Assigning Defined Daily/Course Doses for Antimicrobials in Turkeys to Enable a Cross-Country Quantification and Comparison of Antimicrobial Use
Abstract
1. Introduction
2. Results
2.1. Assigning Defined Daily Doses Turkey (DDDturkey) and Defined Course Dose Turkey (DCDturkey) for France, Germany and Spain
2.1.1. DDDTurkey and DCDTurkey
2.1.2. Deviation from DDDTurkey
Parenteral Products
Feed Products
Water Products
2.2. Quantification of Antimicrobial Usage at Flock Level on 60 Turkey Farms in France, Germany and Spain
2.2.1. AMU Quantification at Flock Level
2.2.2. Qualitative Description of AMU at Flock Level
Administration Route
Antimicrobial Classes
Indication for Treatment
AMU over the Period of one Production Cycle
3. Discussion
3.1. Establishing Defined Daily Doses
3.2. Variation in Recommended Doses
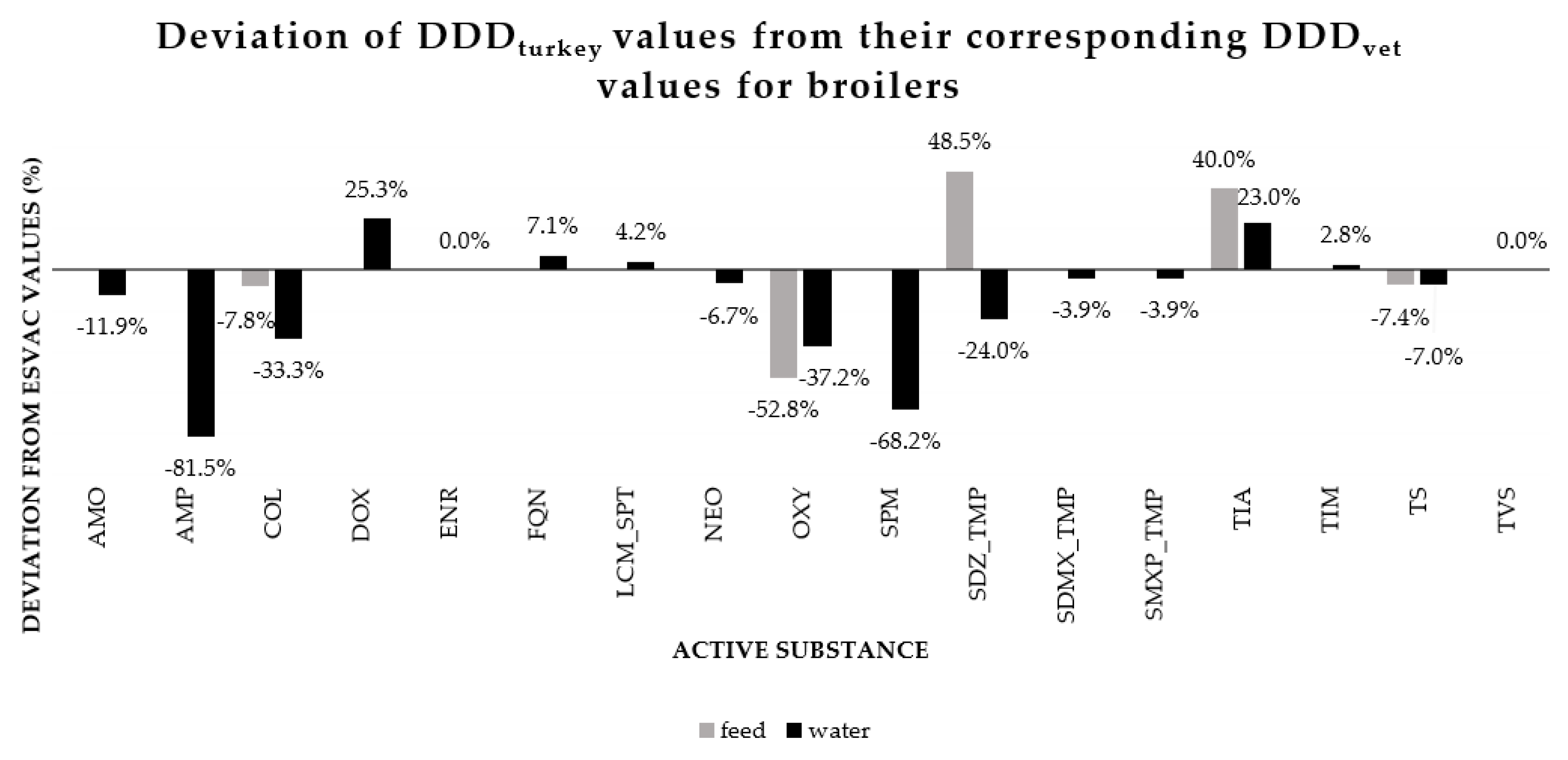
3.3. Comparision of DDDturkey and DDDvet
3.4. AMU at Turkey Farms
4. Materials and Methods
4.1. Assigning Defined Daily Doses Turkey (DDDturkey) and Defined Course Dose (DCDturkey) for France, Germany and Spain
4.1.1. Data Collection
4.1.2. Calculation of Defined Daily Dose Turkey (DDDturkey) and Defined Course Dose (DCDturkey)
4.2. Quantification of Antimicrobial Usage at Flock Level on 60 Turkey Farms in France, Germany and Spain
4.2.1. Study Sample and Data Collection
4.2.2. AMU Quantification
4.3. Data Processing and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cars, O.; Hogberg, L.; Murray, M.; Nordberg, O.; Sivaraman, S.; Lundborg, C.; So, A.; Tomson, G. Meeting the challenge of antibiotic resistance. BMJ 2008, 337, 726–728. [Google Scholar] [CrossRef]
