Study on the Structure-Activity Relationship of an Antimicrobial Peptide, Brevinin-2GUb, from the Skin Secretion of Hylarana guentheri
Abstract
:1. Introduction
2. Results
2.1. Peptide Design
2.2. Peptide Purification and Identification
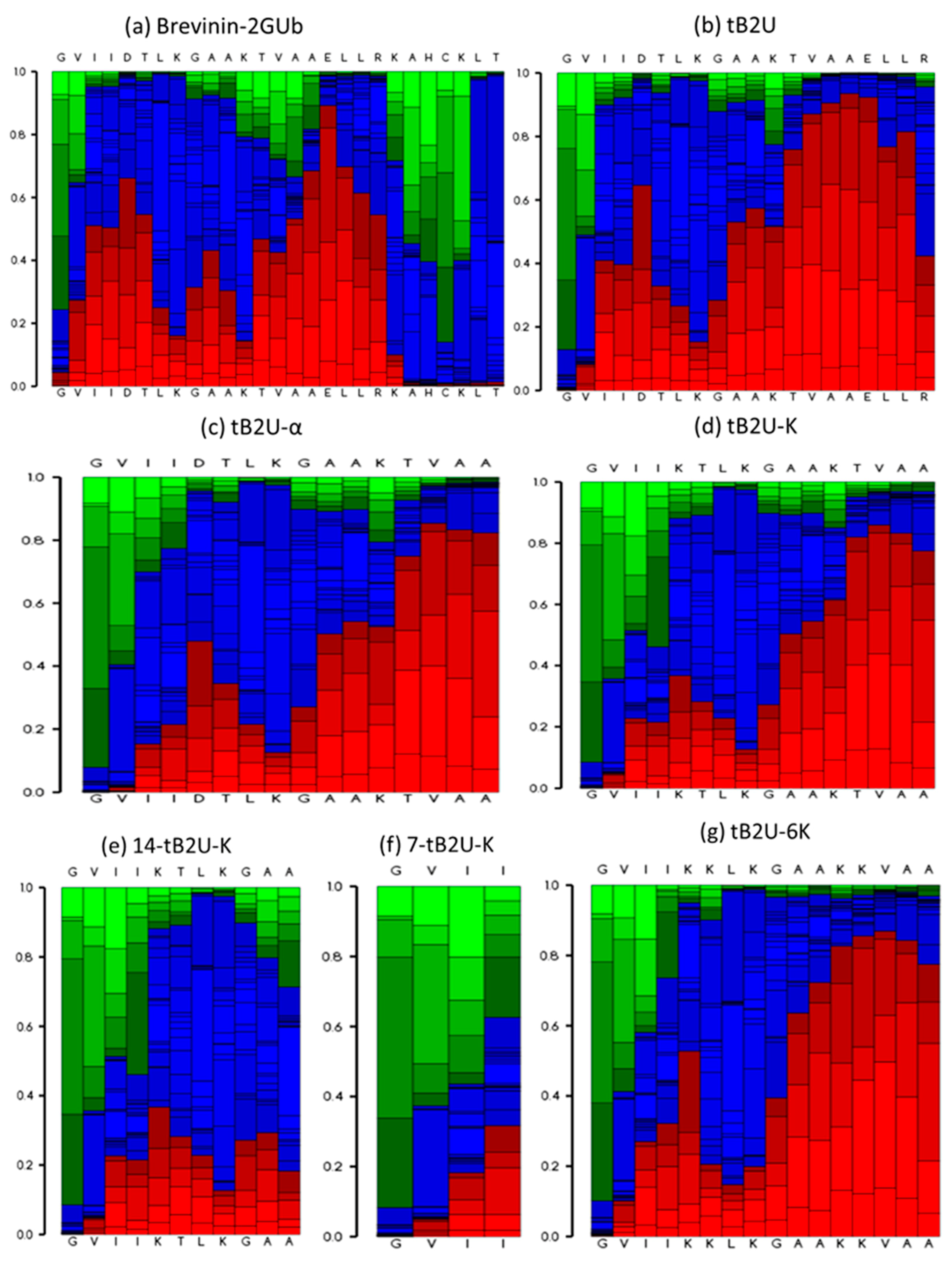
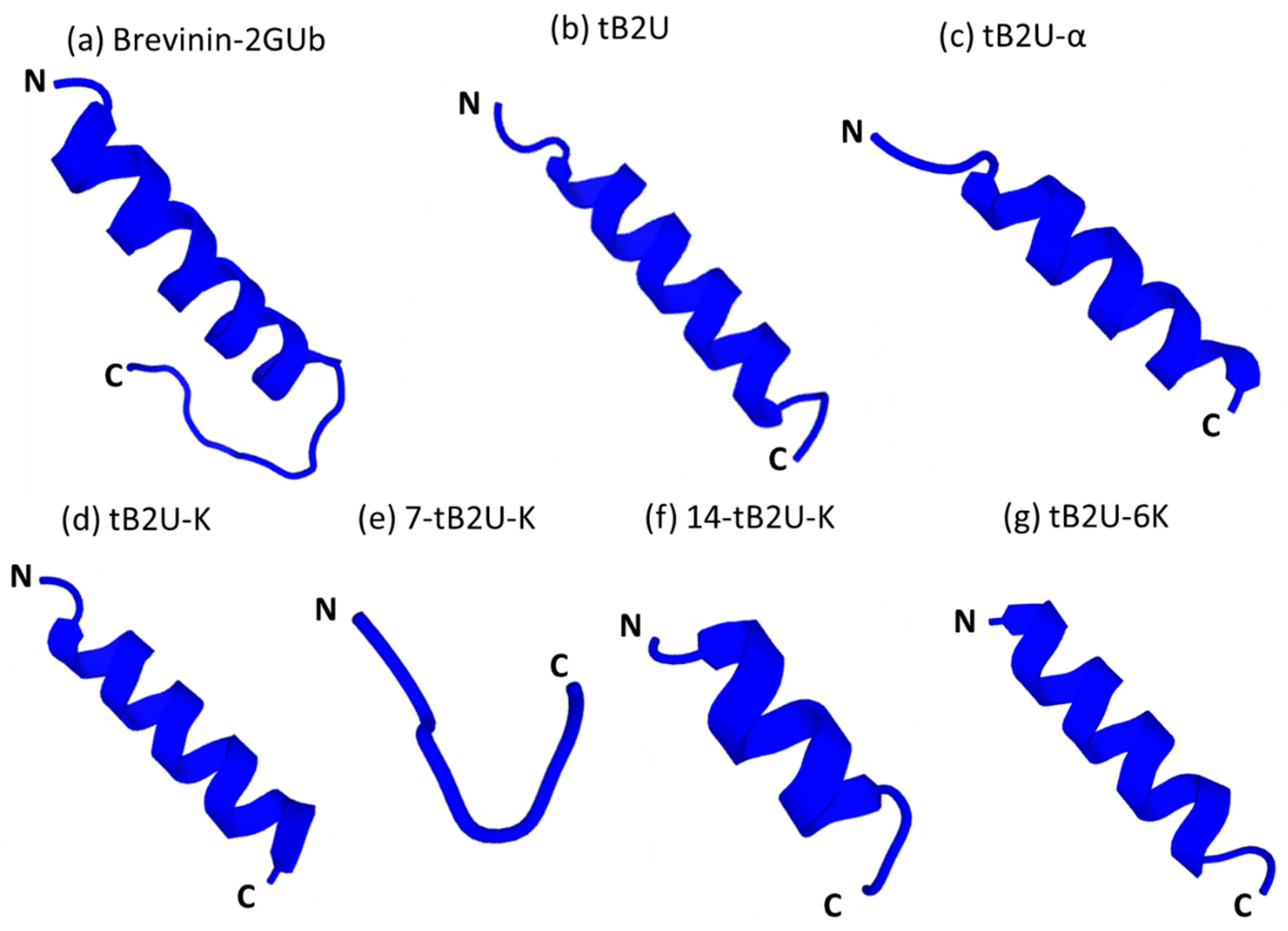
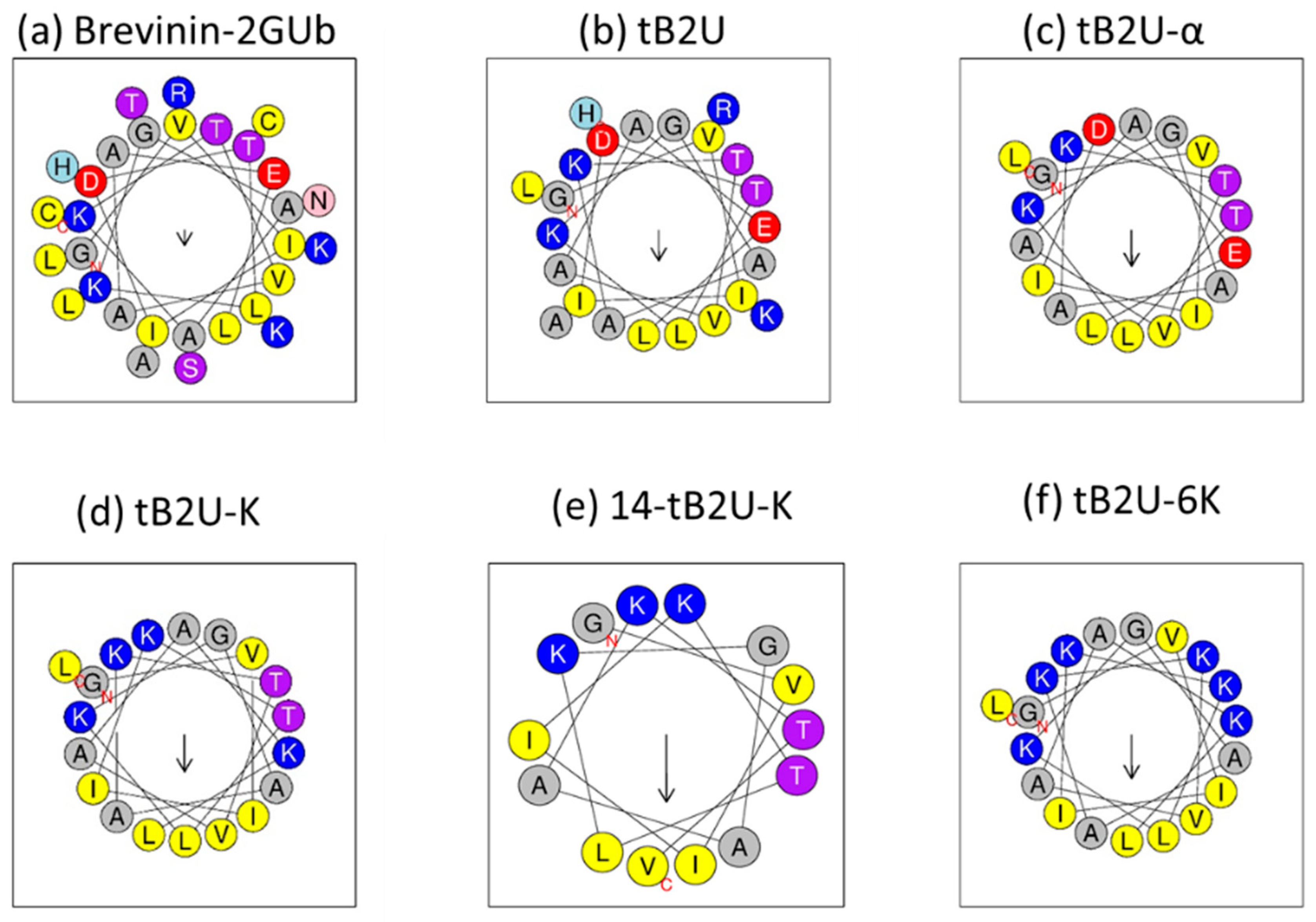
2.3. Secondary Structure Analysis
2.4. Antimicrobial Assays
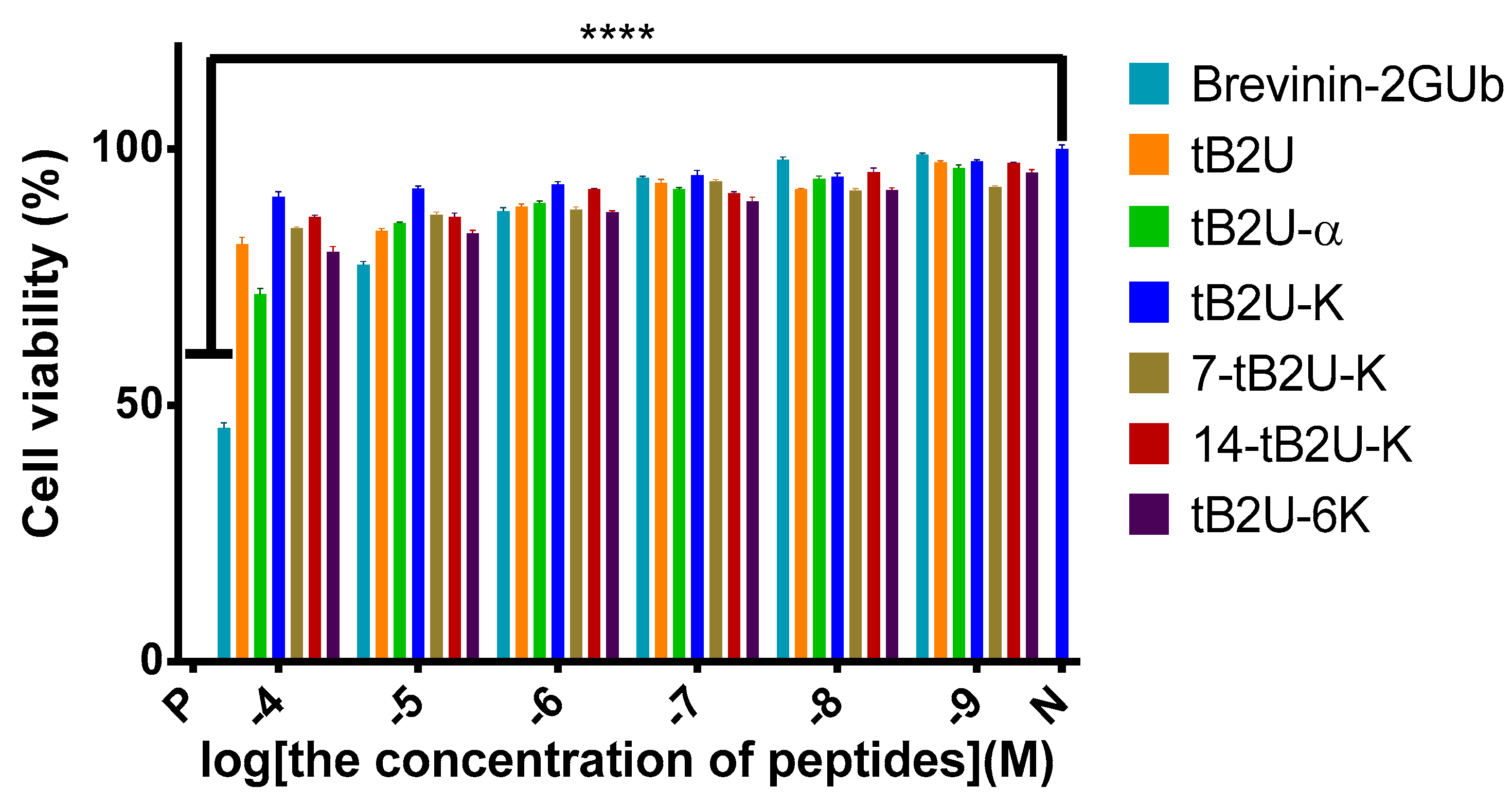
2.5. Determination of Cytotoxicity
2.5.1. Haemolysis Assay
2.5.2. MTT Assay on HaCaT
2.6. Anti-Biofilm Assay
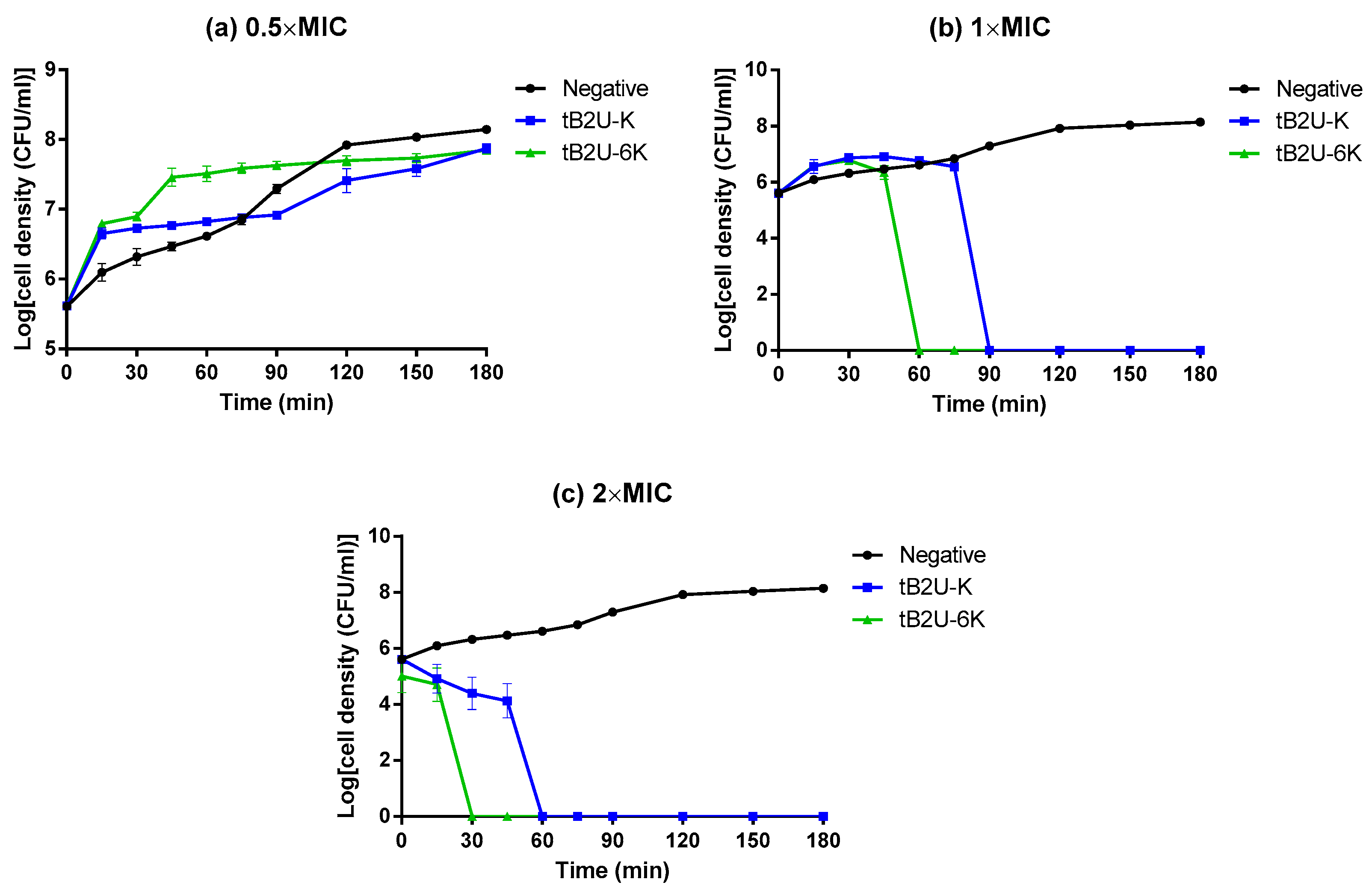
2.7. Time-Killing Assay
2.8. Membrane Permeability Kinetic Assay
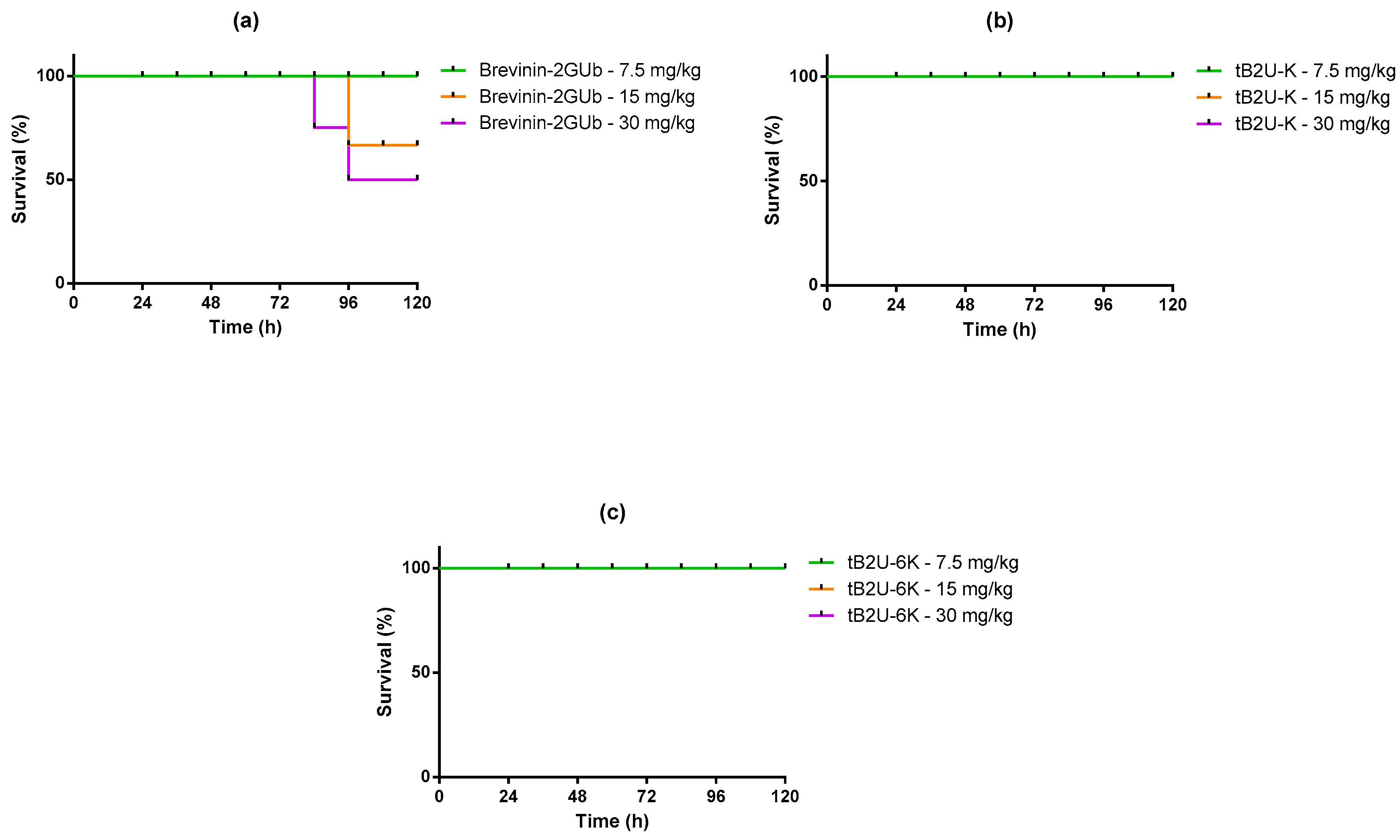
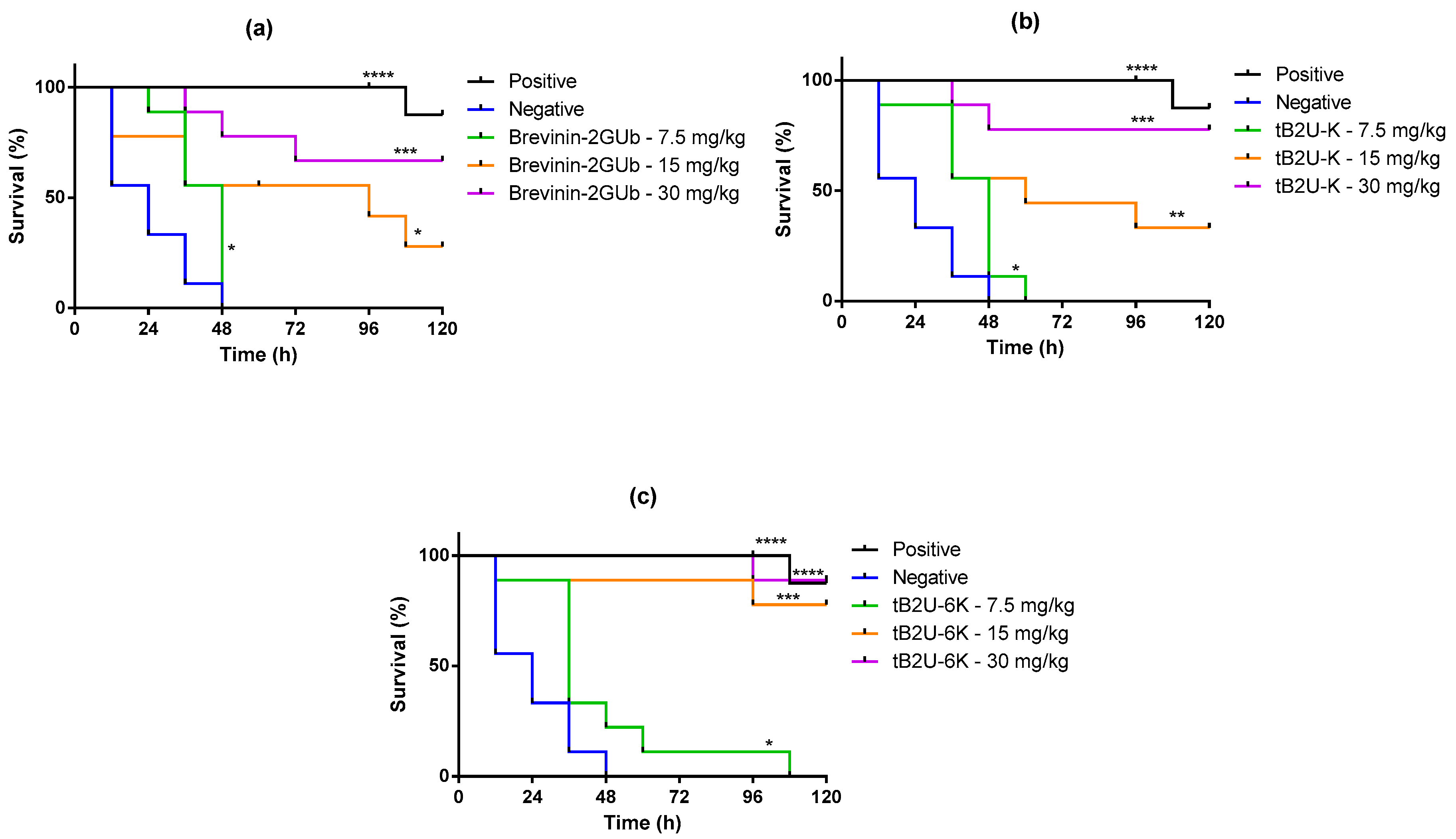
2.9. Evaluation of Efficacy against E. coli In Vivo
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Peptide Purification
4.3. Peptide Identification
4.4. Secondary Structure Prediction and Determination
4.5. Antimicrobial Assay
4.6. Determination of Cytotoxicity
4.6.1. Haemolysis Assay
4.6.2. MTT Assay on HaCaT
4.7. Anti-Biofilm Assay
4.7.1. MBIC
4.7.2. MBEC
4.8. Time-Killing Assay
4.9. Membrane Permeability Kinetic Assay
4.10. Evaluation of Efficacy against E. coli In Vivo
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Aoki, W.; Ueda, M. Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef] [Green Version]
- Bowdish, D.; Davidson, D.J.; Hancock, R. A Re-evaluation of the Role of Host Defence Peptides in Mammalian Immunity. Curr. Protein Pept. Sci. 2005, 6, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Neshani, A.; Sedighian, H.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Jahangiri, A. Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb. Pathog. 2020, 146, 104238. [Google Scholar] [CrossRef]
