Investigating Ghanaian Allium Species for Anti-Infective and Resistance-Reversal Natural Product Leads to Mitigate Multidrug-Resistance in Tuberculosis
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Screening for the Allium Extracts
2.2. Allium Extracts Demonstrated Antibacterial Growth Inhibition
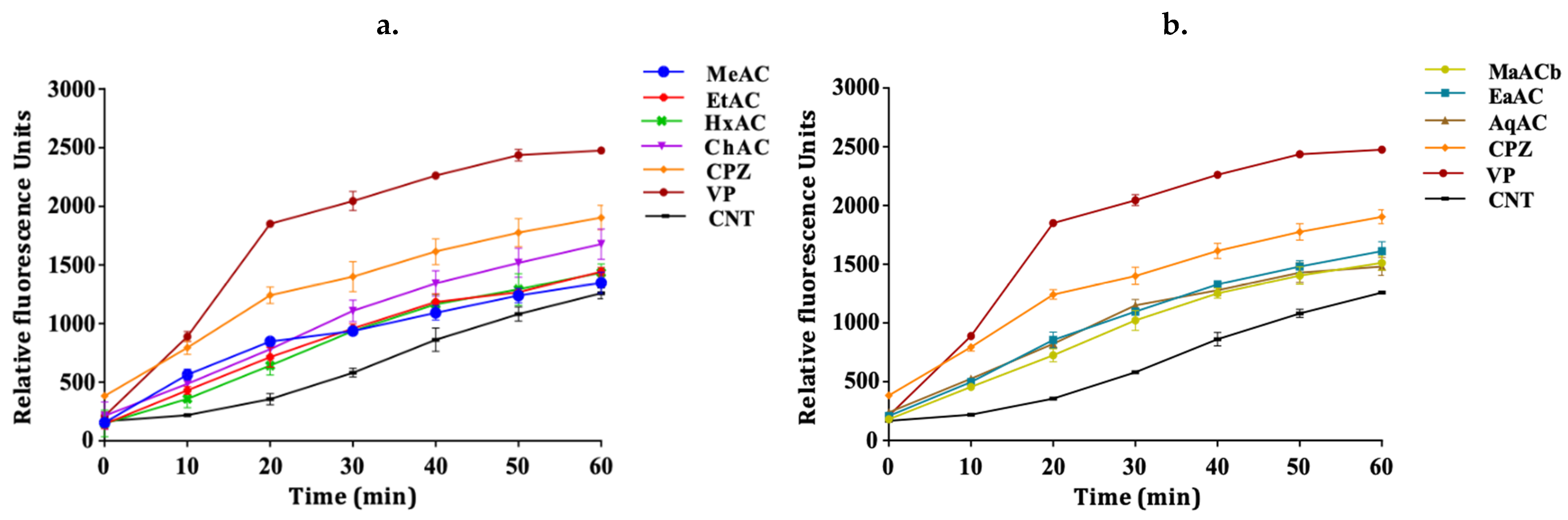
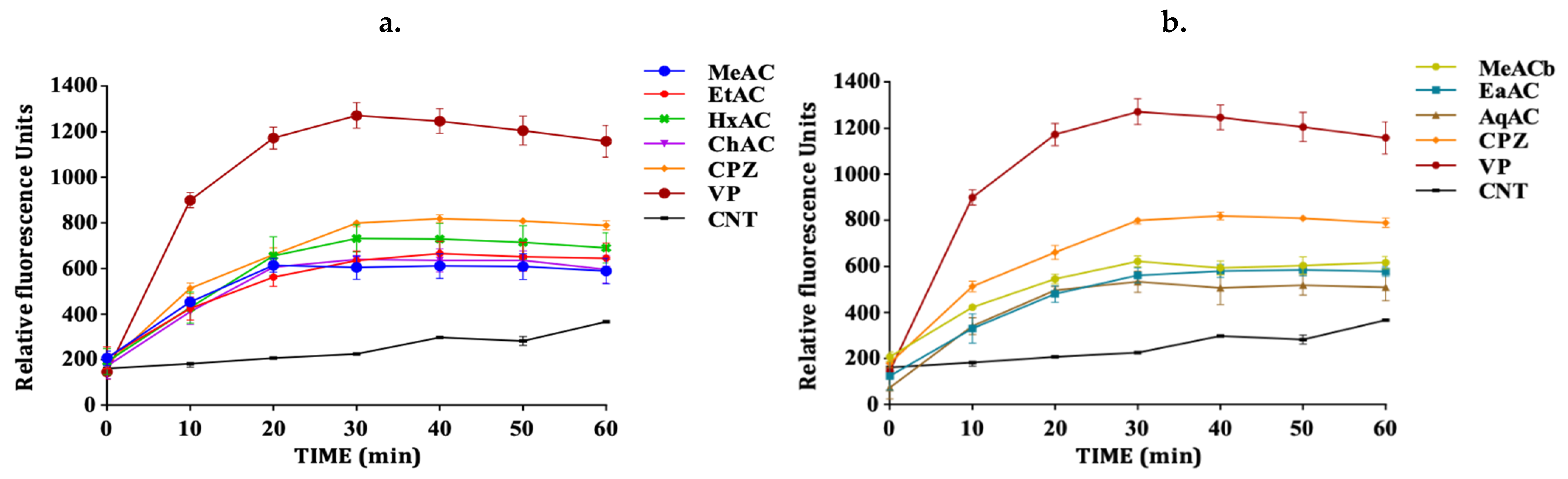
2.3. Allium Extracts Demonstrate Efflux Pump Inhibitory Effect
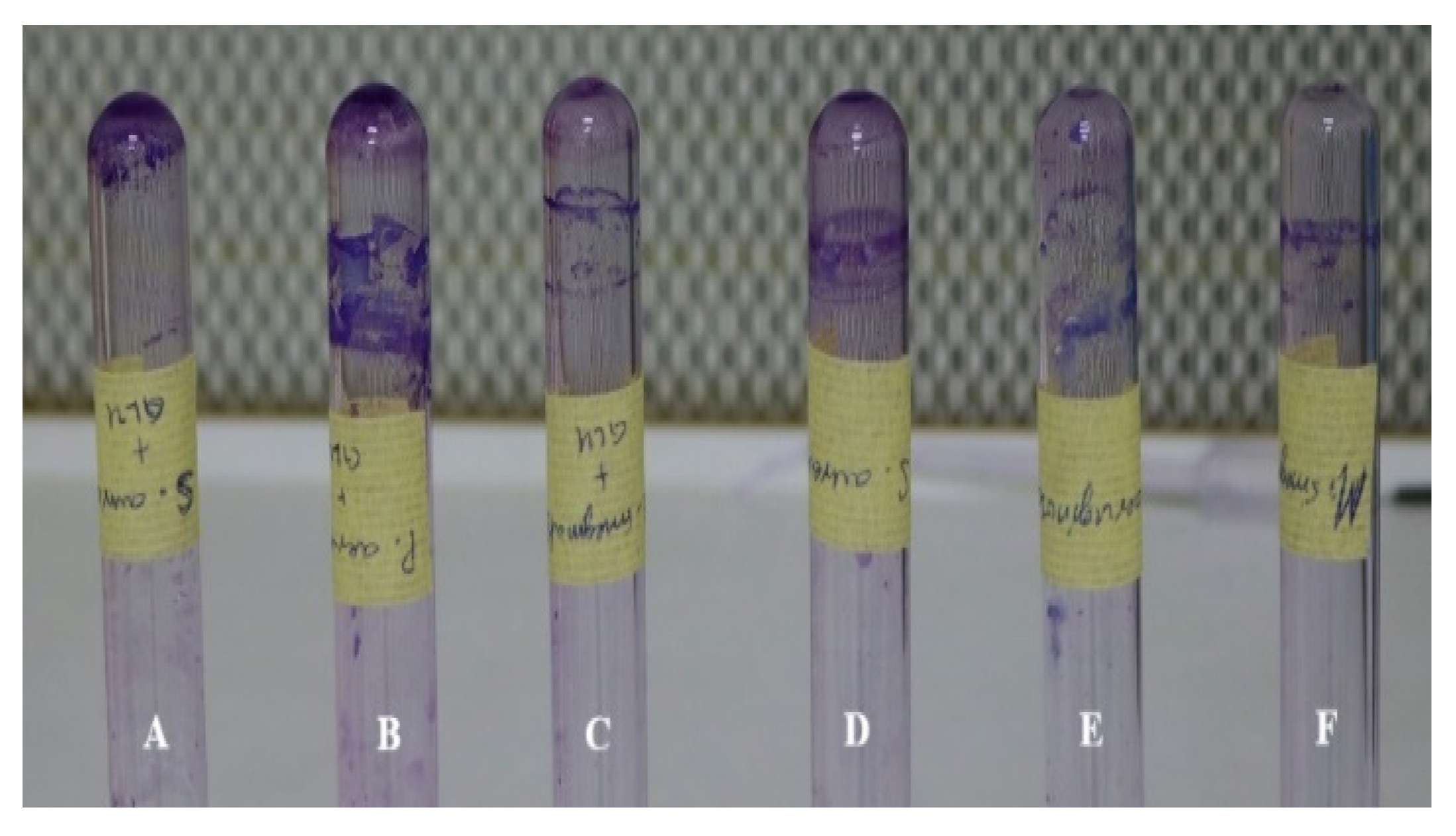
2.4. Biofilm Formation
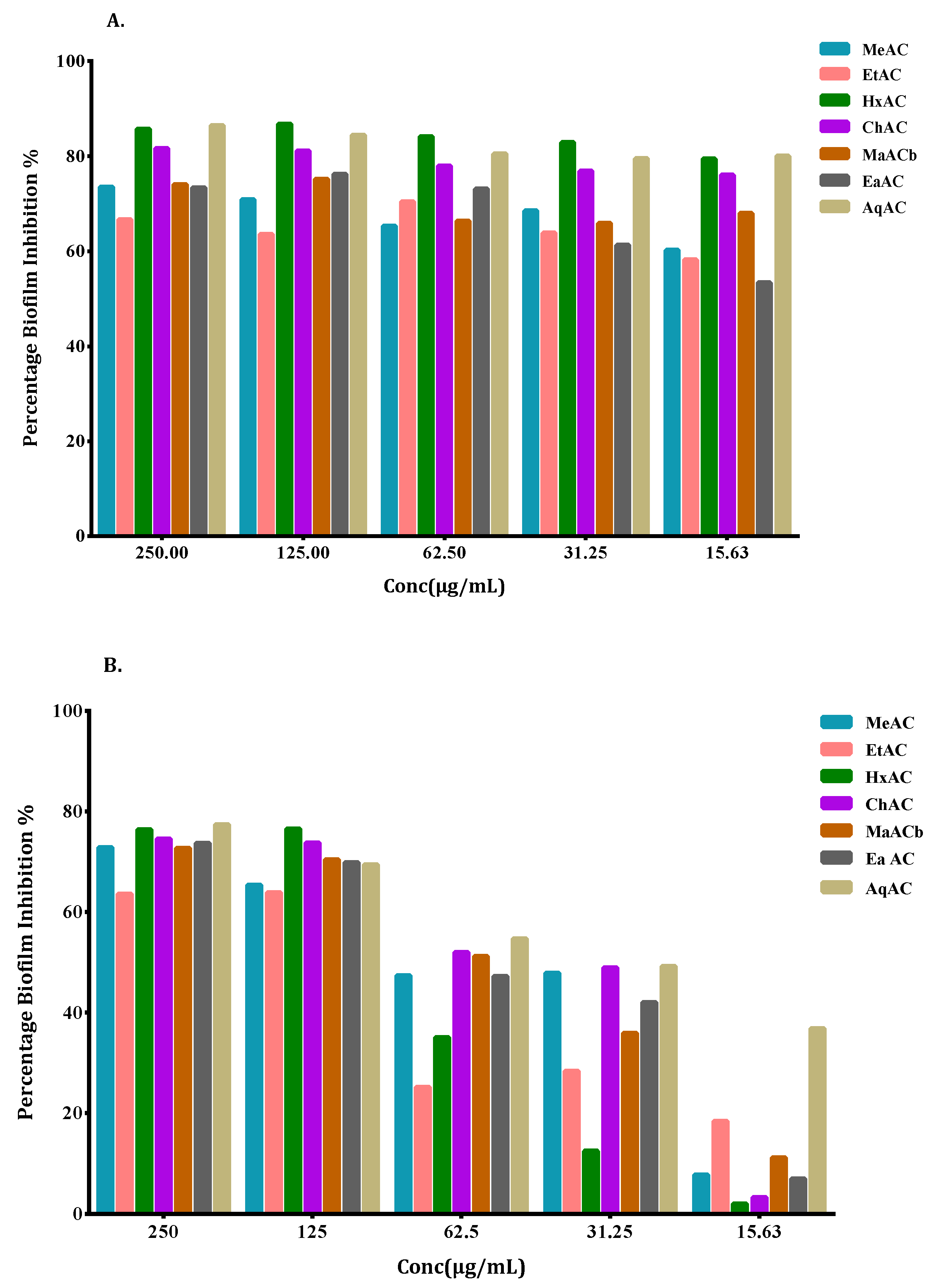
2.5. Allium Extracts Demonstrate Biofilm Inhibition
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Collection of Plant Material and Extraction
4.3. Microbial Strains and Culture Conditions
4.4. Methods
4.4.1. Antimicrobial Susceptibility Assay with HT-SPOTi
4.4.2. Efflux Pump Inhibitory Assay
4.4.3. Biofilm Inhibition Assay
Evaluation of Biofilm Forming Potential
Tube Method
Microtitre-Plate Method
Inhibition of Biofilm Formation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance 2014; World Health Organization: Geneva, Switzerland, 2014; ISBN 9789241564748. [Google Scholar]
- Interagency Coordination Group (IACG). No Time to Wait: Infections from Drug-Resistant Securing the Future from Drug-Resistant Infections; Artforum International Magazine: New York, NY, USA, 2019. [Google Scholar]
- Bretagne, G.; O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- McKeegan, K.S.; Borges-Walmsley, M.I.; Walmsley, A.R. The structure and function of drug pumps: An update. Trends Microbiol. 2003, 11, 21–29. [Google Scholar] [CrossRef]
- Rossi, E.D.; Aínsa, J.A.; Riccardi, G. Role of mycobacterial efflux transporters in drug resistance: An unresolved question. FEMS Microbiol. Rev. 2006, 30, 36–52. [Google Scholar] [CrossRef]
- Viveiros, M.; Leandro, C.; Amaral, L. Mycobacterial efflux pumps and chemotherapeutic implications. Int. J. Antimicrob. Agents 2003, 22, 274–278. [Google Scholar] [CrossRef]