- Snary, E.L.; Kelly, L.A.; Davison, H.C.; Teale, C.J.; Wooldridge, M. Antimicrobial resistance: A microbial risk assessment perspective. J. Antimicrob. Chemother. 2004, 53, 906–917. [Google Scholar] [CrossRef]
- Interagency Coordination Group on Antimicrobial Resistance (IACG). No Time To Wait: Securing the Future from Drug-Resistant Infections Report to the Secretary-General of the United Nations; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- European Medicines Agency (EMA); Committee for Medicinal Products for Veterinary Use (CVMP). Reflection Paper on the Risk of Antimicrobial Resistance Transfer from Companion Animals; EMA: Amsterdam, The Netherlands, 2015.
- Klous, G.; Huss, A.; Heederik, D.J.J.; Coutinho, R.A. Human-livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health 2016, 2, 65–76. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Borck Høg, B.; Dalhoff Andersen, V.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018; EMA: Amsterdam, The Netherlands, 2020.
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 19 EU/EEA Countries in 2010; EMA: Amsterdam, The Netherlands, 2012.
- Collineau, L.; Belloc, C.; Stärk, K.D.C.; Hémonic, A.; Postma, M.; Dewulf, J.; Chauvin, C. Guidance on the Selection of Appropriate Indicators for Quantification of Antimicrobial Usage in Humans and Animals. Zoonoses Public Health 2017, 64, 165–184. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Revised ESVAC Reflection Paper on Collecting Data on Consumption of Antimicrobial Agents per Animal Species, on Technical Units of Measurement and Indicators for Reporting Consumption of Antimicrobial Agents in Animals; EMA: Amsterdam, The Netherlands, 2013.