- Soares, G.M.S.; Figueiredo, L.C.; Faveri, M.; Cortelli, S.C.; Duarte, P.M.; Feres, M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J. Appl. Oral Sci. 2012, 20, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moghaddam, M.M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2015, 40, 133–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control. 2006, 34, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-K.; Shih, L.-Y.; Chang, K.Y. Large-Scale Analysis of Antimicrobial Activities in Relation to Amphipathicity and Charge Reveals Novel Characterization of Antimicrobial Peptides. Molecules 2017, 22, 2037. [Google Scholar] [CrossRef] [Green Version]
- Conlon, J.M. Structural diversity and species distribution of host-defense peptides in frog skin secretions. Cell. Mol. Life Sci. 2011, 68, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Yuan, W.; Ma, C.; Yu, X.; Wang, L.; Hong, M.; Xi, X.; Zhou, M.; Chen, T. Modification Targeting the “Rana Box” Motif of a Novel Nigrocin Peptide From Hylarana latouchii Enhances and Broadens Its Potency Against Multiple Bacteria. Front. Microbiol. 2018, 9, 2846. [Google Scholar] [CrossRef]
- Savelyeva, A.; Ghavami, S.; Davoodpour, P.; Asoodeh, A.; Łos, M.J. An Overview of Brevinin Superfamily: Structure, Function and Clinical Perspectives. Anticancer Genes 2014, 818, 197–212. [Google Scholar] [CrossRef]
- Conlon, J.M.; Power, G.J.; Abdel-Wahab, Y.H.; Flatt, P.; Jiansheng, H.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H. A potent, non-toxic insulin-releasing peptide isolated from an extract of the skin of the Asian frog, Hylarana guntheri (Anura:Ranidae). Regul. Pept. 2008, 151, 153–159. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Yang, Z.; He, S.; Yang, Y.; Feng, X.; Dou, X.; Shan, A. Antimicrobial Peptides with High Proteolytic Resistance for Combating Gram-Negative Bacteria. J. Med. Chem. 2019, 62, 2286–2304. [Google Scholar] [CrossRef] [PubMed]
