Antibiotic Resistance Genes and Associated Phenotypes in Escherichia coli and Enterococcus from Cattle at Different Production Stages on a Dairy Farm in Central California
Abstract
:1. Introduction
2. Results
2.1. AMR Genes Detected in E. coli and Enterococcus spp.
2.2. Associations between AMR Genes, Bacterial Species, and Production Stages
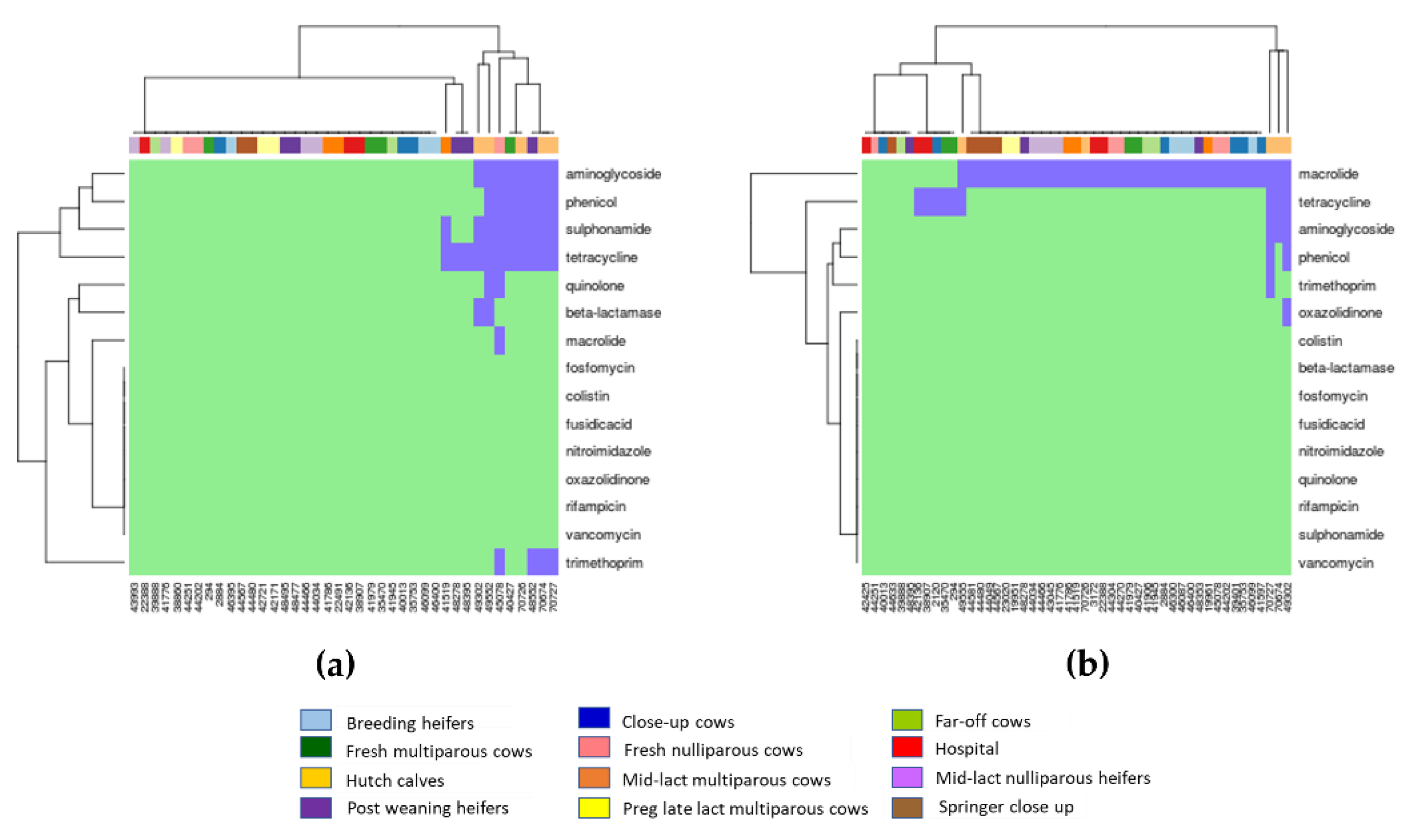
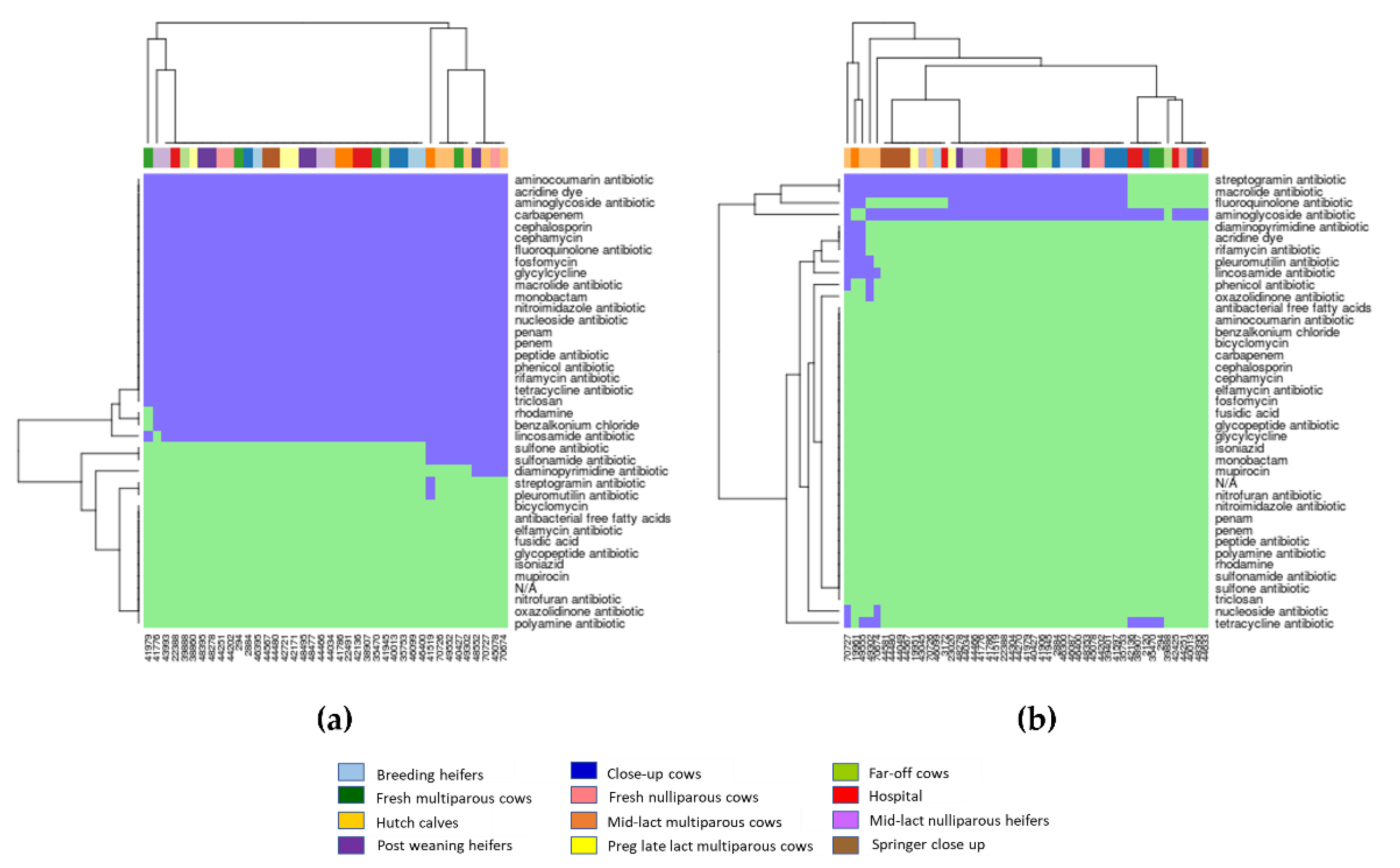
2.3. Associations between AMR Genotypes and Phenotypes
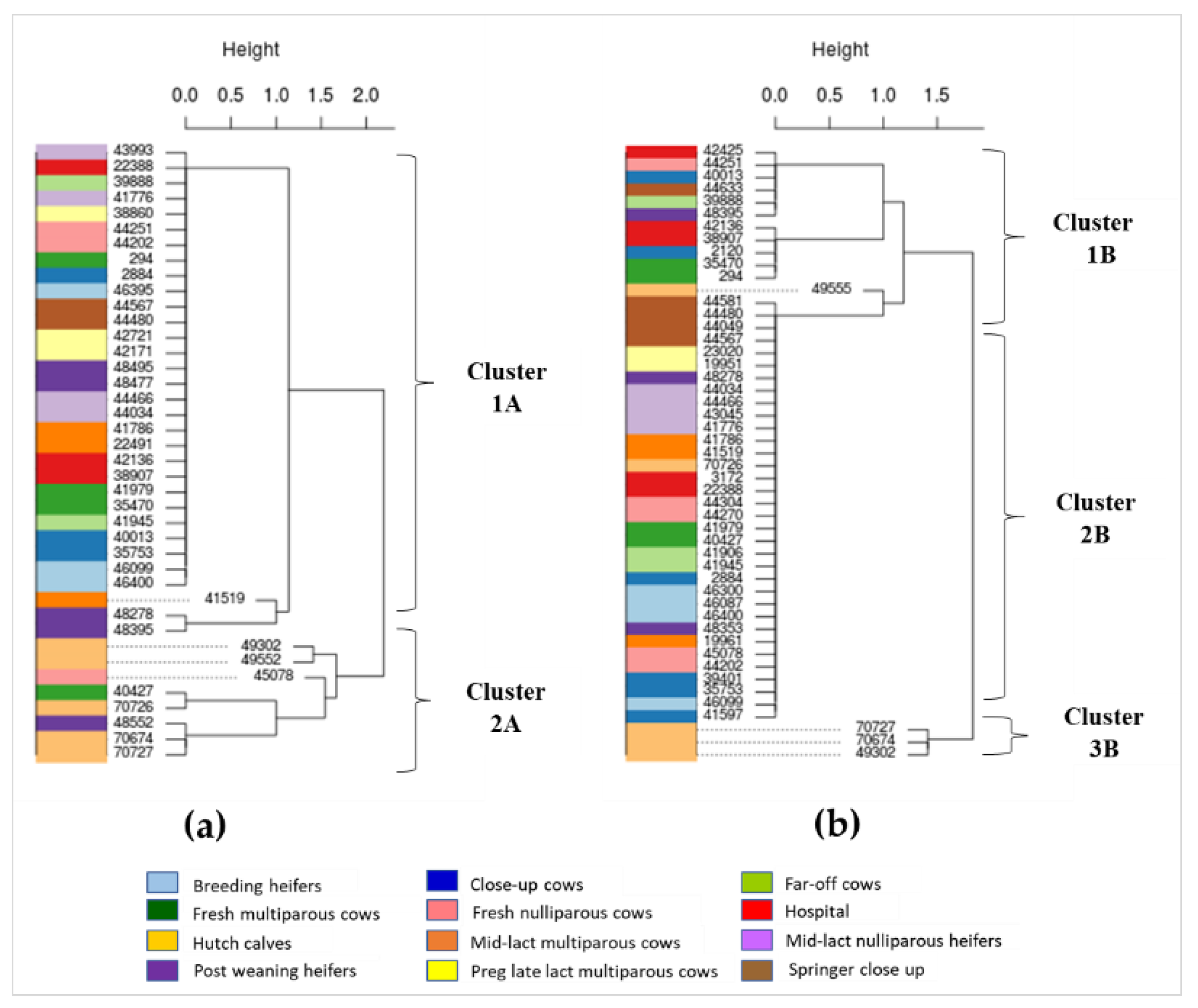
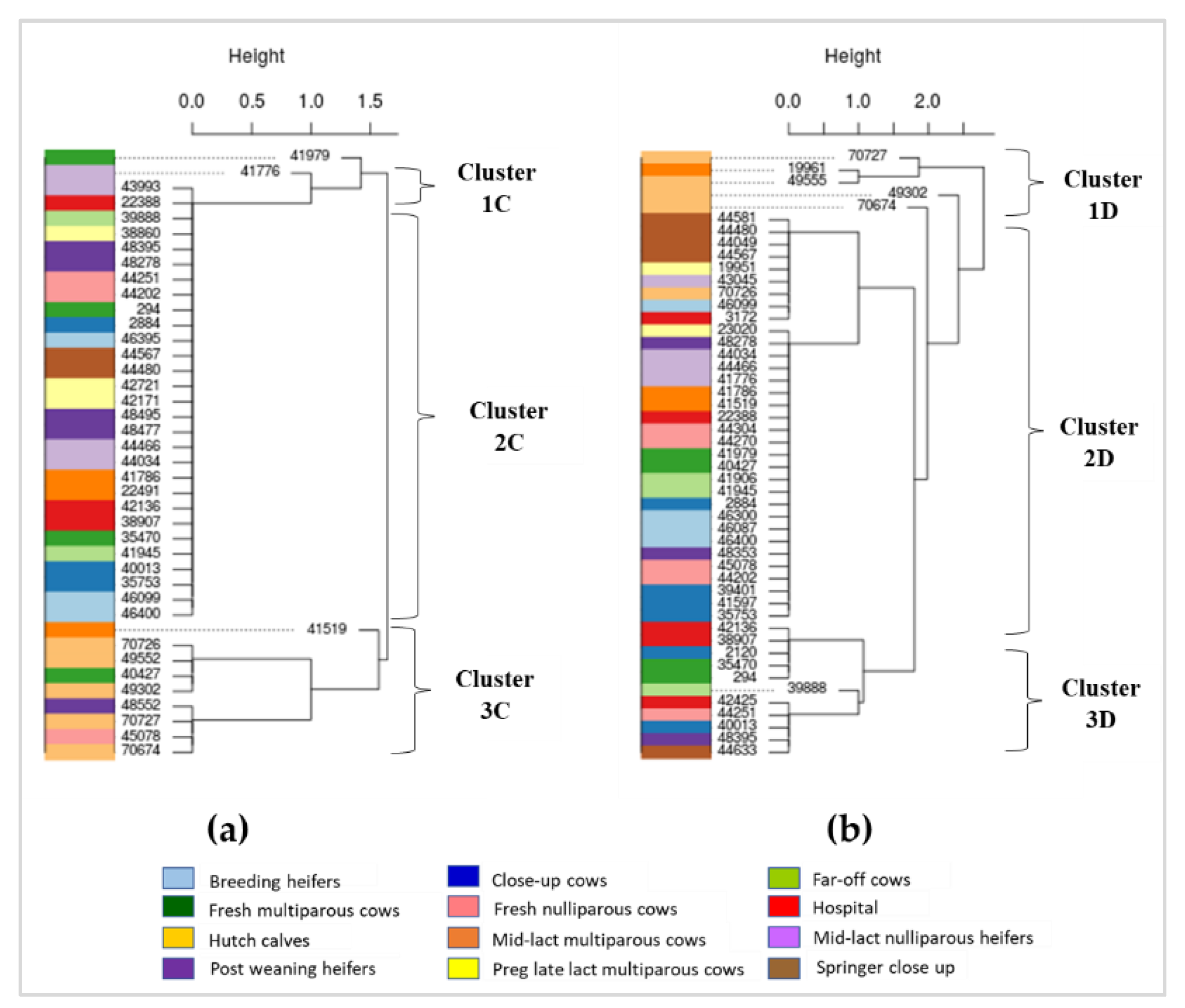
