Trends of Multidrug-Resistant Pathogens, Difficult to Treat Bloodstream Infections, and Antimicrobial Consumption at a Tertiary Care Center in Lebanon from 2015–2020: COVID-19 Aftermath
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Microbiology
2.3. Definitions
2.4. Antimicrobial Consumption
2.5. Statistical Analysis
3. Results
3.1. General Results and Species Repartition
3.2. Trend Analysis of Isolation Densities of All Organisms
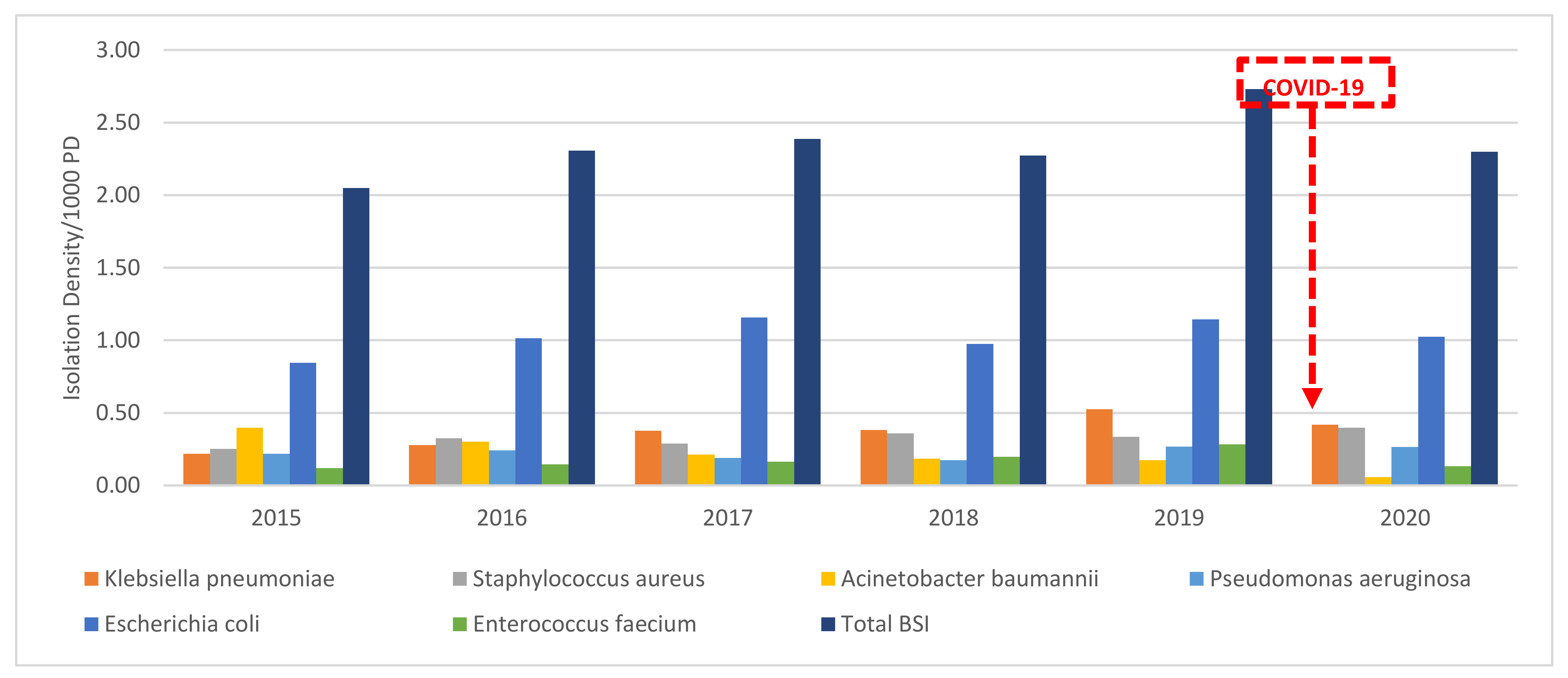