- Agunos, A.; Gow, S.P.; Leger, D.F.; Carson, C.A.; Deckert, A.E.; Bosman, A.L.; Loest, D.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial use and antimicrobial resistance indicators-integration of farm-level surveillance data from broiler chickens and turkeys in British Columbia, Canada. Front. Vet. Sci. 2019, 6, 131. [Google Scholar] [CrossRef]
- Autoriteit Diergeneesmiddelen (SDa). Het Gebruik van Antibiotica bij Landbouwhuisdieren in 2017 Trends, Benchmarken Bedrijven en Dierenartsen; SDa: Utrecht, The Netherlands, 2018. [Google Scholar]
- Chauvin, C.; Le Bouquin-Leneveu, S.; Hardy, A.; Haguet, D.; Orand, J.P.; Sanders, P. An original system for the continuous monitoring of antimicrobial use in poultry production in France. J. Vet. Pharmacol. Ther. 2005, 28, 515–523. [Google Scholar] [CrossRef]
- ITAVI. Réseau Professionnel de Références sur les Usages d’Antibiotiques en Élevage Avicole Synthèse des Résultats du Réseau; ITAVI: Paris, France, 2019. [Google Scholar]
- Caucci, C.; Di Martino, G.; Dalla Costa, A.; Santagiuliana, M.; Lorenzetto, M.; Capello, K.; Mughini-Gras, L.; Gavazzi, L.; Bonfanti, L. Trends and correlates of antimicrobial use in broiler and Turkey farms: A poultry company registry-based study in Italy. J. Antimicrob. Chemother. 2019, 74, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Griesbacher, A.; Schließnig, H.; Weber, S.; Fuchs, K. Antimicrobial use in poultry flocks in Austria between 2013 and 2019. Vet. Rec. 2021, e508. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Defined Daily Doses for Animals (DDDvet ) and Defined Course Doses for Animals (DCDvet): European Surveillance of Veterinary Antimicrobial Consumption (ESVAC); EMA: Amsterdam, The Netherlands, 2016.
- Eurostat Agriculture. Forestry and Fishery Statistics—2020 Edition-Products Statistical Books; Eurostat: Luxembourg, 2015. [Google Scholar]
- European Parliament and The Council Regulation (EU) 2019/of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC; European Parliament: Strasbourg, France, 2018.
- European Medicines Agency (EMA). Principles on Assignment of Defined Daily Dose for Animals (DDDvet) and Defined Course Dose for Animals (DCDvet); EMA: Amsterdam, The Netherlands, 2015.
- Postma, M.; Sjölund, M.; Collineau, L.; Lösken, S.; Stärk, K.D.C.; Dewulf, J.; Andreasen, M.; Ingelheim, B.; Backhans, A.; Belloc, C.; et al. Assigning defined daily doses animal: A European multi-country experience for antimicrobial products authorized for usage in pigs. J. Antimicrob. Chemother. 2015, 70, 294–302. [Google Scholar] [CrossRef]
- Landoni, M.F.; Albarellos, G. The use of antimicrobial agents in broiler chickens. Vet. J. 2015, 205, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Persoons, D.; Dewulf, J.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Butaye, P.; Haesebrouck, F. Antimicrobial use in Belgian broiler production. Prev. Vet. Med. 2012, 105, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Léger, D.F.; Carson, C.A.; Gow, S.P.; Bosman, A.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial use surveillance in broiler chicken flocks in Canada, 2013–2015. PLoS ONE 2017, 12, e0179384. [Google Scholar]
- Taverne, F.J.; Jacobs, J.H.; Heederik, D.J.J.; Mouton, J.W.; Wagenaar, J.A.; van Geijlswijk, I.M. Influence of applying different units of measurement on reporting antimicrobial consumption data for pig farms. BMC Vet. Res. 2015, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Reflection Paper on Dose Review and Adjustment of Established Veterinary Antibiotics in the Context Summary of Product Characteristic (SPC) Harmonization; Committee for Medicinal Products for Veterinary Use (CVMP) Reflection, EMA: Amsterdam, The Netherlands, 2020.
- European Medicines Agency (EMA). Enrofloxacin-Article 35-Annexes I, II, III, IV; EMA: Amsterdam, The Netherlands, 2018.
- VETIDATA. Available online: https://www.vetidata.de/public/index.php (accessed on 7 April 2021).
- Index des RCP. Available online: http://www.ircp.anmv.anses.fr/ (accessed on 7 April 2021).
