene chromophore. J. Antibiot. 1989, 42, 1631–1638. [Google Scholar] [CrossRef]
- Sowinski, P.; Gariboldi, P.; Pawlak, J.K.; Borowski, E. The structure of vacidin A, an aromatic heptaene macrolide antibiotic. II. Stereochemistry of the antibiotic. J. Antibiot. 1989, 42, 1639–1642. [Google Scholar] [CrossRef]
- Sowiński, P.; Pawlak, J.; Borowski, E.; Gariboldi, P. Stereostructure of Gedamycin. Pol. J. Chem. 1995, 69, 213–217. [Google Scholar]
- Cirillo Marucco, E.; Pagliarulo, A.; Piccinno, A.; di Rienzo, U. Mepartricin in the Treatment of Benign Prostatic Hyperplasia. Minerva Urol. Nefrol. 1988, 40, 101–104. [Google Scholar]
- Boehm, S.; Nirnberger, G.; Ferrari, P. Estrogen Suppression as a Pharmacotherapeutic Strategy in the Medical Treat-ment of Benign Prostatic Hyperplasia: Evidence for Its Efficacy from Studies with Mepartricin. Wien. Klin. Wochenschr. 1998, 110, 817–823. [Google Scholar]
- Petrone, U.; Gaspari, G.; Magnocavallo, N.; Petrone, D.; Tucci, C.; Marascia, G. Use of mepartricin in the treatment of benign prostatic hypertrophy. Evaluation of clinical and functional parameters. Minerva Urol. Nefrol. 1988, 40, 89–91. [Google Scholar]
- De Rose, A.F.; Gallo, F.; Giglio, M.; Carmignani, G. Role of mepartricin in category III chronic nonbacterial prostatitis/chronic pelvic pain syndrome: A randomized prospective placebo-controlled trial. Urology 2004, 63, 13–16. [Google Scholar] [CrossRef]
- Lotti, T.; Mirone, V.; Prezioso, D.; de Bernardi, M.; Rapocci, M.P.; Ruozi, P. Observations on Some Hormone Fractions in Patients with Benign Prostatic Hyperplasia Treated with Mepartricin. Curr. Ther. Res. 1988, 44, 402–409. [Google Scholar]
- Szwarc, K.; Szczeblewski, P.; Sowiński, P.; Borowski, E.; Pawlak, J. The stereostructure of candicidin D. J. Antibiot. 2015, 68, 504–510. [Google Scholar] [CrossRef]
- Szczeblewski, P.; Laskowski, T.; Bałka, A.; Borowski, E.; Milewski, S. Light-Induced Transformation of the Aromatic Heptaene Antifungal Antibiotic Candicidin D into Its All-Trans Isomer. J. Nat. Prod. 2018, 81, 1540–1545. [Google Scholar] [CrossRef]
- Szczeblewski, P.; Andrałojć, W.; Polit, J.; Żabka, A.; Winnicki, K.; Laskowski, T. Ipertrofan revisited-The proposal of the complete stereochemistry of mepartricin A and B. Molecules 2021, 26, 5533. [Google Scholar] [CrossRef]
- Waksman, S.A.; Lechevalier, H.A.; Schaffner, C.P. Candicidin and other polyenic antifungal antibiotics. Bull. World Health Organ. 1965, 33, 219–226. [Google Scholar] [PubMed]
- Płosiński, M.; Laskowski, T.; Sowiński, P.; Pawlak, J. Stereostructure of mycoheptin A2. Magn. Reson. Chem. 2012, 50, 818–822. [Google Scholar] [CrossRef]
- Szwarc, K.; Szczeblewski, P.; Sowiński, P.; Borowski, E.; Pawlak, J. The structure, including stereochemistry, of levorin A1. Magn. Reson. Chem. 2015, 53, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Borzyszkowska-Bukowska, J.; Szczeblewski, P.; Konkol, A.; Grynda, J.; Szwarc-Karabyka, K.; Laskowski, T. The complete stereochemistry of the antibiotic candicidin A3 (syn. ascosin A3, levorin A3). Nat. Prod. Res. 2019, 34, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, P.; Pawlak, J.; Borowski, E.; Gariboldi, P. 1H NMR model studies of amphotericin B: Comparison of x-ray and NMR stereochemical data. Magn. Reson. Chem. 1992, 30, 275–279. [Google Scholar] [CrossRef]
- Sowinski, P.; Pawlak, J.; Borowski, E.; Gariboldi, P. Stereostructure of Rimocidin. J. Antibiot. 1995, 48, 1288–1291. [Google Scholar] [CrossRef][Green Version]
- Pawlak, J.; Sowiński, P.; Borowski, E.; Gariboldi, P. Stereostructure of Perimycin A. J. Antibiot. 1995, 48, 1034–1038. [Google Scholar] [CrossRef][Green Version]
- Pawlak, J.; Sowinski, P.; Borowski, E.; Gariboldi, P. Stereostructure and NMR characterization of the antibiotic candidin. J. Antibiot. 1993, 46, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Borzyszkowska-Bukowska, J.; Górska, J.; Szczeblewski, P.; Laskowski, T.; Gabriel, I.; Jurasz, J.; Kozłowska-Tylingo, K.; Milewski, S. Quest for the Molecular Basis of Improved Selective Toxicity of All-Trans Isomers of Aromatic Heptaene Macro-lide Antifungal Antibiotics. Int. J. Mol. Sci. in press.
- Borowski, E.; Ciechocka, K.; Dutkiewicz, M.; Zimiński, T. Sposób Otrzymywania Kandycydyny o Zwiększonej Trwałości, Czystości i Aktywności. Biologicznej. Patent No. 83710, 30 January 1976. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczeblewski, P.; Górska, J.; Andrałojć, W.; Janke, P.; Wąsik, K.; Laskowski, T. Iso-Partricin, an Aromatic Analogue of Amphotericin B: How Shining Light on Old Drugs Might Help Create New Ones. Antibiotics 2021, 10, 1102. https://doi.org/10.3390/antibiotics10091102
Szczeblewski P, Górska J, Andrałojć W, Janke P, Wąsik K, Laskowski T. Iso-Partricin, an Aromatic Analogue of Amphotericin B: How Shining Light on Old Drugs Might Help Create New Ones. Antibiotics. 2021; 10(9):1102. https://doi.org/10.3390/antibiotics10091102
Chicago/Turabian StyleSzczeblewski, Paweł, Justyna Górska, Witold Andrałojć, Patryk Janke, Karolina Wąsik, and Tomasz Laskowski. 2021. "Iso-Partricin, an Aromatic Analogue of Amphotericin B: How Shining Light on Old Drugs Might Help Create New Ones" Antibiotics 10, no. 9: 1102. https://doi.org/10.3390/antibiotics10091102
APA StyleSzczeblewski, P., Górska, J., Andrałojć, W., Janke, P., Wąsik, K., & Laskowski, T. (2021). Iso-Partricin, an Aromatic Analogue of Amphotericin B: How Shining Light on Old Drugs Might Help Create New Ones. Antibiotics, 10(9), 1102. https://doi.org/10.3390/antibiotics10091102