3.3. Trend Analysis of Isolation Densities of BSI
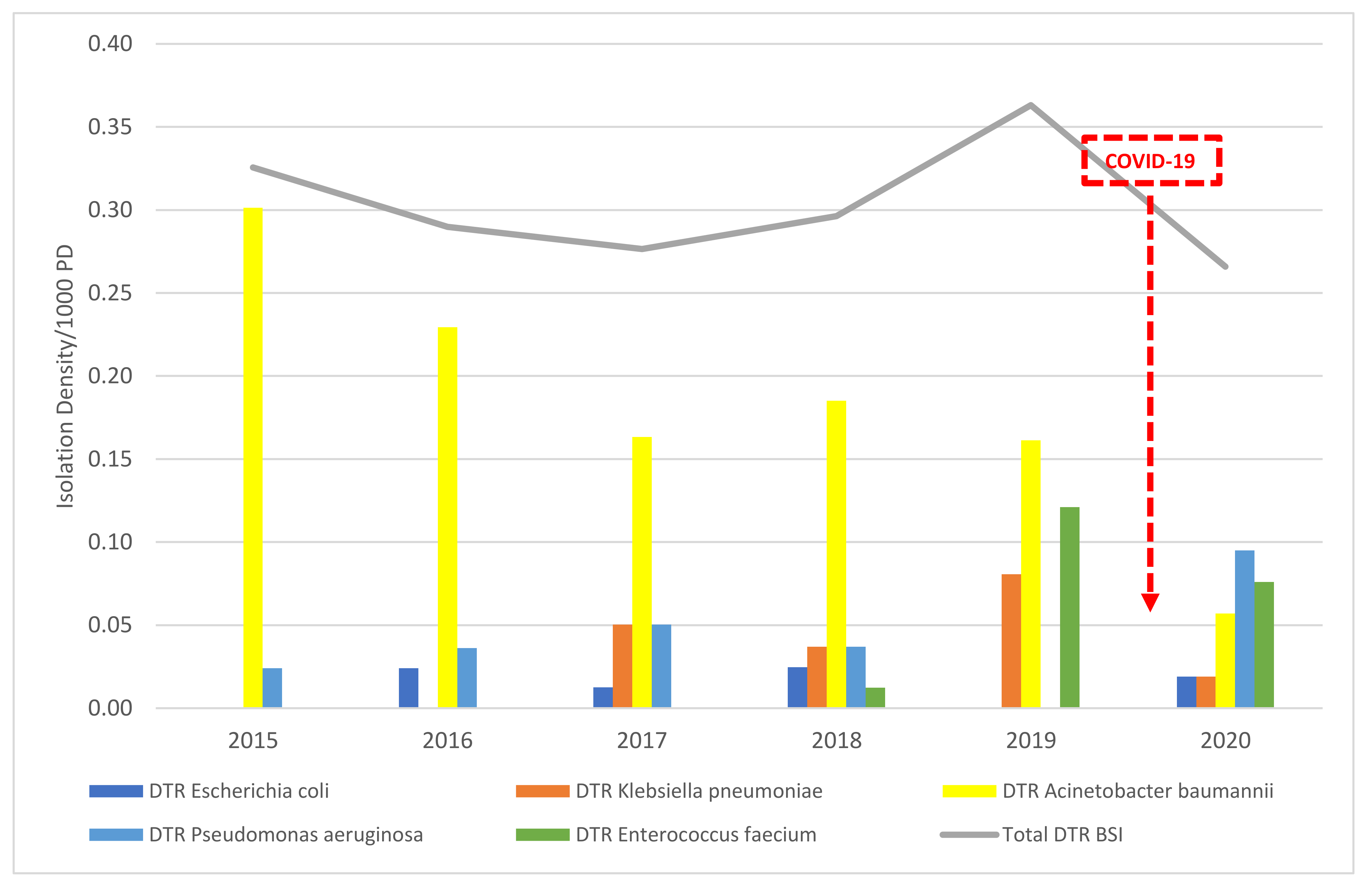
3.4. Difficult-to-Treat Blood Stream Infections
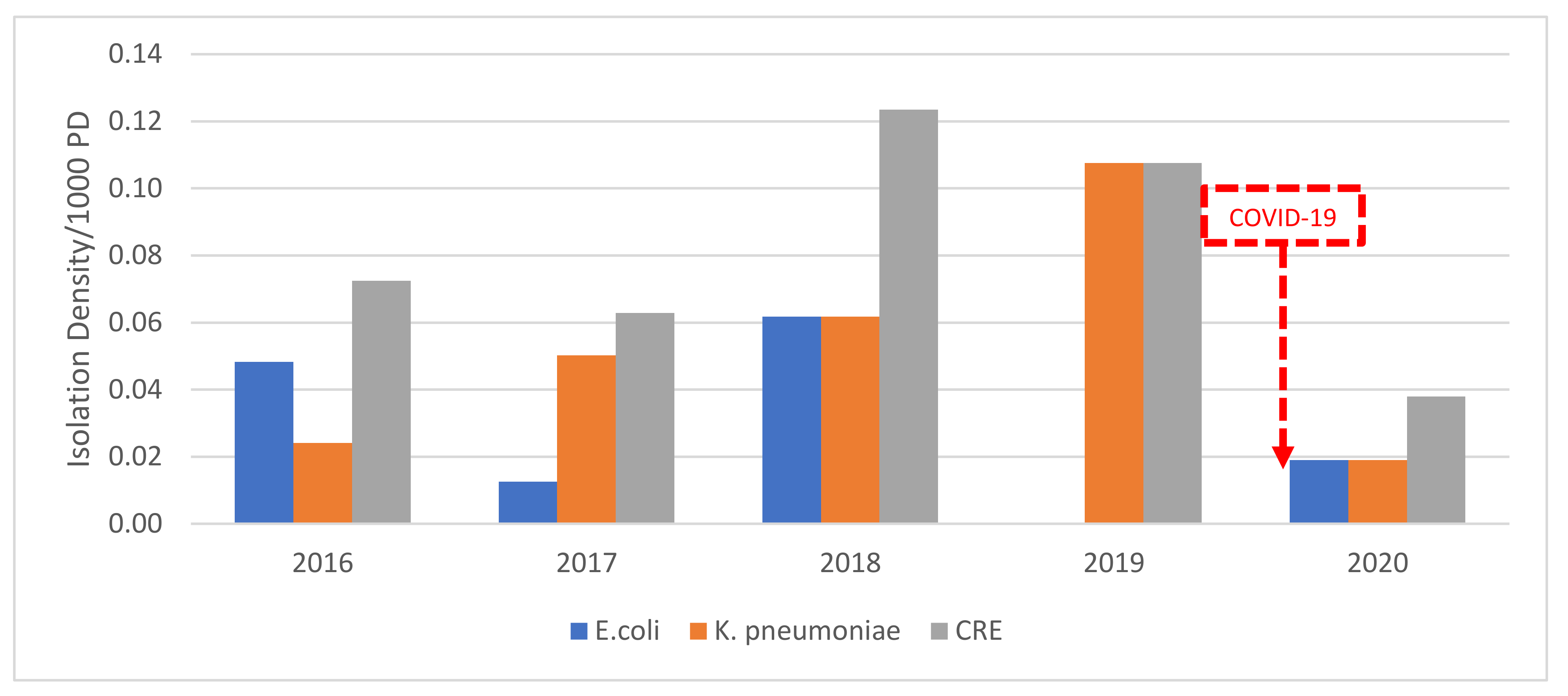
3.5. Carbapenem-Resistant E. coli and K. pneumoniae
3.6. MSSA vs. MRSA BSI
3.7. E. faecium and Vancomycin-Esistant E. faecium BSI
3.8. Antimicrobial Consumption
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination. Available online: https://covid19.who.int/?gclid=CjwKCAjwtpGGBhBJEiwAyRZX2noc7tJdF4ukQHGsEUW9EporJPTgi8Tt1JtC9u0Tlj5aGV2o-FfZvRoCVgAQAvD_BwE (accessed on 12 June 2021).
- Papoutsi, E.; Giannakoulis, V.G.; Ntella, V.; Pappa, S.; Katsaounou, P. Global burden of COVID-19 pandemic on healthcare workers. ERJ Open Res. 2020, 6, 00195–02020. [Google Scholar] [CrossRef]
- Impact of the Covid-19 Pandemic on Healthcare Systems? Available online: https://www2.deloitte.com/fr/fr/pages/covid-insights/articles/impact-covid19-healthcare-systems.html (accessed on 1 May 2021).