- Watteyn, A.; Croubels, S.; De Baere, S.; De Backer, P.; Devreese, M. Pharmacokinetics of florfenicol in Turkey plasma, lung tissue, and pulmonary epithelial lining fluid after single oral bolus or continuous administration in the drinking water. Poult. Sci. 2018, 97, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; El-Kattan, Y.A. Comparative pharmacokinetics of florfenicol in the chicken, pigeon and quail. Br. Poult. Sci. 2009, 50, 144–149. [Google Scholar] [CrossRef]
- Chauvin, C.; Bouvarel, I.; Beloeil, P.-A.; Orand, J.-P.; Guillemot, D.; Sanders, P. A pharmaco-epidemiological analysis of factors associated with antimicrobial consumption level in turkey broiler flocks. Vet. Res. 2005, 36, 199–211. [Google Scholar] [CrossRef][Green Version]
- Joosten, P.; Sarrazin, S.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; Graveland, H.; Schmitt, H.; et al. Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J. Antimicrob. Chemother. 2019, 74, 798–806. [Google Scholar] [CrossRef]
- British Poultry Council BPC. Antibiotics Stewardship Report 2020: Sustainable Use of Antibiotics; British Poultry Council BPC: London, UK, 2020. [Google Scholar]
- National Institute for Public Health and the Environment of the Netherlands. NethMap 2020,Consumption of Antimicrobial Agents and Antimicrobial Resistance among Medically Important Bacteria in the Netherlands; National Institute for Public Health and the Environment of the Netherlands: Bilthoven, The Netherlands, 2020. [Google Scholar]
- Huff, G.; Huff, W.; Rath, N.; Donoghue, A.; Anthony, N.; Nestor, K. Differential effects of sex and genetics on behavior and stress response of Turkeys. Poult. Sci. 2007, 86, 1294–1303. [Google Scholar] [CrossRef]
- Di Martino, G.; Capello, K.; Russo, E.; Mazzucato, M.; Mulatti, P.; Ferrè, N.; Garbo, A.; Brichese, M.; Marangon, S.; Bonfanti, L. Factors associated with pre-slaughter mortality in turkeys and end of lay hens. Animal 2017, 11, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M. Enteric diseases of poultry with special attention to Clostridium perfringens. Pak. Vet. J. 2011, 31, 175–184. [Google Scholar]
- Sarrazin, S.; Joosten, P.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; Wagenaar, J.; Graveland, H.; et al. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J. Antimicrob. Chemother. 2019, 74, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Moreki, J. Commercial Turkey Production. 2007. Available online: https://www.researchgate.net/publication/228503047 (accessed on 1 June 2021).
- Hafez, H.M.; Shehata, A.A. Turkey production and health: Current challenges. Ger. J. Vet. Res. 2021, 1, 3–14. [Google Scholar] [CrossRef]
- CIMAVet. Centro de Información de Medicamentos para Veterinaria. Available online: https://cimavet.aemps.es/cimavet/publico/home.html (accessed on 7 April 2021).
- WHO Collaborating Centre for Drug Statistics Methodology WHOCC-ATCvet Index. Available online: https://www.whocc.no/atcvet/atcvet_index/?code=QJ (accessed on 7 April 2021).
- European Medicines Agency (EMA). European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Web Based Sales Data and Animal Population Data Collection Protocol (version 2); EMA: Amsterdam, The Netherlands, 2016.
- World Health Organisation (WHO). Critically Important Antimicrobials for Human Medicine 5th Revision 2016 Ranking of Medically Important Antimicrobials for Risk Management of Antimicrobial Resistance Due to non-Human Use; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- World organisation for animal health (OIE). OIE list of Antimicrobial Agents of Veterinary Importance; OIE: Paris, France, 2018. [Google Scholar]
- Hybrid Turkeys Performance Goals XL Commercial Males. Available online: https://www.hybridturkeys.com/documents/369/PG_XL_CS_E_L_KG_08_17.pdf (accessed on 17 July 2021).
- Eurostat Eurostat—Data Explorer. Available online: http://appsso.eurostat.ec.europa.eu/nui/show.do (accessed on 6 August 2021).