- Ojha, A.K.; Baughn, A.D.; Sambandan, D.; Hsu, T.; Trivelli, X.; Guerardel, Y.; Alahari, A.; Kremer, L.; Jacobs, W.R., Jr.; Hatfull, G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008, 69, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.G.; Lister, T.; May-Dracka, T.L. New natural products as new leads for antibacterial drug discovery. Bioorganic Med. Chem. Lett. 2014, 24, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Karbasizade, V.; Dehghan, P.; Sichani, M.M.; Shahanipoor, K.; Sepahvand, S.; Jafari, R.; Yousefian, R. Evaluation of three plant extracts against biofilm formation and expression of quorum sensing regulated virulence factors in Pseudomonas aeruginosa. Pak. J. Pharm. Sci. 2017, 3, 585–589. [Google Scholar]
- Shriram, V.; Khare, T.; Bhagwat, R.; Shukla, R.; Kumar, V. Inhibiting bacterial drug efflux pumps via phyto-therapeutics to combat threatening antimicrobial resistance. Front. Microbiol. 2018, 9, 2990. [Google Scholar] [CrossRef]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G.A. Medicinal plants used to treat TB in Ghana. Int. J. Mycobacteriol. 2015, 4, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Minkah, P.A.B.; Danquah, C.A. Anti-infective, anti-inflammatory and antipyretic activities of the bulb extracts of Crinum jagus (J. Thomps.) Dandy (Amaryllidaceae). Sci. Afr. 2021, 12, e00723. [Google Scholar] [CrossRef]
- Bruns, M.M.; Kakarla, P.; Floyd, J.T.; Mukherjee, M.M.; Ponce, R.C.; Garcia, J.A.; Ranaweera, I.; Sanford, L.M.; Hernandez, A.J.; Willmon, M.T.; et al. Modulation of the multidrug efflux pump EmrD-3 from Vibrio cholerae by Allium sativum extract and the bioactive agent allyl sulfide plus synergistic enhancement of antimicrobial susceptibility by A. sativum extract. Arch. Microbiol. 2017, 99, 1103–1112. [Google Scholar] [CrossRef]
- Danquah, C.A.; Kakagianni, E.; Khondkar, P.; Maitra, A.; Rahman, M.; Evangelopoulos, D.; McHugh, T.D.; Stapleton, P.; Malkinson, J.; Bhakta, S.; et al. Analogues of Disulfides from Allium stipitatum demonstrate potent anti-tubercular activities through drug efflux pump and biofilm inhibition. Sci. Rep. 2018, 8, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria: A n update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Schindler, B.D.; Kaatz, G.W. Multidrug efflux pumps of Gram-positive bacteria. Drug Resist. Updates 2016, 27, 1–3. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mohsenipour, Z. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J. Microbiol. 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, M.C.; Ponce, A.G. Beet (Beta vulgaris) and Leek (Allium porrum) Leaves as a Source of Bioactive Compounds with Anti-quorum Sensing and Anti-biofilm Activity. Waste Biomass Valorization 2019, 1–9. [Google Scholar] [CrossRef]
- Ceylan, O.; Alic, H. Antibiofilm, antioxidant, antimutagenic activities and phenolic compounds of Allium orientale BOISS. Braz. Arch. Biol. Technol. 2015, 58, 935–943. [Google Scholar] [CrossRef]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Hamat, R.A.; Pichika, M.R.; Lung, L.T.T.; Mohd Fauzi, F.; Chigurupati, S.; van Belkum, A.; Neela, V. Antibacterial and Antibiofilm Activities of Nonpolar Extracts of Allium stipitatum Regel. against Multidrug Resistant Bacteria. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382. [Google Scholar] [CrossRef] [Green Version]
- Szumowski, J.D.; Adams, K.N.; Edelstein, P.H.; Ramakrishnan, L. Antimicrobial efflux pumps and Mycobacterium tuberculosis drug tolerance: Evolutionary considerations. Curr. Top. Microbiol. Immunol. 2012, 81–108. [Google Scholar] [CrossRef]
- Richards, J.P.; Ojha, A.K. Mycobacterial Biofilms. Microbiol. Spectr. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danquah, C.A.; Maitra, A.; Gibbons, S.; Faull, J.; Bhakta, S. HT-SPOTi: A rapid drug susceptibility test (DST) to evaluate antibiotic resistance profiles and novel chemicals for anti-infective drug discovery. Curr. Protoc. Microbiol. 2016. [Google Scholar] [CrossRef]
- Rodrigues, L.; Wagner, D.; Viveiros, M.; Sampaio, D.; Couto, I.; Vavra, M.; Kern, W.V.; Amaral, L. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 61. [Google Scholar] [CrossRef]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Prasch, S.; Duran, A.G.; Chinchilla, N.; Molinillo, J.M.G.; Macías, F.A.; Bucar, F. Resistance modulatory and efflux-inhibitory activities of capsaicinoids and capsinoids. Bioorganic Chem. 2019, 82, 378–384. [Google Scholar] [CrossRef]