- Kmeck, A.; Tancer, R.J.; Ventura, C.R.; Wiedman, G.R. Synergies with and Resistance to Membrane-Active Peptides. Antibiotics 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Choudhary, M.; Vashistt, J.; Shrivastava, R.; Bisht, G.S. Cationic antimicrobial peptide and its poly-N-substituted glycine congener: Antibacterial and antibiofilm potential against A. baumannii. Biochem. Biophys. Res. Commun. 2019, 518, 472–478. [Google Scholar] [CrossRef]
- Chen, G.; Miao, Y.; Ma, C.; Zhou, M.; Shi, Z.; Chen, X.; Burrows, J.F.; Xi, X.; Chen, T.; Wang, L. Brevinin-2GHk from Sylvirana guentheri and the Design of Truncated Analogs Exhibiting the Enhancement of Antimicrobial Activity. Antibiotics 2020, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Dennison, S.R.; Mura, M.; Harris, F.; Morton, L.H.; Zvelindovsky, A.; Phoenix, D.A. The role of C-terminal amidation in the membrane interactions of the anionic antimicrobial peptide, maximin H5. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Tao, K.; Jia, Y.; Yan, X.; Zou, Q.; Gazit, E.; Li, J. Photoactive properties of supramolecular assembled short peptides. Chem. Soc. Rev. 2019, 48, 4387–4400. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, X.; Yuan, Y.; Bao, Y.; Xiong, M. Role and modulation of the secondary structure of antimicrobial peptides to improve selectivity. Biomater. Sci. 2020, 8, 6858–6866. [Google Scholar] [CrossRef]
- Kshtriya, V.; Koshti, B.; Gour, N. Controlled Morphological Changes in Self-Assembled Structures Formed by Fmoc Variants of Threonine and Serine. ChemRxiv 2021. pre-print. [Google Scholar]
- Huang, Y.; He, L.; Li, G.; Zhai, N.; Jiang, H.; Chen, Y. Role of helicity of α-helical antimicrobial peptides to improve specificity. Protein Cell 2014, 5, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chou, S.; Xu, L.; Zhu, X.; Dong, N.; Shan, A.; Chen, Z. High specific selectivity and Membrane-Active Mechanism of the synthetic centrosymmetric α-helical peptides with Gly-Gly pairs. Sci. Rep. 2015, 5, 