A High-Throughput Metabolic Microarray Assay Reveals Antibacterial Effects of Black and Red Raspberries and Blackberries against Helicobacter pylori Infection
Abstract
:1. Introduction
2. Results
2.1. Analysis of Powders and Extracts of Black and Red Raspberries and Blackberries for Anthocyanin Content and Composition
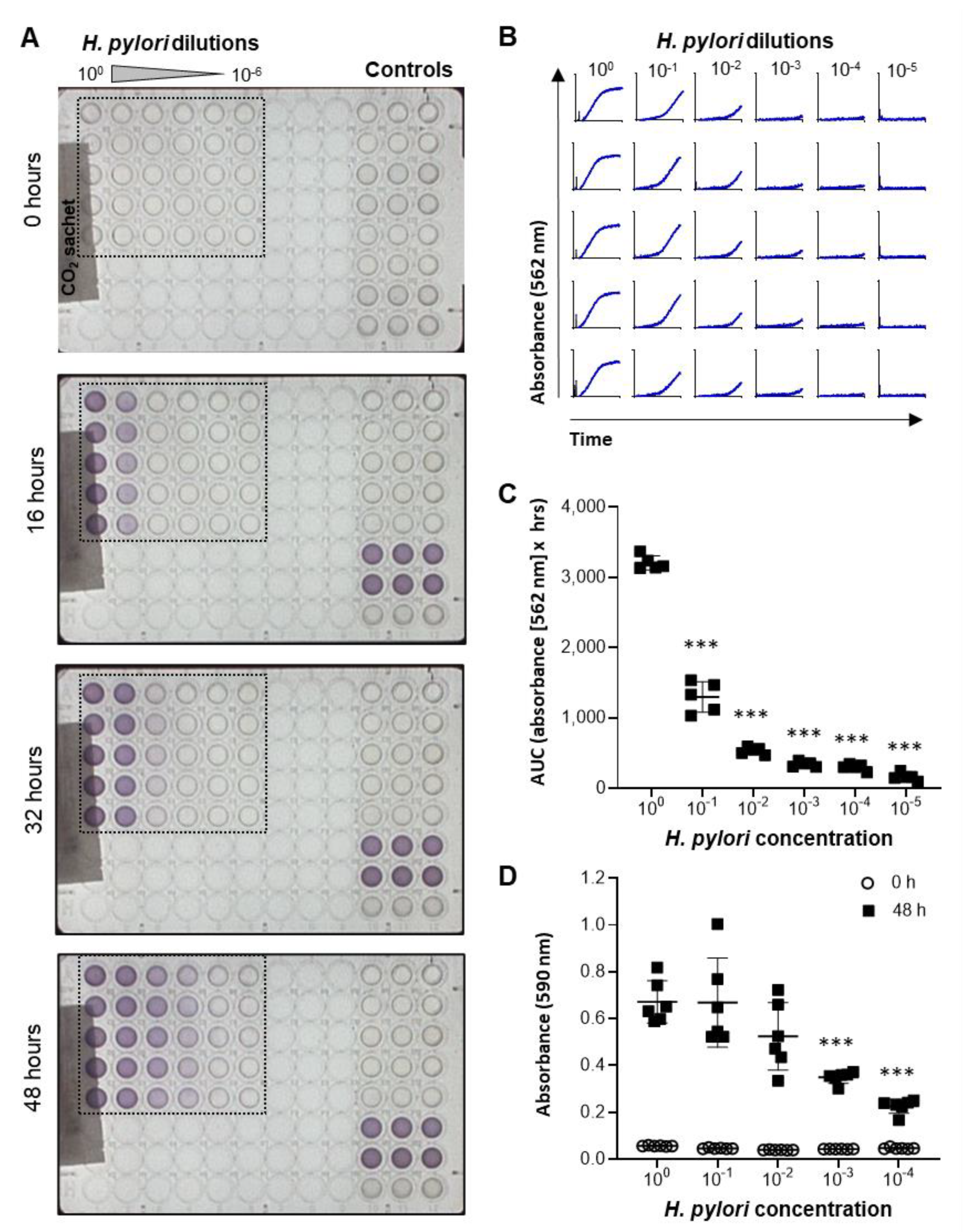