- Machado, D.; Couto, I.; Perdigão, J.; Rodrigues, L.; Portugal, I.; Baptista, P.; Veigas, B.; Amaral, L.; Viveiros, M. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PLoS ONE 2012, 7, e34538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, G.A. Microtiter dish Biofilm formation assay. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Abidi, S.H.; Ahmed, K.; Sherwani, S.K.; Bibi, N.; Kazmi, U.S. Detection of Mycobacterium Smegmatis Biofilm and its Control by Natural Agents. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 801–812. [Google Scholar]
- Kwasny, S.M.; Opperman, T.J. Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr. Protoc. Pharmacol. 2010, 50, 13A.8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Short Biography of Authors
 | Dr. (Mrs.) Cynthia Amaning Danquah is a Senior Lecturer at the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana. She pursued an interdisciplinary PhD in Drug Discovery to tackle antimicrobial resistance at the School of Pharmacy, University College London, UK and Birkbeck, University of London, UK. Her MPhil (Pharmacology) and Bachelor of Pharmacy (Hons) were obtained from KNUST. Natural product drug discovery, tuberculosis, antimicrobial resistance and natural product pharmacology and toxicology are her research areas of interest. She is a Pharmacist with passion for infectious diseases of public health concern and a member of a range of professional bodies including the British Pharmacological Society (BPS), the British Society for Antimicrobial Chemotherapy (BSAC), International Society for Pharmacovigilance (ISOP), the West African Network of Natural Products Research Scientists (WANPRESS), International Pharmaceutical Federation (FIP), Pharmaceutical Society of Ghana (PSGH), an affiliate of the African Academy of Science (AAS) and the Country ambassador for the American Society for Microbiology (ASM). |