15963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziato, G.; Costantino, G. Antimicrobial peptides (AMPs): A patent review (2015–2020). Expert Opin. Ther. Patents 2020, 30, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Kumar, T.V.V.; Reshmy, V.; Kumar, K.S.; George, S. A mini review on the antimicrobial peptides isolated from the genus Hylarana (Amphibia: Anura) with a proposed nomenclature for amphibian skin peptides. Mol. Biol. Rep. 2012, 39, 6943–6947. [Google Scholar] [CrossRef] [PubMed]
- Tamba, Y.; Yamazaki, M. Single Giant Unilamellar Vesicle Method Reveals Effect of Antimicrobial Peptide Magainin 2 on Membrane Permeability. Biochemistry 2005, 44, 15823–15833. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Wang, J.; Ma, Z.; Xu, W.; Li, J.; Shan, A. Characterization of antimicrobial activity and mechanisms of low amphipathic peptides with different α-helical propensity. Acta Biomater. 2015, 18, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cheng, P.; Ma, C.; Xi, X.; Wang, L.; Zhou, M.; Bian, H.; Chen, T. Evaluating the Bioactivity of a Novel Broad-Spectrum Antimicrobial Peptide Brevinin-1GHa from the Frog Skin Secretion of Hylarana guentheri and Its Analogues. Toxins 2018, 10, 413. [Google Scholar] [CrossRef] [Green Version]
- Casciaro, B.; Cappiello, F.; Cacciafesta, M.; Mangoni, M.L. Promising Approaches to Optimize the Biological Properties of the Antimicrobial Peptide Esculentin-1a(1–21)NH2: Amino Acids Substitution and Conjugation to Nanoparticles. Front. Chem. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim-Carmo, B.; Daniele-Silva, A.; Parente, A.M.S.; Furtado, A.A.; Carvalho, E.; Oliveira, J.W.F.; Santos, E.C.G.; Silva, M.S.; Silva, S.R.B.; Silva-Júnior, A.A.; et al. Potent and Broad-Spectrum Antimicrobial Activity of Analogs from the Scorpion Peptide Stigmurin. Int. J. Mol. Sci. 2019, 20, 623. [Google Scholar] [CrossRef] [Green Version]