2.4. Phylogenetic Characterization of AMR Gens in Dairy Cattle Production Line
3. Discussion
3.1. Abundance of AMR Genes in E. coli and Enterococcus spp.
3.2. Associations between AMR Genes, Bacteria, and Production Stages
3.3. Correlations between AMR Genotypes and Phenotypes
3.4. Phylogenetics of AMR Genes in Cattle at Different Production Stages
4. Materials and Methods
4.1. Bacterial Isolates and Resistance Phenotypes
4.2. DNA Sequencing and Assembly
4.3. Bioinformatics Analysis
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of Antibiotic Use in Adult Dairy Cows on Antimicrobial Resistance of Veterinary and Human Pathogens: A Comprehensive Review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, H.; Finney, S.; Muñoz-Vargas, L.; Feicht, S.; Masterson, M.; Habing, G. Prevalence and Transmission of Antimicrobial Resistance in a Vertically Integrated Veal Calf Production System. Foodborne Pathog. Dis. 2017, 14, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Springer, H.R.; Denagamage, T.N.; Fenton, G.D.; Haley, B.J.; Van Kessel, J.A.S.; Hovingh, E.P. Antimicrobial Resistance in Fecal Escherichia coli and Salmonella enterica from Dairy Calves: A Systematic Review. Foodborne Pathog. Dis. 2019, 16, 23–34. [Google Scholar] [CrossRef]
- Li, X.; Aly, S.S.; Su, Z.; Pereira, R.; Williams, D.R.; Rossitto, P.; Champagne, J.D.; Chase, J.; Nguyen, T.; Atwill, E.R. Phenotypic Antimicrobial Resistance Profiles of E. coli and Enterococcus from Dairy Cattle in Different Management Units on a Central California Dairy. Clin. Microbiol. Open Access 2018, 7, 1–7. [Google Scholar] [CrossRef]
- USEPA. Ag 101 Lifecycle Production Phases. Available online: https://p2infohouse.org/ref/02/01244/www.epa.gov/agriculture/ag101/dairyphases.html (accessed on 21 August 2021).