 | Michael Tetteh is a research assistant at the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST). He graduated with a BSc. (Hon) in Herbal Medicine at KNUST in 2016. He worked as a teaching and research assistant in the Department of Pharmacology KNUST, a medical intern at Tetteh Quarshie Memorial Hospital and a research intern at the Center for Plant Medicine Research all in Ghana. Natural product drug discovery against resistant infectious disease is his research area of interest. He is a member of the British Society for Antimicrobial Chemotherapy, the American Society for Microbiology and the Ghana Association of Medical Herbalist. |
 | Dr. Isaac Kingsley Amponsah is a Senior lecturer in Pharmacognosy at the Kwame Nkrumah University of Science and Technology (KNUST). He holds a Doctor of philosophy in Pharmacognosy (completed 2012) and a Bachelor of Pharmacy degree (completed 2005), all from KNUST. He has a certificate in Good manufacturing practices (GMP) and good agricultural collection practices (GCAP) from Hanoi University of Pharmacy in Vietnam and i+solutions, Amsterdam, Netherlands. His research focuses on phytochemical investigation of biologically active plant medicines and the quality control of herbal products using various analytical methods. His current research includes plant constituents as biofilm inhibitors for the mitigation of microbial resistance, plant constituents and formulations against Mycobacterium ulcerans (implicated in Buruli ulcer) and standardisation of herbal products. Dr Isaac Kingsley Amponsah is a member of the society for medicinal plants and natural product research. He serves as an ECOWAS expert committee member on traditional medicine. He is also a member of the governing board of the traditional medicine practice council of Ghana and Chairman of its education committee. |