- Yeaman, M.R.; Yount, N.; Hauger, R.L.; Grigoriadis, D.E.; Dallman, M.F.; Plotsky, P.M.; Vale, W.W.; Dautzenberg, F.M. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslouches, B.; Phadke, S.M.; Lazarevic, V.; Cascio, M.; Islam, K.; Montelaro, R.C.; Mietzner, T.A. De Novo Generation of Cationic Antimicrobial Peptides: Influence of Length and Tryptophan Substitution on Antimicrobial Activity. Antimicrob. Agents Chemother. 2005, 49, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Mattila, J.-P.; Sabatini, K.; Kinnunen, P.K. Oxidized phospholipids as potential molecular targets for antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 2041–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, G.; Shi, Y.-H.; Tang, Y.-L.; Le, G.-W. The membrane action mechanism of analogs of the antimicrobial peptide Buforin 2. Peptides 2009, 30, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Wiradharma, N.; Sng, M.Y.S.; Khan, M.; Ong, Z.Y.; Yang, Y.-Y. Rationally Designed α-Helical Broad-Spectrum Antimicrobial Peptides with Idealized Facial Amphiphilicity. Macromol. Rapid Commun. 2012, 34, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.; Sonnevend, A.; Patel, M.; Al-Dhaheri, K.; Nielsen, P.F.; Kolodziejek, J.; Nowotny, N.; Iwamuro, S.; Pal, T. A family of brevinin-2 peptides with potent activity against Pseudomonas aeruginosa from the skin of the Hokkaido frog, Rana pirica. Regul. Pept. 2004, 118, 135–141. [Google Scholar] [CrossRef]
- Tamargo, J.; Le Heuzey, J.-Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. [Google Scholar] [CrossRef] [Green Version]
- Stanley, T.H. Anesthesia for the 21st century. Bayl. Univ. Med. Cent. Proc. 2000, 13, 7–10. [Google Scholar] [CrossRef]