- Noyes, N.R.; Yang, X.; Linke, L.M.; Magnuson, R.J.; Cook, S.R.; Zaheer, R.; Yang, H.; Woerner, D.R.; Geornaras, I.; McArt, J.; et al. Characterization of the resistome in manure, soil and wastewater from dairy and beef production systems. Sci. Rep. 2016, 6, 24645. [Google Scholar] [CrossRef]
- USDA. Dairy Cattle Management Practices in the United States, 2014. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_PartI_1.pdf (accessed on 13 January 2021).
- Khachatryan, A.R.; Hancock, D.D.; Besser, T.E.; Call, D.R. Role of Calf-Adapted Escherichia coli in Maintenance of Antimicrobial Drug Resistance in Dairy Calves. Appl. Environ. Microbiol. 2004, 70, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Chambers, L.; Yang, Y.; Littier, H.; Ray, P.; Zhang, T.; Pruden, A.; Strickland, M.; Knowlton, K. Metagenomic Analysis of Antibiotic Resistance Genes in Dairy Cow Feces following Therapeutic Administration of Third Generation Cephalosporin. PLoS ONE 2015, 10, e0133764. [Google Scholar] [CrossRef] [Green Version]
- Brennan, E.; Martins, M.; McCusker, M.P.; Wang, J.; Alves, B.M.; Hurley, D.; El Garch, F.; Woehrlé, F.; Miossec, C.; McGrath, L.; et al. Multidrug-ResistantEscherichia coliin Bovine Animals, Europe. Emerg. Infect. Dis. 2016, 22, 1650–1652. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, M.; Iglesias, M.R.; Rodriguez-Lazaro, D.; Gallardo, A.; Quijada, N.M.; Miguela-Villoldo, P.; Campos, M.J.; Píriz, S.; López-Orozco, G.; De Frutos, C.; et al. Co-occurrence of colistin-resistance genes mcr-1 and mcr-3 among multidrug-resistant Escherichia coli isolated from cattle, Spain, September 2015. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- Smith, R.; O’Hara, M.; Hobman, J.L.; Millard, A.D. Draft Genome Sequences of 14 Escherichia coli Phages Isolated from Cattle Slurry. Genome Announc. 2015, 3, e01364-15. [Google Scholar] [CrossRef] [Green Version]
- Webb, H.E.; Bugarel, M.; Bakker, H.C.D.; Nightingale, K.K.; Granier, S.A.; Scott, H.; Loneragan, G.H. Carbapenem-Resistant Bacteria Recovered from Faeces of Dairy Cattle in the High Plains Region of the USA. PLoS ONE 2016, 11, e0147363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDFA. California Agricultural Statistics Review 2018–2019. Available online: https://www.cdfa.ca.gov/statistics/PDFs/2018-2019AgReportnass.pdf (accessed on 13 January 2021).
- CDFA. California Agricultural Statistics Review 2017–2018. Available online: https://www.cdfa.ca.gov/statistics/PDFs/2017-18AgReport.pdf (accessed on 13 January 2021).