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef]
- Haug, N.; Geyrhofer, L.; Londei, A.; Dervic, E.; Desvars-Larrive, A.; Loreto, V.; Pinior, B.; Thurner, S.; Klimek, P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat. Hum. Behav. 2020, 4, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.P. Complex scenarios with competing factors. Nonlinear Dyn. 2021, 104, 2961–2975. [Google Scholar] [CrossRef] [PubMed]
- Shahi, G.K.; Dirkson, A.; Majchrzak, T.A. An exploratory study of COVID-19 misinformation on Twitter. Online Soc. Netw. Media 2021, 22, 100104. [Google Scholar] [CrossRef]
- Cinelli, M.; Quattrociocchi, W.; Galeazzi, A.; Valensise, C.M.; Brugnoli, E.; Schmidt, A.L.; Zola, P.; Zollo, F.; Scala, A. The COVID-19 social media infodemic. Sci. Rep. 2020, 10, 16598. [Google Scholar] [CrossRef]
- Bessi, A.; Coletto, M.; Davidescu, G.A.; Scala, A.; Caldarelli, G.; Quattrociocchi, W. Science vs Conspiracy: Collective Narratives in the Age of Misinformation. PLoS ONE 2015, 10, e0118093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, F.; Shaar, S.; Nikolov, A.; Mubarak, H.; da San Martino, G.; Abdelali, A.; Dalvi, F.; Durrani, N.; Sajjad, H.; Darwish, K.; et al. Fighting the covid-19 infodemic: Modeling the perspective of journalists, fact-checkers, social media platforms, policy makers, and the society. arXiv 2020, arXiv:2005.00033. [Google Scholar]
- Orben, A.; Tomova, L.; Blakemore, S.-J. The effects of social deprivation on adolescent development and mental health. Lancet Child Adolesc. Health 2020, 4, 634–640. [Google Scholar] [CrossRef]
- Melki, J.; Tamim, H.; Hadid, D.; Farhat, S.; Makki, M.; Ghandour, L.; Hitti, E. Media Exposure and Health Behavior during Pandemics: The Mediating Effect of Perceived Knowledge and Fear on Compliance with COVID-19 Prevention Measures. Health Commun. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Mahmood, S.F.; Khan, M.; Hasan, R. Covid -19, misinformation, and antimicrobial resistance. BMJ 2020, 371, m4501. [Google Scholar] [CrossRef]
- Ma, X.; Vervoort, D. Critical care capacity during the COVID-19 pandemic: Global availability of intensive care beds. J. Crit. Care 2020, 58, 96–97. [Google Scholar] [CrossRef]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef]
- Khoury, P.; Azar, E.; Hitti, E. COVID-19 response in Lebanon: Current experience and challenges in a low-resource setting. JAMA 2020, 324, 548–549. [Google Scholar] [CrossRef]
- Kamar, A.; Maalouf, N.; Hitti, E.; El Eid, G.; Isma’Eel, H.; Elhajj, I.H. Challenge of forecasting demand of medical resources and supplies during a pandemic: A comparative evaluation of three surge calculators for COVID-19. Epidemiol. Infect. 2021, 149, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, M.A.; Tetaj, N.; Selleri, M.; Marchioni, L.; Capone, A.; Caraffa, E.; Di Caro, A.; Petrosillo, N. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: An alarming “collateral effect”. J. Glob. Antimicrob. Resist. 2020, 23, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Healthcare-Associated Infections|HAI|CDC. Available online: https://www.cdc.gov/hai/ (accessed on 12 June 2021).
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: A retrospective analysis. Antimicrob. Resist. Infect. Control. 2020, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Flandre, P.L.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensiv. Care 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Sharifipour, E.; Shams, S.; Esmkhani, M.; Khodadadi, J.; Fotouhi-Ardakani, R.; Koohpaei, A.; Doosti, Z.; Golzari, S.E. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis. 2020, 20, 646. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, Q.; Xu, M.; Kong, H.; Chen, H.; Fu, Y.; Yao, Y.; Zhou, H.; Zhou, J. Secondary Bacterial Infections in Critical Ill Patients With Coronavirus Disease 2019. Open Forum Infect. Dis. 2020, 7, ofaa220. [Google Scholar] [CrossRef] [PubMed]
- Nori, P.; Szymczak, W.; Puius, Y.; Sharma, A.; Cowman, K.; Gialanella, P.; Fleischner, Z.; Corpuz, M.; Torres-Isasiga, J.; Bartash, R.; et al. Emerging Co-Pathogens: New Delhi Metallo-beta-lactamase producing Enterobacterales Infections in New York City COVID-19 Patients. Int. J. Antimicrob. Agents 2020, 56, 106179. [Google Scholar] [CrossRef] [PubMed]
- Farfour, E.; Lecuru, M.; Dortet, L.; Le Guen, M.; Cerf, C.; Karnycheff, F.; Bonnin, R.A.; Vasse, M.; Lesprit, P. Carbapenemase-producing Enterobacterales outbreak: Another dark side of COVID-19. Am. J. Infect. Control. 2020, 48, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [Green Version]
- Diallo, O.O.; Baron, S.A.; Dubourg, G.; Chaudet, H.; Halfon, P.; Camiade, S.; Comte, B.; Joubert, S.; François, A.; Seyral, P.; et al. Major discrepancy between factual antibiotic resistance and consumption in South of France: Analysis of 539,037 bacterial strains. Sci. Rep. 2020, 10, 18262. [Google Scholar] [CrossRef]
- Echols, R.M.; Tillotson, G.S. Difficult to Treat: Do We Need a New Definition? Clin. Infect. Dis. 2019, 69, 1641–1642. [Google Scholar] [CrossRef]
- WHO. WHO Collaborating Centre for Drug Statistics Methodology. Nor. Inst. Public Health 2013. [Google Scholar]
- Azar, E.; Afif, C.; Lakis, C.; Diab, N.; Daoud, Z.; Irani, J. Antimicrobial Stewardship: The impact of instant electronic notifiation at the initiation of therapy on antimicrobial consumption. In Proceedings of the ECCMID 2013; ECCMID 2013: Berlin, Germany, 2013. [Google Scholar]
- Wagner, A.K.; Soumerai, S.B.; Zhang, F.; Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002, 27, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Addinsoft XLSTAT 2014: Data Analysis and Statistical Solution for Microsoft Excel; Addinsoft: Paris, France, 2014.