 | Professor Kwame Ohene Buabeng is a Clinical Pharmacologist and Associate Professor of Pharmacy Practice at Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana. He is the Vice President of the Ghana College of Pharmacists (GCPharm) and Chair of the Division of Pharmaceutical Care, GCPharm. He is also Deputy Chair of the multi-sectoral, multi-stakeholder coordinating platform on Antimicrobial Resistance in Ghana, and a Member of the National Medicines Selection Committee for the Development of Standard Treatment Guidelines (STG) and Essential Medicines List (EML). Prof Ohene Buabeng has B. Pharm degree, MSc in Clinical Pharmacology and PhD in Pharmacy (Social Pharmacy-Health Systems and Drug Utilization Research) as well as Certificate in Public Health Science, Research and Ethics. His research interest is in prevention, pharmacotherapy and control of Infectious Diseases and Cardio-metabolic disorders. |
 | Professor Abraham Yeboah Mensah obtained his PhD at King’s College, London, United Kingdom in1999 and his Bachelor of Pharmacy (Hons) in 1990 from KNUST. He is a Pharmacist and a lecturer in Pharmacognosy. His research interest is in natural products with biological activities. Prof Yeboah Mensah is a Commonwealth Scholar and Fellow and a recipient of the prestigious Welcome Trust (United Kingdom) Research Development Award in Tropical Medicine (2002–2004). He is also a scholar of the University of Michigan African Presidential Scholar Program. Other awards received by Prof Abraham Yeboah Mensah include the German Academic Exchange Service Award (DAAD). He collaborates with a number of scientists from several countries including Germany, United States and the United Kingdom. He is a Fellow of the Pharmaceutical Society of Ghana (PSGH) and a Fellow of the Ghana College of Pharmacist. |
 | Professor Simon Gibbons is a Professor of Natural Product Chemistry at the University of East Anglia School of Pharmacy, UK. He is founding Editor-in-Chief of Phytochemistry Letters and Co-editor of Progress in the Chemistry of Organic Natural Products (“Zechmeister”). Simon was past President of the Phytochemical Society of Europe and has served on the UK Government’s Advisory Council on the Misuse of Drugs (ACMD). He currently is a member of the Home Office’s