The Emergence of Multidrug-Resistant Helicobacter pylori in Southeast Asia: A Systematic Review on the Trends and Intervention Strategies Using Antimicrobial Peptides
Abstract
1. Introduction
2. Results
2.1. Summary of Primary Antibiotic Resistance Rate in H. pylori
2.1.1. Clarithromycin Resistance
2.1.2. Metronidazole Resistance
2.1.3. Levofloxacin Resistance
2.1.4. Amoxicillin Resistance
2.1.5. Tetracycline Resistance
2.1.6. Multidrug Resistance Rate of H. pylori
2.2. A Review on Intervention Strategy Using AMP
3. Discussion
4. Methods
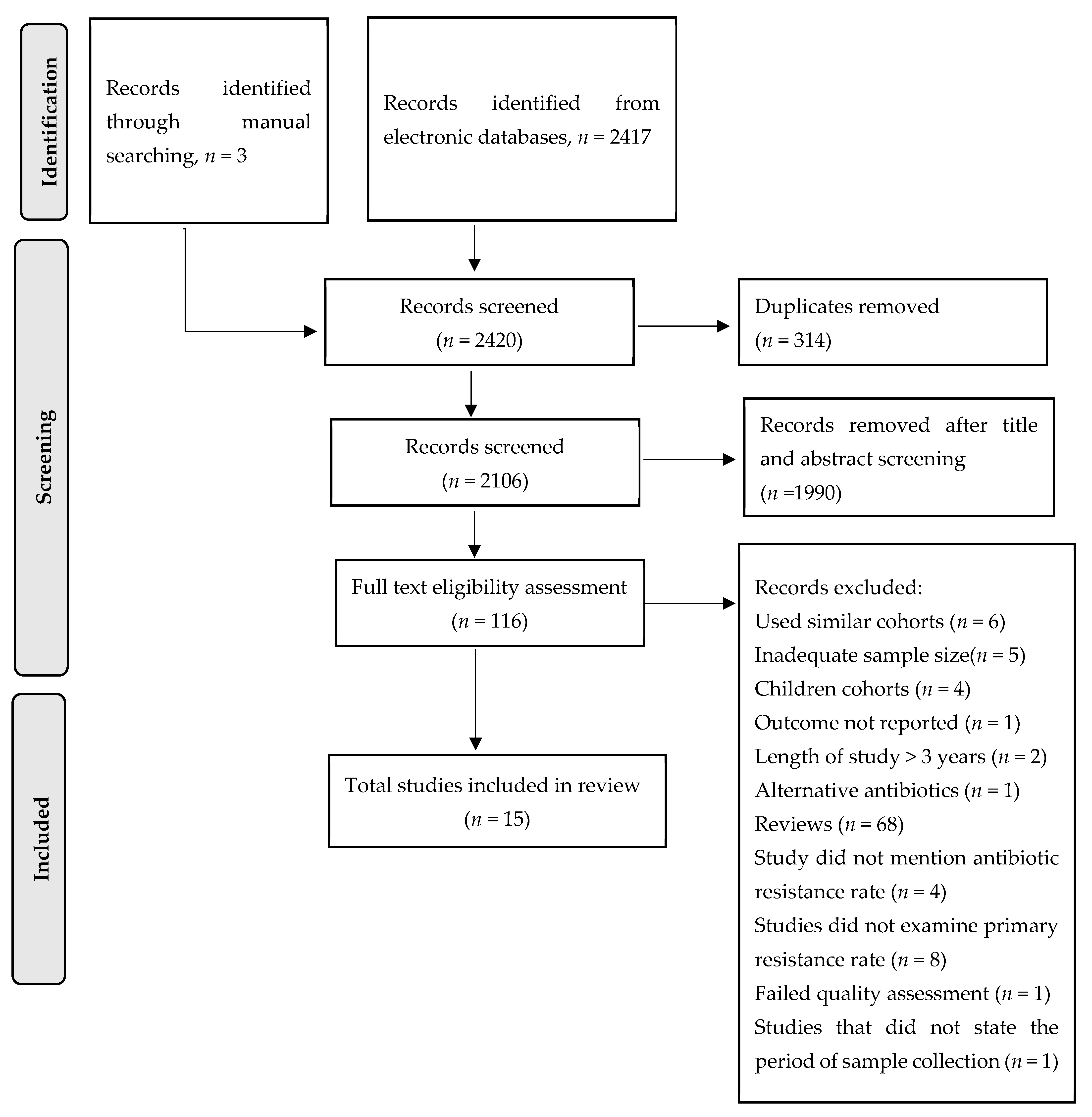
4.1. Search Strategy and Study Selection
4.2. Definitions of Diagnostic Tools and Antibiotic Resistance
4.3. Data Extraction
4.4. Quality Assessment of Studies
4.5. A Narrative Review on AMP Studies in H. pylori
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’Angelis, G.L. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Biomed. 2018, 89, 72–76. [Google Scholar] [PubMed]
- Sukri, A.; Hanafiah, A.; Mohamad Zin, N.; Kosai, N.R. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS 2020, 128, 150–161. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rugge, M.; Fassan, M.; Graham, D. Epidemiology of Gastric Cancer. In Gastric Cancer; Strong, V.E., Ed.; Springer: Cham, Switzerland, 2015; pp. 23–34. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- The World Bank. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 29 June 2021).