- Sawant, A.A.; Hegde, N.V.; Straley, B.A.; Donaldson, S.C.; Love, B.C.; Knabel, S.J.; Jayarao, B.M. Antimicrobial-Resistant Enteric Bacteria from Dairy Cattle. Appl. Environ. Microbiol. 2007, 73, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathers, J.J.; Flick, S.C.; Cox, L.A., Jr. Longer-duration uses of tetracyclines and penicillins in U.S. food-producing animals: Indications and microbiologic effects. Environ. Int. 2011, 37, 991–1004. [Google Scholar] [CrossRef]
- Jackson, C.R.; Lombard, J.E.; Dargatz, D.A.; Cray, P. Prevalence, species distribution and antimicrobial resistance of enterococci isolated from US dairy cattle. Lett. Appl. Microbiol. 2010, 52, 41–48. [Google Scholar] [CrossRef]
- Nam, H.M.; Lim, S.K.; Moon, J.S.; Kang, H.M.; Kim, J.M.; Jang, K.C.; Kang, M.; Joo, Y.S.; Jung, S.C. Antimicrobial Resistance of Enterococci Isolated from Mastitic Bovine Milk Samples in Korea. Zoonoses Public Health 2009, 57, e59–e64. [Google Scholar] [CrossRef]
- Collignon, P.; Powers, J.H.; Chiller, T.M.; Aidara-Kane, A.; Aarestrup, F. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies for the Use of Antimicrobials in Food Production Animals. Clin. Infect. Dis. 2009, 49, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Marosevic, D.; Kaevska, M.; Jaglic, Z. Resistance to the tetracyclines and macrolide-lincosamide-streptogramin group of antibiotics and its genetic linkage—A review. Ann. Agric. Environ. Med. 2017, 24, 338–344. [Google Scholar] [CrossRef]
- Kyselkovã, M.; Jirout, J.; Vrchotovã, N.; Schmitt, H.; Elhottovã, D. Spread of tetracycline resistance genes at a conventional dairy farm. Front. Microbiol. 2015, 6, 536. [Google Scholar] [CrossRef] [Green Version]
- Fàbrega-Santamaria, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2008, 2, 40–61. [Google Scholar] [CrossRef] [Green Version]
- Carroll, L.M.; Wiedmann, M.; Bakker, H.D.; Siler, J.; Warchocki, S.; Kent, D.; Lyalina, S.; Davis, M.; Sischo, W.; Besser, T.; et al. Whole-Genome Sequencing of Drug-Resistant Salmonella enterica Isolates from Dairy Cattle and Humans in New York and Washington States Reveals Source and Geographic Associations. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-Genome Sequencing for Detecting Antimicrobial Resistance in Nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef] [Green Version]
- Whitehouse, C.A.; Young, S.; Li, C.; Hsu, C.-H.; Martin, G.; Zhao, S. Use of whole-genome sequencing for Campylobacter surveillance from NARMS retail poultry in the United States in 2015. Food Microbiol. 2018, 73, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.H.; Sabo, J.L.; Rice-Trujillo, C.; Hernandez, J.; McDermott, P.F. Whole-genome sequencing based characterization of antimicrobial resistance in Enterococcus. Pathog. Dis. 2018, 76, 76. [Google Scholar] [CrossRef]
- Yamaba, Y.; Takakuwa, O.; Saito, M.; Kawae, D.; Yoshihara, M.; Mori, Y.; Kunii, E.; Ito, Y.; Yoshida, S.; Akita, K. Pulmonary Mycobacterium abscessus Subspecies abscessus Disease That Showed a Discrepancy Between the Genotype and Phenotype of Clarithromycin Resistance. Intern. Med. 2019, 58, 2675–2678. [Google Scholar] [CrossRef] [Green Version]
- Lob, S.H.; Biedenbach, D.J.; Badal, R.E.; Kazmierczak, K.M.; Sahm, D.F. Discrepancy between genotypic and phenotypic extended-spectrum β-lactamase rates in Escherichia coli from intra-abdominal infections in the USA. J. Med. Microbiol. 2016, 65, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.; Bard, J.D.; Simner, P.J. The Genotype-to-Phenotype Dilemma: How Should Laboratories Approach Discordant Susceptibility Results? J. Clin. Microbiol. 2021, 59, e00138-20. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Tsuyuguchi, K.; Kobayashi, T.; Tomita, M.; Inoue, Y.; Hayashi, S.; Suzuki, K. Discrepancies between the genotypes and phenotypes of clarithromycin-resistant Mycobacterium abscessus complex. Int. J. Tuberc. Lung Dis. 2018, 22, 413–418. [Google Scholar] [CrossRef]