- Yoon, S.; Park, K.R.; Lee, S.; Song, S.; Park, W.B.; Jang, I.; Yu, K. Assessment of Appropriateness of an Initial Dosing Regimen of Vancomycin and Development of a New Dosing Nomogram. Basic Clin. Pharmacol. Toxicol. 2017, 122, 233–238. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Vergis, J.; Malik, S.S.; Pathak, R.; Kumar, M.; Ramanjaneya, S.; Kurkure, N.V.; Barbuddhe, S.B.; Rawool, D.B. Antimicrobial Efficacy of Indolicidin Against Multi-Drug Resistant Enteroaggregative Escherichia coli in a Galleria mellonella Model. Front. Microbiol. 2019, 10, 2723. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, N.; Nielsen-Leroux, C.; Lereclus, D. The Insect Galleria mellonella as a Powerful Infection Model to Investigate Bacterial Pathogenesis. J. Vis. Exp. 2012, e4392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A.; Coote, P.J. Wax moth larva (Galleria mellonella): An in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, K.; Amsel, D.; Kalsy, M.; Billion, A.; Dobrindt, U.; Vilcinskas, A. MicroRNAs regulate innate immunity against uropathogenic and commensal-like Escherichia coli infections in the surrogate insect model Galleria mellonella. Sci. Rep. 2020, 10, 2570. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Loh, J.M.S.; Proft, T. The Use of Galleria mellonella (Wax Moth) as an Infection Model for Group A Streptococcus. Methods Mol. Biol. 2020, 2136, 279–286. [Google Scholar] [CrossRef]
- Frankel, G.; Schroeder, G.N. The Galleria mellonella Infection Model for Investigating the Molecular Mechanisms of Legionella Virulence. Methods Mol. Biol. 2019, 1921, 333–346. [Google Scholar] [CrossRef]


| Peptide | Sequence | Hydrophobicity <H> | Hydrophobic Moment <μH> | Net Charge (z) |
|---|---|---|---|---|
| Brevinin-2GUb | GVIIDTLKGAAKTVAAELLRKAHCKLTNSC | 0.379 | 0.144 | +3 |
| tB2U | GVIIDTLKGAAKTVAAELLRKAH-NH2 | 0.346 | 0.288 | +2 |
| tB2U-α | GVIIDTLKGAAKTVAAELL-NH2 | 0.501 | 0.339 | 0 |
| tB2U-K | GVIIKTLKGAAKTVAAKLL-NH2 | 0.471 | 0.347 | +4 |
| 7-tB2U-K 1 | GVIIKTL-NH2 | - | - | +1 |
| 14-tB2U-K | GVIIKTLKGAAKTV-NH2 | 0.422 | 0.506 | +3 |
| tB2U-6K | GVIIKKLKGAAKKVAAKLL-NH2 | 0.339 | 0.410 | +6 |
| Peptide | 20 mM NH4Ac | 50% TFE in NH4Ac | ||||