- Hughes, A. Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere 2017, 8, e01624. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.M.B.; Dias, S.C.; Franco, O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol 2018, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Auttajaroon, J.; Chotivitayatarakorn, P.; Yamaoka, Y.; Vilaichone, R.K. CYP2C19 Genotype, CagA genotype and antibiotic resistant strain of Helicobacter pylori infection. Asian Pac. J. Cancer Prev. 2019, 20, 1243–1247. [Google Scholar] [CrossRef]
- Vilaichone, R.K.; Ratanachu ek, T.; Gamnarai, P.; Subsomwong, P.; Uchida, T.; Yamaoka, Y.; Mahachai, V. High fluoroquinolone resistant strains of Helicobacter pylori in the Golden triangle. Asian Pac. J. Cancer Prev. 2017, 18, 455–458. [Google Scholar] [PubMed]
- Vilaichone, R.K.; Ratanachu ek, T.; Gamnarai, P.; Chaithongrat, S.; Uchida, T.; Yamaoka, Y.; Mahachai, V. Extremely high prevalence of metronidazole-resistant Helicobacter pylori strains in mountain people (Karen and Hmong) in Thailand. Am. J. Trop Med. Hyg. 2016, 94, 717–720. [Google Scholar] [CrossRef][Green Version]
- Vilaichone, R.K.; Mahacahai, V.; Tumwasorn, S.; Kachintorn, U. CagA genotype and metronidazole resistant strain of Helicobacter pylori in functional dyspepsia in Thailand. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S3), 46–48. [Google Scholar] [CrossRef] [PubMed]
- Tongtawee, T.; Dechsukhum, C.; Matrakool, L.; Panpimanmas, S.; Loyd, R.A.; Kaewpitoon, S.J.; Kaewpitoon, N. High Prevalence of Helicobacter pylori resistance to clarithromycin: A hospital-based cross-sectional study in Nakhon Ratchasima Province, Northeast of Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 8281–8285. [Google Scholar] [CrossRef]
- Tuan, V.P.; Narith, D.; Tshibangu-Kabamba, E.; Dung, H.D.Q.; Viet, P.T.; Sokomoth, S.; Binh, T.T.; Sokhem, S.; Tri, T.D.; Ngov, S.; et al. A next-generation sequencing-based approach to identify genetic determinants of antibiotic resistance in Cambodian Helicobacter pylori clinical isolates. J. Clin. Med. 2019, 8, 858. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Syam, A.F.; Nusi, I.A.; Makmun, D.; Waskito, L.A.; Zein, L.H.; Akil, F.; Uwan, W.B.; Simanjuntak, D.; Wibawa, I.D.; et al. Surveillance of Helicobacter pylori antibiotic susceptibility in Indonesia: Different resistance types among regions and with novel genetic mutations. PLoS ONE 2016, 11, e0166199. [Google Scholar] [CrossRef]
- Vannarath, S.; Vilaichone, R.K.; Rasachak, B.; Mairiang, P.; Yamaoka, Y.; Mahachai, V. Antibiotic resistant pattern of Helicobacter pylori infection based on molecular tests in Laos. Asian Pac. J. Cancer Prev. 2016, 17, 285–287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanafiah, A.; Binmaeil, H.; Raja Ali, R.A.; Mohamed Rose, I.; Lopes, B.S. Molecular characterization and prevalence of antibiotic resistance in Helicobacter pylori isolates in Kuala Lumpur, Malaysia. Infect. Drug Resist. 2019, 12, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Navaratnam, P. High Helicobacter pylori resistance to metronidazole but zero or low resistance to clarithromycin, levofloxacin, and other antibiotics in Malaysia. Helicobacter 2011, 16, 241–245. [Google Scholar] [CrossRef]