| Active Substance | Consensus DDDturkey (n) | Consensus DCDturkey | DDDvet | DCDvet | ||||
|---|---|---|---|---|---|---|---|---|
| Feed | Injection | Water | Feed | Injection | Water | Feed/Water | Feed/Water | |
| Amoxicillin | - | - | 14.1 (25) | - | - | 60.0 | 16.0 | 74.0 |
| Ampicillin * | - | 10.5 (1) | 20.0 (4) | - | 31.5 | 60.0 | 108.0 | 453.0 |
| Benzylpenicillin * | - | - | 28.8 (1) | - | - | 115 | - | - |
| Colistin | 4.7 (3) | 2.5 (2) | 3.4 (31) | 32.7 | 7.5 | 13.8 | 5.1 | 27.0 |
| Doxycycline | - | - | 18.8 (37) | - | - | 90.4 | 15.0 | 61.0 |
| Enrofloxacin | - | - | 10.0 (29) | - | - | 40.0 | 10.0 | 41.0 |
| Flumequine | - | - | 15.0 (10) | - | - | 66.0 | 14.0 | 60.0 |
| Linco_spec | - | 30.0 (2) | 62.5 (2) | - | 90.0 | 287.2 | 60.0 | 112.0 |
| Neomycin | - | - | 22.4 (9) | - | - | 93.7 | 24.0 | 114.0 |
| Oxytetracycline * | 18.4 (9) | 7.5 (1) | 24.5 (18) | 180.9 | 30.0 | 106.7 | 39.0 | 207.0 |
| Phenoxymethylpenicillin ** | - | - | 16.8 (6) | - | - | 83.8 | 17.0 | 84.0 |
| Spiramycin | - | - | 23.3 (2) | - | - | 89.1 | 73.0 | 459.0 |
| Sulfadiazine_TMP | 60.0 (3) | - | 30.7 (5) | 420.0 | - | 165.6 | 40.4 | 219.0 |
| Sulfadimethoxine_TMP | - | - | 36.0 (7) | - | - | 190.3 | 37.4 | 161.0 |
| Sulfamethoxypyridazine_TMP * | - | - | 28.2 (1) | - | - | 113.0 | 29.4 | 93.0 |
| Tiamulin | 32.2 (4) | - | 28.3 (12) | 224.8 | - | 130.2 | 23.0 | 88.0 |
| Tilmicosin | - | - | 18.5 (6) | - | - | 55.5 | 18.0 | 53.0 |
| Tylosin | 75 (6) | - | 75.3 (17) | 262.5 | - | 280.2 | 81.0 | 342.0 |
| Tylvalosin | - | - | 25.0 (3) | - | - | 125.0 | 25.0 | 75.0 |
| No. | Active Substance | Administration Route | Country | Product Name | Consensus DDDturkey | Mean Recommended Dose from SPC ** | Deviation from DDDturkey (%) * |
|---|---|---|---|---|---|---|---|
| 1 | oxytetracycline | water | Germany | Ursocyclin-Pulver® 20% | 24.5 | 80 | +227% |
| 2 | oxytetracycline | feed | France | VO 31-2 Oxytetracycline® | 18.4 | 40 | +117% |
| 3 | oxytetracycline | feed | France | Oxytetracycline® 40-CR® | 18.4 | 40 | +117% |
| 4 | oxytetracycline | feed | France | PM 17-A Oxytetracycline® 40 | 18.4 | 40 | +117% |
| 5 | oxytetracycline | water | Spain | Terramicina® 55 | 24.5 | 50 | +104% |
| 6 | sulfadimethoxine | water | France | Trimethosulfa® V | 36.0 | 46.7 | +58% |
| 7 | neomycine | water | Spain | Nemicina® | 22.4 | 33.3 | +48% |
| 8 | colistin | water | Germany | Belacol® 100% | 3.4 | 4.8 | +41% |
| 9 | colistin | water | Germany | Belacol® 24% | 3.4 | 4.8 | +41% |
| 10 | colistin | water | Germany | Colistin® 2,4 | 3.4 | 4.8 | +41% |
| 11 | doxycycline | water | France | Ronaxan® P.S. 5% | 18.8 | 10 | −47% |
| 12 | doxycycline | water | Germany | Doxipulvis® 500 | 18.8 | 10 | −47% |
| 13 | doxycycline | water | Spain | Doxi® 100 | 18.8 | 10 | −47% |
| 14 | doxycycline | water | Spain | Doxipulvis® 500 | 18.8 | 10 | −47% |
| 15 | oxytetracycline | water | Spain | Oxitetraciclina® lagro | 24.5 | 12.5 | −49% |
| 16 | neomycine | water | Spain | Nisocline® | 22.4 | 7.5 | −67% |