- Pitta, D.W.; Dou, Z.; Kumar, S.; Indugu, N.; Toth, J.D.; Vecchiarelli, B.; Bhukya, B. Metagenomic Evidence of the Prevalence and Distribution Patterns of Antimicrobial Resistance Genes in Dairy Agroecosystems. Foodborne Pathog. Dis. 2016, 13, 296–302. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Source | Resistance Gene | Factor | Coeff. | Std. Err. | C.I. | p-Value |
|---|---|---|---|---|---|---|
| ResFinder | Macrolide | constant tetracycline | 1.735 −1.958 | 0.443 0.804 | 0.867 to 2.602 −3.533 to −0.382 | <0.0001 0.015 |
| CARD | Fluoroquinolone | constant tetracycline | 0.446 −1.700 | 0.320 0.863 | −0.181 to 1.074 −3.391 to −0.007 | 0.163 0.049 |
| Source | Genotype | Phenotype | Agreement (%) | Expected Agreement (%) | Kappa (%) | Std. Err. | p-Value |
|---|---|---|---|---|---|---|---|
| ResFinder a | TET | TET | 95.00 | 57.88 | 88.13 | 0.157 | <0.0001 |
| ResFinder a | SUL | SXT | 97.50 | 88.25 | 78.72 | 0.155 | <0.0001 |
| ResFinder a | TRI | SXT | 87.50 | 75.50 | 48.98 | 0.158 | 0.0002 |
| ResFinder a | BET | AMP | 97.50 | 88.25 | 78.72 | 0.136 | <0.0001 |
| ResFinder a | AMI | STR | 95.00 | 71.00 | 82.76 | 0.155 | <0.0001 |
| CARD a | TET | TET | 85.71 | 63.56 | 60.80 | 0.156 | <0.0001 |
| CARD a | AMI | STR | 89.80 | 83.13 | 39.51 | 0.137 | 0.0015 |
| ResFinder b | TET | TET | 85.71 | 63.56 | 60.80 | 0.133 | <0.0001 |
| Management Units | E. coli | Enterococcus |
|---|---|---|
| Hutch calves | 5 | 5 |
| Post-weaning heifers | 5 | 3 |
| Breeding heifers | 3 | 4 |
| Springers/Close-up yearlings | 2 | 5 |
| Fresh uniparous cows | 3 | 6 |
| Fresh multiparous cows | 4 | 3 |
| Mid lactation uniparous cows | 3 | 3 |
| Mid-lactation multiparous cows | 4 | 4 |
| Pregnant late lactation multiparous cows | 3 | 2 |
| Far-off multiparous cows | 2 | 3 |
| Close up multiparous cows | 3 | 6 |
| Hospital animals | 3 | 5 |
| Total | 40 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeamsripong, S.; Li, X.; Aly, S.S.; Su, Z.; Pereira, R.V.; Atwill, E.R. Antibiotic Resistance Genes and Associated Phenotypes in Escherichia coli and Enterococcus from Cattle at Different Production Stages on a Dairy Farm in Central California. Antibiotics 2021, 10, 1042. https://doi.org/10.3390/antibiotics10091042
Jeamsripong S, Li X, Aly SS, Su Z, Pereira RV, Atwill ER. Antibiotic Resistance Genes and Associated Phenotypes in Escherichia coli and Enterococcus from Cattle at Different Production Stages on a Dairy Farm in Central California. Antibiotics. 2021; 10(9):1042. https://doi.org/10.3390/antibiotics10091042
Chicago/Turabian StyleJeamsripong, Saharuetai, Xunde Li, Sharif S. Aly, Zhengchang Su, Richard V. Pereira, and Edward R. Atwill. 2021. "Antibiotic Resistance Genes and Associated Phenotypes in Escherichia coli and Enterococcus from Cattle at Different Production Stages on a Dairy Farm in Central California" Antibiotics 10, no. 9: 1042. https://doi.org/10.3390/antibiotics10091042
APA StyleJeamsripong, S., Li, X., Aly, S. S., Su, Z., Pereira, R. V., & Atwill, E. R. (2021). Antibiotic Resistance Genes and Associated Phenotypes in Escherichia coli and Enterococcus from Cattle at Different Production Stages on a Dairy Farm in Central California. Antibiotics, 10(9), 1042. https://doi.org/10.3390/antibiotics10091042