- Chamieh, A.; Nawfal, T.D.; Ballouz, T.; Afif, C.; Juvelekian, G.; Hlais, S.; Rolain, J.-M.; Azar, E. Control and Elimination of Extensively Drug-Resistant Acinetobacter baumanii in an Intensive Care Unit. Emerg. Infect. Dis. 2019, 25, 1928–1931. [Google Scholar] [CrossRef] [Green Version]
- Dagher, T.N.; Azar, E.; Al-Bayssari, C.; Chamieh, A.S.; Rolain, J.-M. First Detection of Colistin-Resistant Klebsiella pneumoniae in Association with NDM-5 Carbapenemase Isolated from Clinical Lebanese Patients. Microb. Drug Resist. 2019, 25, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Kampmeier, S.; Tönnies, H.; Correa-Martinez, C.L.; Mellmann, A.; Schwierzeck, V. A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob. Resist. Infect. Control. 2020, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Chamieh, A.; El-Hajj, G.; Zmerli, O.; Afif, C.; Azar, E. Carbapenem resistant organisms: A 9-year surveillance and trends at Saint George University Medical Center. J. Infect. Public Health 2020, 13, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Guidance: Businesses and Employers|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/community/guidance-business-response.html (accessed on 12 June 2021).
- Management of Visitors to Healthcare Facilities in the Context of COVID-19: Non-US Healthcare Settings|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/hcf-visitors.html (accessed on 12 June 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamieh, A.; Zgheib, R.; El-Sawalhi, S.; Yammine, L.; El-Hajj, G.; Zmerli, O.; Afif, C.; Rolain, J.-M.; Azar, E. Trends of Multidrug-Resistant Pathogens, Difficult to Treat Bloodstream Infections, and Antimicrobial Consumption at a Tertiary Care Center in Lebanon from 2015–2020: COVID-19 Aftermath. Antibiotics 2021, 10, 1016. https://doi.org/10.3390/antibiotics10081016
Chamieh A, Zgheib R, El-Sawalhi S, Yammine L, El-Hajj G, Zmerli O, Afif C, Rolain J-M, Azar E. Trends of Multidrug-Resistant Pathogens, Difficult to Treat Bloodstream Infections, and Antimicrobial Consumption at a Tertiary Care Center in Lebanon from 2015–2020: COVID-19 Aftermath. Antibiotics. 2021; 10(8):1016. https://doi.org/10.3390/antibiotics10081016
Chicago/Turabian StyleChamieh, Amanda, Rita Zgheib, Sabah El-Sawalhi, Laure Yammine, Gerard El-Hajj, Omar Zmerli, Claude Afif, Jean-Marc Rolain, and Eid Azar. 2021. "Trends of Multidrug-Resistant Pathogens, Difficult to Treat Bloodstream Infections, and Antimicrobial Consumption at a Tertiary Care Center in Lebanon from 2015–2020: COVID-19 Aftermath" Antibiotics 10, no. 8: 1016. https://doi.org/10.3390/antibiotics10081016
APA StyleChamieh, A., Zgheib, R., El-Sawalhi, S., Yammine, L., El-Hajj, G., Zmerli, O., Afif, C., Rolain, J.-M., & Azar, E. (2021). Trends of Multidrug-Resistant Pathogens, Difficult to Treat Bloodstream Infections, and Antimicrobial Consumption at a Tertiary Care Center in Lebanon from 2015–2020: COVID-19 Aftermath. Antibiotics, 10(8), 1016. https://doi.org/10.3390/antibiotics10081016