|---|---|---|---|---|---|---|
| Name | Helix (%) | Antiparallel (%) | Turn (%) | Helix (%) | Antiparallel (%) | Turn (%) |
| Brevinin-2GUb | 62.9 | 0.0 | 6.5 | 73.2 | 0.0 | 4.9 |
| tB2U | 58.7 | 0.0 | 6.4 | 72.7 | 0.0 | 4.6 |
| tB2U-α | 39.9 | 10.3 | 12.1 | 50.3 | 2.6 | 10.6 |
| tB2U-K | 50.3 | 0.0 | 1.1 | 89.6 | 0.0 | 0.0 |
| 7-tB2U-K | 25.9 | 23.0 | 0.0 | 51.0 | 8.2 | 0.0 |
| 14-tB2U-K | 15.9 | 23.8 | 10.9 | 31.0 | 18.8 | 9.1 |
| tB2U-6K | 51.5 | 0.0 | 7.8 | 66.0 | 0.0 | 3.2 |
| Bacteria | MIC/MBC(μM) | ||||||
|---|---|---|---|---|---|---|---|
| Brevinin-2GUb | tB2U | tB2U-α | tB2U-K | 7-tB2U-K | 14-tB2U-K | tB2U-6K | |
| S. aureus | 32/32 | 256/>512 | >512 | 32/32 | 512/>512 | 256/>512 | 32/32 |
| E. coli | 16/16 | 128/128 | >512 | 8/8 | >512 | >512 | 2/2 |
| C. albicans | >512 | >512 | >512 | 64/64 | >512 | >512 | 256/256 |
| Methicillin-resistant Staphylococcus aureus (MRSA) | >512 | >512 | >512 | 128/128 | 256/>512 | >512 | 64/128 |
| K. pneumoniae | 128/128 | 512/>512 | >512 | 128/128 | >512 | >512 | 32/32 |
| Pseudomonas aeruginosa (P. aeruginosa) | 128/512 | >512 | >512 | 32/32 | >512 | >512 | 16/16 |
| Enterococcus faecalis (E. faecalis) | >512 | >512 | >512 | 32/32 | >512 | >512 | 64/64 |
| Clinical strains | |||||||
| MRSA (B042 V2E1 A) | >512 | >512 | >512 | >512 | >512 | >512 | 256/256 |
| E. coli (ATCC BAA-2340) | 32/32 | 512 | >512 | 16/16 | >512 | >512 | 8/8 |
| P. aeruginosa (B004 V2S2 B) | >512 | >512 | >512 | >512 | >512 | >512 | 64/64 |
| K. pneumoniae (ATCC BAA-1705) | 512/512 | >512 | >512 | 128/128 | >512 | >512 | 128/128 |
| HC50 (μM) | 2010 | 16,393 | 15,609 | 3056 | 11,008 | 10,834 | 6506 |
| TI 2,3 (Overall) | 27.98 | 16.01 | 15.24 | 70.18 | 10.75 | 10.58 | 158.02 |
| Normal Cell Lines | IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|
| Brevinin-2GUb | tB2U | tB2U-α | tB2U-K | tB2U-6K | 7-tB2U-K | 14-tB2U-K | |
| HaCaT | 68.0 | 381.6 | 228.0 | 883.2 | 346.8 | 485.3 | 572.6 |