- Ahmad, N.; Zakaria, W.R.; Mohamed, R. Analysis of antibiotic susceptibility patterns of Helicobacter pylori isolates from Malaysia. Helicobacter 2011, 16, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.L.; Fock, K.M.; Song, M.; Ang, D.; Kwek, A.B.; Ong, J.; Tan, J.; Teo, E.K.; Dhamodaran, S. Ten-day triple therapy versus sequential therapy versus concomitant therapy as first-line treatment for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2015, 30, 1134–1139. [Google Scholar] [CrossRef]
- Dang, N.Q.H.; Ha, T.M.T.; Nguyen, S.T.; Le, N.D.K.; Nguyen, T.M.T.; Nguyen, T.H.; Pham, T.T.H.; Tran, V.H. High rates of clarithromycin and levofloxacin resistance of Helicobacter pylori in patients with chronic gastritis in the south east area of Vietnam. J. Glob. Antimicrob. Resist. 2020, 22, 620–624. [Google Scholar] [CrossRef]
- Binh, T.T.; Shiota, S.; Nguyen, L.T.; Ho, D.D.; Hoang, H.H.; Ta, L.; Trinh, D.T.; Fujioka, T.; Yamaoka, Y. The incidence of primary antibiotic resistance of Helicobacter pylori in Vietnam. J. Clin. Gastroenterol. 2013, 47, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.N.; Santona, A.; Tran, V.H.; Tran, T.N.; Le, V.A.; Cappuccinelli, P.; Rubino, S.; Paglietti, B. High rate of levofloxacin resistance in a background of clarithromycin- and metronidazole-resistant Helicobacter pylori in Vietnam. Int. J. Antimicrob. Agents 2015, 45, 244–248. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.K.; Gallo, R.L.; Fang, E.F.; Hu, W.; Ling, T.K.; Shen, J.; Chan, R.L.; Lu, L.; Luo, X.M.; et al. Critical role of antimicrobial peptide cathelicidin for controlling Helicobacter pylori survival and infection. J. Immunol. 2016, 196, 1799–1809. [Google Scholar] [CrossRef]
- Guzman, J.; Téné, N.; Touchard, A.; Castillo, D.; Belkhelfa, H.; Haddioui-Hbabi, L.; Treilhou, M.; Sauvain, M. Anti-Helicobacter pylori properties of the ant-venom peptide bicarinalin. Toxins 2017, 10, 21. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, J.; Xu, X.; Lai, R.; Zou, Q. An antimicrobial peptide with antimicrobial activity against Helicobacter pylori. Peptides 2007, 28, 1527–1531. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.N.; Wu, C.J.; Chen, J.Y. Efficacy of the antimicrobial peptide TP4 against Helicobacter pylori infection: In vitro membrane perturbsation via micellization and in vivo suppression of host immune responses in a mouse model. Oncotarget 2015, 6, 12936–12954. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Li, J.; Liu, H.; Xu, X.; Lai, R.; Zhang, K. A new family of antimicrobial peptides from skin secretions of Rana pleuraden. Peptides 2007, 28, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ma, L.; Huang, Y.; Wu, H.; Dou, J.; Zhou, C. Antimicrobial activities of peptide Cbf-K16 against drug-resistant Helicobacter pylori infection in vitro and in vivo. Microb. Pathog. 2020, 138, 103847. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, A.M.; Ma, Z.Y.; Li, X.B.; Xiong, Y.Y.; Dou, J.F.; Wang, J.F. The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-helicobacter pylori activity in vitro and in vivo. Molecules 2015, 20, 3972–3985. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.N.; Wu, C.J.; Chen, J.Y. Epinecidin-1 antimicrobial activity: In vitro membrane lysis and in vivo efficacy against Helicobacter pylori infection in a mouse model. Biomaterials 2015, 61, 41–51. [Google Scholar] [CrossRef]