Cannabis Based Medicinal Products Committee. He has special interests in medicinal natural products, phytochemistry and antimicrobial natural products from plants and microbes. His group specialises in the isolation and structure elucidation of natural products that are active against drug-resistant bacteria. He also is interested in compounds that modify bacterial resistance. These may be plasmid transfer inhibitors or efflux inhibitors |
 | Professor Sanjib Bhakta is a Professor of Molecular Microbiology and Biochemistry, Department of Biological Sciences, Institute of Structural and Molecular Biology (ISMB), Birkbeck, University of London, UK. He is the Programme Director of MRes Global Infectious Diseases, Department of Biological Sciences, Assistant Dean (Strategic) Internationalisation and Partnership, School of Science, Birkbeck. Professor Bhakta’s research group at the ISMB-Mycobacteria Research Laboratory works on global infectious diseases such as tuberculosis (TB) and international health challenges with antibiotic resistance. His main research interests are focused around characterising the physiology of the different metabolic states of Mycobacterium spp., tackling antimicrobial resistance through validating novel therapeutic targets, from identifying hits to optimising novel leads and repurposing existing immunomodulatory drugs to cure TB. |


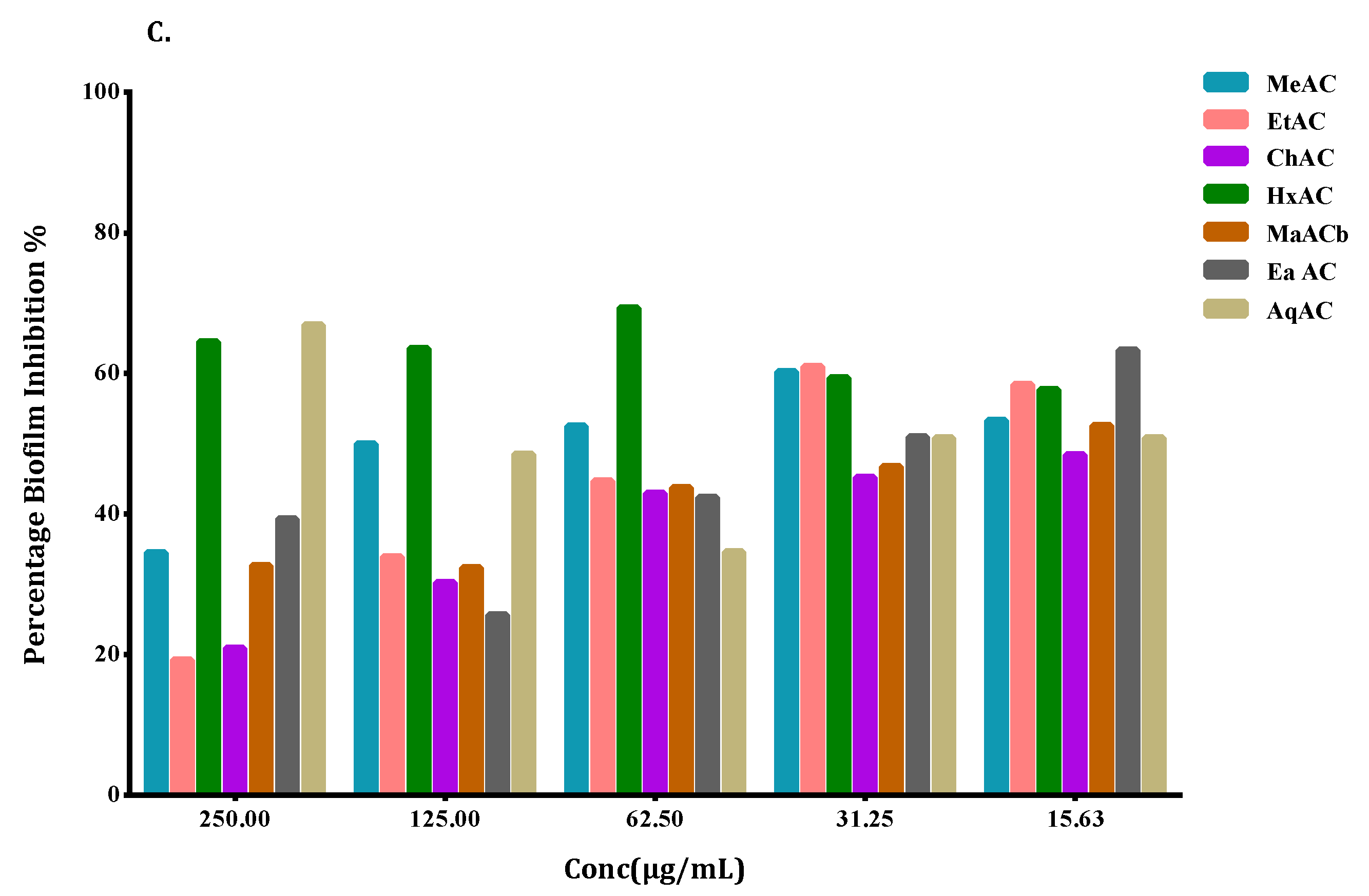
| MeAC | HxAC | ChAC | EtAC | AqAC | MeACb | EaAC | |
|---|---|---|---|---|---|---|---|
| Tannins | Condensed | Condensed | Condensed | Condensed | Condensed | Condensed | Condensed |
| Saponins | + | − | + | + | + | + | + |
| Flavonoids | + | − | − | + | − | + | − |
| Reducing sugars | + | − | + | + | + | + | + |
| Alkaloids | + | + | + | + | + | +− | − |
| Triterpenoids | + | + | + | + | + | + | + |
| Phytosterols | + | + | + | + | + | + | + |
| Coumarins | + | + | + | + | + | + | + |
| Minimum Inhibitory Concentration MIC (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. mirabilis | S. aureus | K. pneumoniae | P. aeruginosa | S. typhi | S. epidermidis | M. smegmatis | |