| Bacteria Strains | MBIC/MBEC (μM) | ||||||
|---|---|---|---|---|---|---|---|
| Brevinin-2GUb | tB2U | tB2U-α | tB2U-K | tB2U-6K | 7-tB2U-K | 14-tB2U-K | |
| S. aureus | 256/>512 | >512 | >512 | 16/>512 | 64/>512 | 512/>512 | >512 |
| E. coli | 128/>512 | >512 | >512 | 16/>512 | 2/>512 | >512 | >512 |
| K. pneumoniae | >512 | >512 | >512 | 128/>512 | 32/>512 | >512 | >512 |
| P. aeruginosa | >512 | >512 | >512 | 128/>512 | 32/>512 | >512 | >512 |
| MRSA | 256/>512 | 128/>512 | >512 | 16/>512 | 32/>512 | 512/>512 | >512 |
| E. faecalis | >512 | 256/>512 | >512 | 32/>512 | 64/>512 | >512 | >512 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Liu, S.; Xi, X.; Ma, C.; Wang, L.; Chen, X.; Shi, Z.; Chen, T.; Shaw, C.; Zhou, M. Study on the Structure-Activity Relationship of an Antimicrobial Peptide, Brevinin-2GUb, from the Skin Secretion of Hylarana guentheri. Antibiotics 2021, 10, 895. https://doi.org/10.3390/antibiotics10080895
Lin Y, Liu S, Xi X, Ma C, Wang L, Chen X, Shi Z, Chen T, Shaw C, Zhou M. Study on the Structure-Activity Relationship of an Antimicrobial Peptide, Brevinin-2GUb, from the Skin Secretion of Hylarana guentheri. Antibiotics. 2021; 10(8):895. https://doi.org/10.3390/antibiotics10080895
Chicago/Turabian StyleLin, Yaxian, Siyan Liu, Xinping Xi, Chengbang Ma, Lei Wang, Xiaoling Chen, Zhanzhong Shi, Tianbao Chen, Chris Shaw, and Mei Zhou. 2021. "Study on the Structure-Activity Relationship of an Antimicrobial Peptide, Brevinin-2GUb, from the Skin Secretion of Hylarana guentheri" Antibiotics 10, no. 8: 895. https://doi.org/10.3390/antibiotics10080895
APA StyleLin, Y., Liu, S., Xi, X., Ma, C., Wang, L., Chen, X., Shi, Z., Chen, T., Shaw, C., & Zhou, M. (2021). Study on the Structure-Activity Relationship of an Antimicrobial Peptide, Brevinin-2GUb, from the Skin Secretion of Hylarana guentheri. Antibiotics, 10(8), 895. https://doi.org/10.3390/antibiotics10080895