| 17 | neomycine | water | Spain | Neocil® premix | 22.4 | 7.5 | −67% |
| 18 | oxytetracycline | feed | Spain | Oxitetraciclina® BMP | 18.4 | 10.2 | −72% |
| 19 | oxytetracycline | feed | Spain | Oxitetraciclina® 100 | 18.4 | 10.2 | −72% |
| 20 | oxytetracycline | feed | Spain | Z-19® | 18.4 | 10.2 | −72% |
| Country (n) | NNUF* | TIDDDturkey-FL Med [95% CI] (Min-Max) | TIDDDvet-FL Med [95% CI] (Min-Max) | TIDCDturkey-FL Med [95% CI] (Min-Max) | TIDCDvet-FL Med [95% CI] (Min-Max) | TIUDD-FL Med [95% CI] (Min-Max) |
|---|---|---|---|---|---|---|
| B | 3 | 8.0 [4.3–11.6] | 6.0 [3.9–11.2] | 1.9 [1.1–2.6] | 1.3 [0.9–2.9] | 2.7 [2.1–3.1] |
| (20) | (0.0; 16.9) | (0.0; 17.6) | (0.0; 4.0) | (0.0; 4.1) | (0.0; 4.3) | |
| E | 3 | 8.5 [1.5–12.2] | 6.0 [1.3–10.5] | 2.1 [0.8–3.7] | 1.2 [0.3–2.0] | 2.4 [1.8–3.1] |
| (20) | (0.0; 25.9) | (0.0; 17.0) | (0.0; 6.8) | (0.0; 3.9) | (0.0; 5.7) | |
| H | 1 | 12.0 [10.1–15.1] | 11.3 [9.0–13.7] | 2.9 [2.4–3.5] | 2.5 [2.1–3.2] | 4.6 [3.5–4.8] |
| (20) | (0.0; 65.7) | (0.0; 57.8) | (0.0; 16.1) | (0.0; 13.4) | (0.0; 6.6) | |
| Total | 7 | 10.0 [8.0–11.9] | 8.7 [6.1–11.4] | 2.4. [1.9–2.8] | 1.9 [1.4–2.5] | 3.0 [2.7–3.6] |
| (60) | (0.0; 65.7) | (0.0; 57.8) | (0.0; 16.1) | (0.0; 13.4) | (0.0; 6.6) |
| Formula | Result | |
|---|---|---|
| TIDDDturkey 1 | Nr. of DDDturkey/100 days at risk/day | |
| TIDCDturkey 2 | Nr. of DCDturkey/100 days at risk | |
| TIUDD | Nr. of UDD/100 days at risk/day | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joosten, P.; Sarrazin, S.; Chauvin, C.; Moyano, G.; Wadepohl, K.; Van Gompel, L.; Wagenaar, J.A.; Dewulf, J.; on behalf of the [EFFORT group]. Assigning Defined Daily/Course Doses for Antimicrobials in Turkeys to Enable a Cross-Country Quantification and Comparison of Antimicrobial Use. Antibiotics 2021, 10, 971. https://doi.org/10.3390/antibiotics10080971
Joosten P, Sarrazin S, Chauvin C, Moyano G, Wadepohl K, Van Gompel L, Wagenaar JA, Dewulf J, on behalf of the [EFFORT group]. Assigning Defined Daily/Course Doses for Antimicrobials in Turkeys to Enable a Cross-Country Quantification and Comparison of Antimicrobial Use. Antibiotics. 2021; 10(8):971. https://doi.org/10.3390/antibiotics10080971
Chicago/Turabian StyleJoosten, Philip, Steven Sarrazin, Claire Chauvin, Gabriel Moyano, Katharina Wadepohl, Liese Van Gompel, Jaap A. Wagenaar, Jeroen Dewulf, and on behalf of the [EFFORT group]. 2021. "Assigning Defined Daily/Course Doses for Antimicrobials in Turkeys to Enable a Cross-Country Quantification and Comparison of Antimicrobial Use" Antibiotics 10, no. 8: 971. https://doi.org/10.3390/antibiotics10080971
APA StyleJoosten, P., Sarrazin, S., Chauvin, C., Moyano, G., Wadepohl, K., Van Gompel, L., Wagenaar, J. A., Dewulf, J., & on behalf of the [EFFORT group]. (2021). Assigning Defined Daily/Course Doses for Antimicrobials in Turkeys to Enable a Cross-Country Quantification and Comparison of Antimicrobial Use. Antibiotics, 10(8), 971. https://doi.org/10.3390/antibiotics10080971