- Xiong, M.; Bao, Y.; Xu, X.; Wang, H.; Han, Z.; Wang, Z.; Liu, Y.; Huang, S.; Song, Z.; Chen, J.; et al. Selective killing of Helicobacter pylori with pH-responsive helix-coil conformation transitionable antimicrobial polypeptides. Proc. Natl. Acad. Sci. USA 2017, 114, 12675–12680. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, A.; Qi, B.; Yu, H.; Xiong, Y.; Zhou, G.; Qin, M.; Dou, J.; Wang, J. Secretion expression of human neutrophil peptide 1 (HNP1) in Pichia pastoris and its functional analysis against antibiotic-resistant Helicobacter pylori. Appl. Microbiol. Biotechnol. 2018, 102, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, A.; Wang, G.; Yu, H.; Qi, B.; Xiong, Y.; Zhou, G.; Qin, M.; Dou, J.; Wang, J. Fusion expression of the PGLa-AM1 with native structure and evaluation of its anti-Helicobacter pylori activity. Appl. Microbiol. Biotechnol. 2017, 101, 5667–5675. [Google Scholar] [CrossRef]
- Makobongo, M.O.; Gancz, H.; Carpenter, B.M.; McDaniel, D.P.; Merrell, D.S. The oligo-acyl lysyl antimicrobial peptide C₁₂K-2β₁₂ exhibits a dual mechanism of action and demonstrates strong in vivo efficacy against Helicobacter pylori. Antimicrob. Agents Chemother. 2012, 56, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Rigano, M.M.; Romanelli, A.; Fulgione, A.; Nocerino, N.; D’Agostino, N.; Avitabile, C.; Frusciante, L.; Barone, A.; Capuano, F.; Capparelli, R. A novel synthetic peptide from a tomato defensin exhibits antibacterial activities against Helicobacter pylori. J. Pept. Sci. 2012, 18, 755–762. [Google Scholar] [CrossRef]
- Iwahori, A.; Hirota, Y.; Sampe, R.; Miyano, S.; Takahashi, N.; Sasatsu, M.; Kondo, I.; Numao, N. On the antibacterial activity of normal and reversed magainin 2 analogs against Helicobacter pylori. Biol. Pharm. Bull. 1997, 20, 805–808. [Google Scholar] [CrossRef]
- Kuo, Y.T.; Liou, J.M.; El-Omar, E.M.; Wu, J.Y.; Leow, A.H.R.; Goh, K.L.; Das, R.; Lu, H.; Lin, J.T.; Tu, Y.K.; et al. Asian Pacific Alliance on Helicobacter and Microbiota. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 707–715. [Google Scholar] [CrossRef]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar]
- Mahachai, V.; Vilaichone, R.K.; Pittayanon, R.; Rojborwonwitaya, J.; Leelakusolvong, S.; Maneerattanaporn, M.; Chotivitayatarakorn, P.; Treeprasertsuk, S.; Kositchaiwat, C.; Pisespongsa, P.; et al. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J. Gastroenterol. Hepatol. 2018, 33, 37–56. [Google Scholar] [CrossRef]
- Levy, S.B. The 2000 Garrod lecture. Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother. 2002, 49, 25–30. [Google Scholar] [CrossRef]
- Losurdo, G.; Leandro, G.; Principi, M.; Giorgio, F.; Montenegro, L.; Sorrentino, C.; Ierardi, E.; Di Leo, A. Sequential vs. prolonged 14-day triple therapy for Helicobacter pylori eradication: The meta-analysis may be influenced by ‘geographical weighting’. Int. J. Clin. Pract. 2015, 69, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tang, G.; Pan, L.; Zhu, H.; Zhou, S.; Wei, Z. Clinical factors associated with initial Helicobacter pylori eradication therapy: A retrospective study in China. Sci. Rep. 2020, 10, 15403. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. Available online: http://www.prisma-statement.org/ (accessed on 1 May 2021).
- United Nations. Available online: https://www.un.org/press/en/2020/sc14093.doc.htm (accessed on 29 June 2021).