| MeAC | 500 | 500 | 250 | >500 | 250 | >500 | 500 | >500 |
| EtAC | >500 | >500 | 500 | 500 | 500 | >500 | 500 | 250 |
| HxAC | >500 | >500 | 500 | >500 | 500 | >500 | >500 | >500 |
| ChAC | >500 | >500 | >500 | >500 | 500 | >500 | 500 | >500 |
| MeACb | 500 | >500 | >500 | >500 | 500 | >500 | 500 | >500 |
| EaAC | >500 | 500 | >500 | >500 | >500 | >500 | 500 | >500 |
| AqAC | 250 | 500 | >500 | >500 | 500 | 500 | 500 | >500 |
| Ciprofloxacin | <0.49 | <0.49 | <0.49 | <0.49 | <0.49 | 7.81 | <0.49 | <0.49 |
| Amoxicillin | 15.6 | 62.5 | 62.5 | >500 | 62.5 | >500 | 7.81 | NT |
| Rifampicin | NT | NT | NT | NT | NT | NT | NT | 7.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danquah, C.A.; Tetteh, M.; Amponsah, I.K.; Mensah, A.Y.; Buabeng, K.O.; Gibbons, S.; Bhakta, S. Investigating Ghanaian Allium Species for Anti-Infective and Resistance-Reversal Natural Product Leads to Mitigate Multidrug-Resistance in Tuberculosis. Antibiotics 2021, 10, 902. https://doi.org/10.3390/antibiotics10080902
Danquah CA, Tetteh M, Amponsah IK, Mensah AY, Buabeng KO, Gibbons S, Bhakta S. Investigating Ghanaian Allium Species for Anti-Infective and Resistance-Reversal Natural Product Leads to Mitigate Multidrug-Resistance in Tuberculosis. Antibiotics. 2021; 10(8):902. https://doi.org/10.3390/antibiotics10080902
Chicago/Turabian StyleDanquah, Cynthia Amaning, Michael Tetteh, Isaac Kingsley Amponsah, Abraham Yeboah Mensah, Kwame Ohene Buabeng, Simon Gibbons, and Sanjib Bhakta. 2021. "Investigating Ghanaian Allium Species for Anti-Infective and Resistance-Reversal Natural Product Leads to Mitigate Multidrug-Resistance in Tuberculosis" Antibiotics 10, no. 8: 902. https://doi.org/10.3390/antibiotics10080902
APA StyleDanquah, C. A., Tetteh, M., Amponsah, I. K., Mensah, A. Y., Buabeng, K. O., Gibbons, S., & Bhakta, S. (2021). Investigating Ghanaian Allium Species for Anti-Infective and Resistance-Reversal Natural Product Leads to Mitigate Multidrug-Resistance in Tuberculosis. Antibiotics, 10(8), 902. https://doi.org/10.3390/antibiotics10080902