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef] [PubMed]
| Author | Year Published | Patient’s Age (Mean in Years) | Country | No. ofPatients | HP Diagnostic Test | Patients’ Diseases | Antibiotic Resistance Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| Auttajaroon et al. | 2019 | 54.5 | Thailand | 93 | RUT and culture | Functional dyspepsia | E-test | [13] |
| Vilaichone et al. | 2017 | 55.9 | Thailand | 148 | RUT and culture | Dyspepsia | E-test | [14] |
| Vilaichone et al. | 2016 | 46.7 | Thailand | 291 | RUT and culture | Dyspepsia | E-test | [15] |
| Vilaichone et al. | 2011 | 49.1 | Thailand | 412 | Culture and CLO test | Dyspepsia | E-test | [16] |
| Tongtawee et al. | 2015 | 45.2 | Thailand | 300 | HPE and RUT | Dyspepsia | Molecular technique a | [17] |
| Tuan et al. | 2019 | 45.3 | Cambodia | 206 | Culture | NA | Agar dilution assay | [18] |
| Miftahussurur et al. | 2016 | 49.6 (male) and 48.9 (female) | Indonesia | 849 | Culture and HPE | Dyspepsia | E-test | [19] |
| Vannarath et al. | 2016 | 46 | Laos | 329 | CLO and HPE | Dyspepsia | Molecular technique a | [20] |
| Hanafiah et al. | 2019 | 52.41 | Malaysia | 288 | Culture, HPE and RUT | Chronic dyspepsia | E-test | [21] |
| Goh et al. | 2011 | 50.5 | Malaysia | 90 | Culture | NA | E-test | [22] |
| Ahmad et al. | 2011 | NA | Malaysia | 777 | Culture, RUT and HPE | Gastritis, peptic ulcer and gastric cancer | E-test | [23] |
| Ang et al. | 2015 | 48 | Singapore | 462 | UBT, RUT and HPE | NA | Agar dilution method | [24] |
| Dang et al. | 2020 | 38.3 | Vietnam | 153 | Culture and RUT | Chronic gastritis | E-test | [25] |
| Binh et al. | 2013 | 47.3 (male) and 42.3 (female) | Vietnam | NA | Culture | Dyspepsia | E-test | [26] |
| Phan et al. | 2015 | 44.1 | Vietnam | 92 | Culture | Dyspepsia | E-test | [27] |
| Author | Period of Sample Collection | Country | No. of HP Isolates | Primary Antibiotic Resistance Rate, % | MDR Rate, % (No. of Isolates) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLA | MET | LEVO | AMX | TET | ||||||
| Auttajaroon et al. | 2017 | Thailand | 93 a | 12.9 | 62.8 | - | - | - | - | [13] |
| Vilaichone et al. | 2016 | Thailand | 50 | 2 | 26 | 22 | 0 | 0 | 4% (2/50) | [14] |
| Vilaichone et al. | 2013 | Thailand | 124 | 5.6 | 71.8 | 19.4 | 0.8 | 0 | 21.8% (27/124) | [15] |
| Vilaichone et al. | 2008–2010 | Thailand | 100 | - | 30 | - | - | - | - | [16] |
| Tongtawee et al. | 2014–2015 | Thailand | 300 b,* | 76.2 c | - | - | - | - | - | [17] |
| Tuan et al. | 2015 | Cambodia | 55 | 25.5 | 96.4 | 67.3 | 9.1 | 0 | 76.4% (42/55) | [18] |
| Miftahussurur et al. | 2012–2015 | Indonesia | 77 | 9.1 | 46.8 | 31.2 | 5.2 | 2.6 | - | [19] |
| Vannarath et al. | 2010–2012 | Laos | 119 b,** | 12.6 | - | - | - | - | - | [20] |
| Hanafiah et al. | 2014–2015 | Malaysia | 59 d | 12.2 | 56.1 | 17.1 | 0 | 0 | 7.4% (2/27) | [21] |
| Goh et al. | 2009 | Malaysia | 90 | 0 | 75.5 | 0 | 0 | - | - | [22] |
| Ahmad et al. | 2004–2007 | Malaysia | 187 | 2.1 | 36.9 | - | 0 | 0 | 2.1% (4/187) | [23] |
| Ang et al. | 2011–2014 | Singapore | 106 | 17.9 | 48.1 | - | 4.7 | - | 7.5% (8/106) | [24] |
| Dang et al. | 2014–2016 | Vietnam | 153 d | 66.1 | - | 38.1 | - | - | 30.7% e (47/153) | [25] |
| Binh et al. | 2008 | Vietnam | 103 | 33 | 69.9 | 18.4 | 0 | 5.8 | 24.3% (25/103) | [26] |
| Phan et al. | 2012–2014 | Vietnam | 92 d | 34.2 | 75.3 | 35.6 | 0 | - | 50.7% (37/73) for primary resistance and 78.9% (15/19) for secondary resistance | [27] |
| Author | Year | Name of AMP | Source | Finding on Antibacterial Activity against H. pylori | Reference |
|---|---|---|---|---|---|
| Zhang et al. | 2016 | Cathelicidin | Mouse and human | Bactericidal activity against clarithromycin-resistant H. pylori; anti-biofilm activity against H. pylori SS1 strain; protected mouse from H. pylori orchestrated inflammation and reduced H. pylori colonization | [28] |
| Guzman et al. | 2018 | Bicarinalin | Synthetically synthesized in lab (anti venom Tetramorium bicarinatum) | Perturbation of membrane permeability against drug-resistant H. pylori | [29] |
| Chen et al. | 2007 | Odorranain-HP | Synthetically synthesized in lab (Diskless odorous frog, Odorrana graham) | Showed antimicrobial activity against H. pylori (MIC of 20 µg/mL) | [30] |
| Narayana et al. | 2015 | Tilapia Piscidin 4 (TP4) | Synthetically synthesized in lab (Nile tilapia, Oreochromis niloticus) | Demonstrated potential lytic activity against H. pylori surface membrane; disrupted the bacterial cell membrane | [31] |
| Wang et al. | 2007 | Pleurain-A | Synthetically synthesized in lab (Yunnan frog, Rana pleuraden) | Inhibited growth of H. pylori in vitro (30 µg/mL) | [32] |
| Jiang et al. | 2020 | Cbf-K16(cathelicidin-like AMP) | Synthetically synthesized | Demonstrated bactericidal activity against clarithromycin- and amoxicillin-resistant H. pylori; reduced intercellular and intracellular drug-resistant H. pylori in cell culture; showed increased membrane permeation in drug-resistant H. pylori | [33] |
| Zhang et al. | 2018 | Fusion human neutrophil peptide 1 | Expression system in yeast | Eradication of wild type and drug-resistant H. pylori in animal model | [34] |
| Narayana et al. | 2015 | Epinecidin-1 | Synthetically synthesized in lab | Showed bactericidal activity against drug-resistant H. pylori and modulated immune response in mouse-infected H. pylori for bacterial clearance | [35] |
| Xiong et al. | 2017 | Helix–coil conformation transitional antimicrobial polypeptides | Synthetically synthesized in lab | Displayed bactericidal activity against H. pylori at low pH, both in vitro and in vivo | [36] |
| Zhang et al. | 2015 | Pexiganan | Synthetically synthesized in lab | Inhibited the growth of H. pylori (MIC = 4 µg/mL); decreased H. pylori colonization in animal model | [37] |
| Zhang et al. | 2017 | Fusion PGLa-AM1 | Synthetically synthesized in lab | Showed bactericidal activity against H. pylori in vitro and clearance of the bacteria in vivo | [38] |
| Makobongo et al. | 2012 | C12K-2β12 | Synthetically synthesized in lab | Ruptured H. pylori surface membrane for bactericidal effect; reduced colonization of H. pylori in gerbil model | [39] |
| Rigano et al. | 2012 | Tomato defensin | Synthetically synthesized in lab | Showed antibacterial activity against H. pylori at MIC: 15 µg/mL | [40] |
| Iwahori et al. | 1997 | Magainin 2 analog | Synthetically synthesized in lab | Inhibited growth of H. pylori in vitro | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukri, A.; Lopes, B.S.; Hanafiah, A. The Emergence of Multidrug-Resistant Helicobacter pylori in Southeast Asia: A Systematic Review on the Trends and Intervention Strategies Using Antimicrobial Peptides. Antibiotics 2021, 10, 1061. https://doi.org/10.3390/antibiotics10091061
Sukri A, Lopes BS, Hanafiah A. The Emergence of Multidrug-Resistant Helicobacter pylori in Southeast Asia: A Systematic Review on the Trends and Intervention Strategies Using Antimicrobial Peptides. Antibiotics. 2021; 10(9):1061. https://doi.org/10.3390/antibiotics10091061
Chicago/Turabian StyleSukri, Asif, Bruno S. Lopes, and Alfizah Hanafiah. 2021. "The Emergence of Multidrug-Resistant Helicobacter pylori in Southeast Asia: A Systematic Review on the Trends and Intervention Strategies Using Antimicrobial Peptides" Antibiotics 10, no. 9: 1061. https://doi.org/10.3390/antibiotics10091061
APA StyleSukri, A., Lopes, B. S., & Hanafiah, A. (2021). The Emergence of Multidrug-Resistant Helicobacter pylori in Southeast Asia: A Systematic Review on the Trends and Intervention Strategies Using Antimicrobial Peptides. Antibiotics, 10(9), 1061. https://doi.org/10.3390/antibiotics10091061