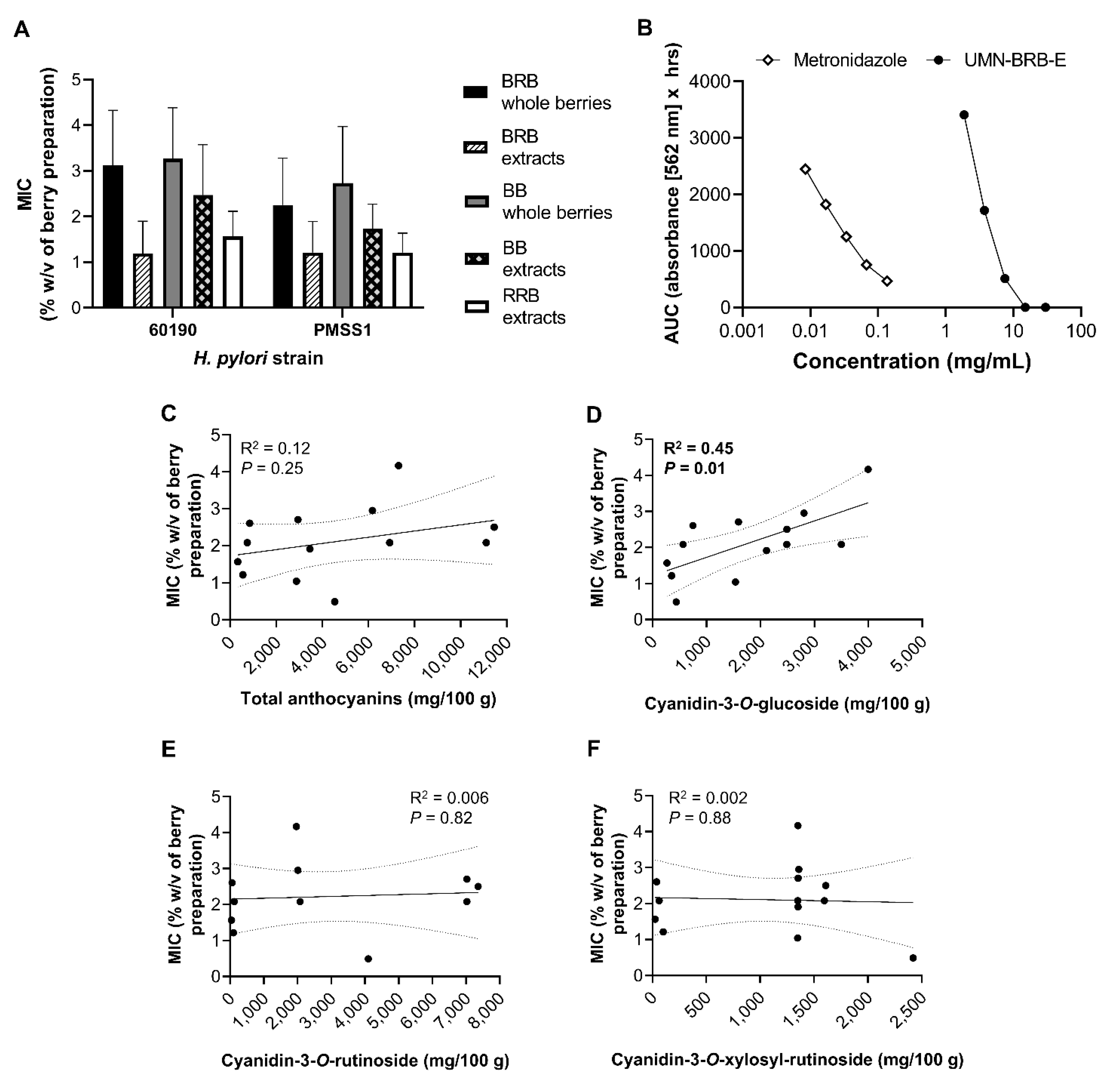
2.2. Development and Validation of a High-Throughput Assay to Measure H. pylori Growth
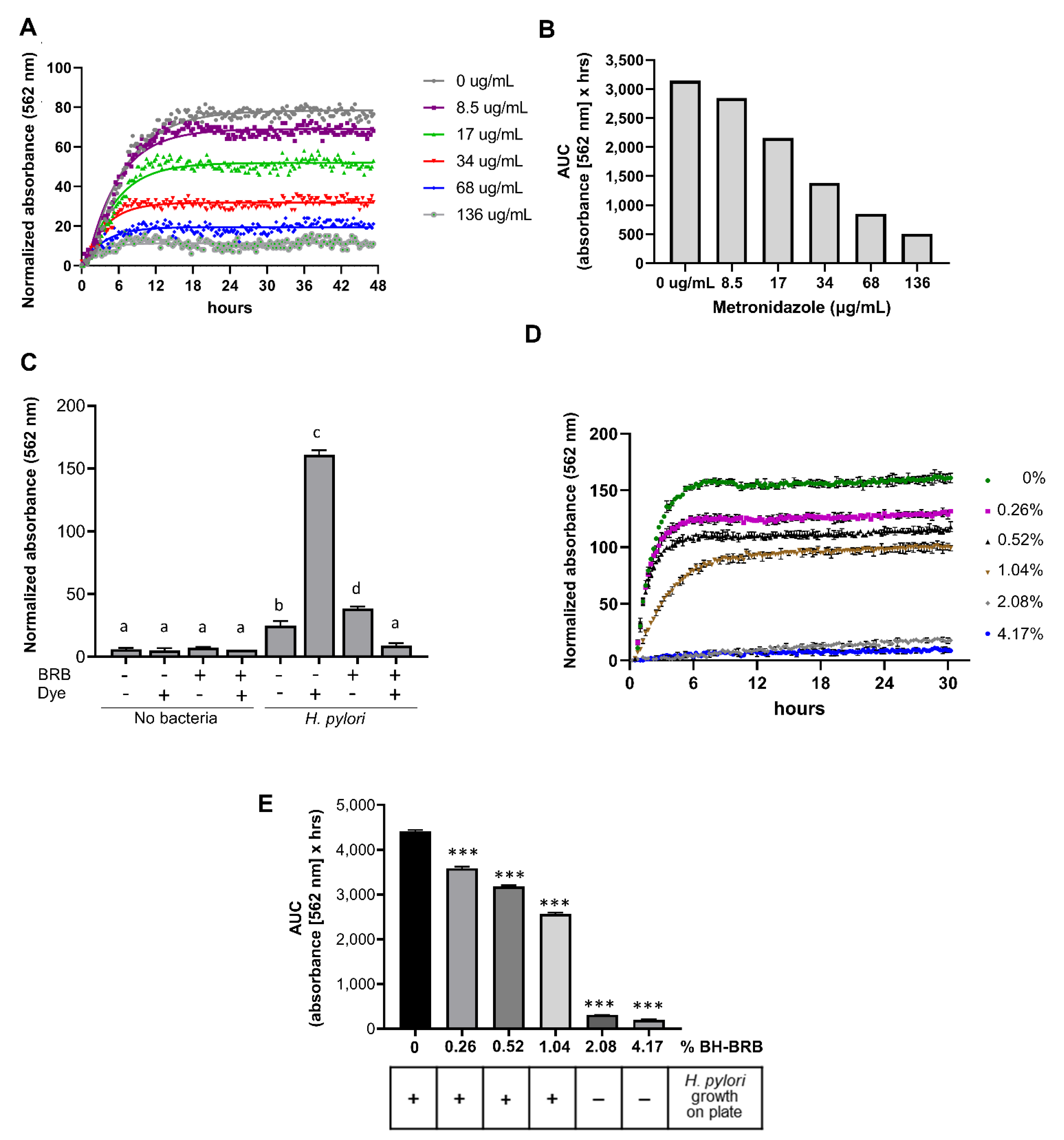
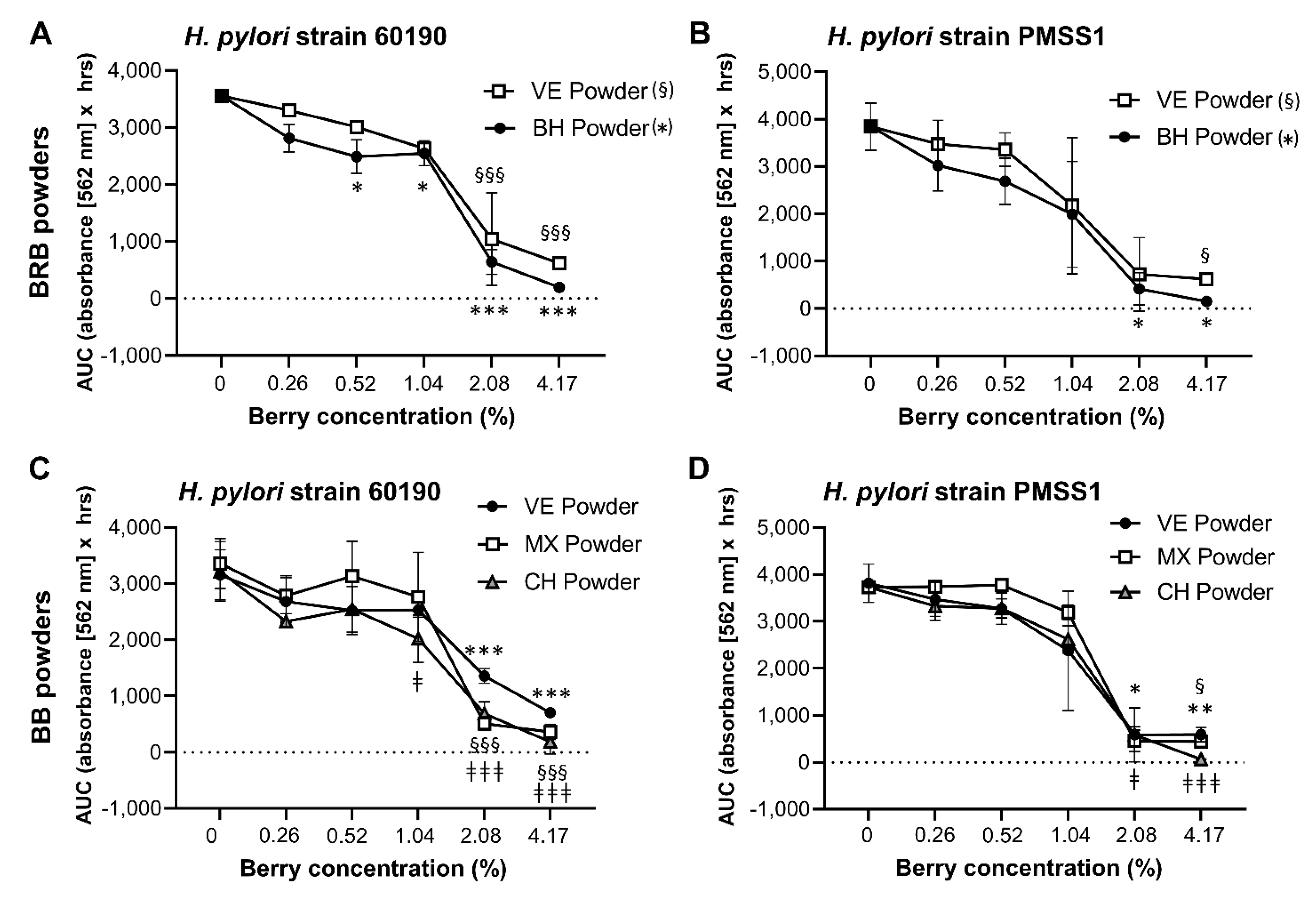
2.3. Analysis of the Antibacterial Effects of Black and Red Raspberry and Blackberry Powders and Extracts on H. pylori
2.4. Effect of BRB Extract on Gastric Epithelial Cell Viability in a Human Gastric Organoid Model
3. Discussion
4. Materials and Methods
4.1. Berry Powders and Preparation of Extracts
4.2. Analysis of Anthocyanin Content
4.3. Helicobacter pylori Strains and Culture Conditions
4.4. High-Throughput Helicobacter pylori Growth Assay
4.5. Data Analysis for Bacterial Growth Assays
4.6. Human Gastric Organoid Culture and Viability Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017, 22, e12403. [Google Scholar] [CrossRef] [PubMed]
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori–Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, T.G.; Czinn, S.J. Current Status and Prospects for a Helicobacter pylori Vaccine. Gastroenterol. Clin. N. Am. 2015, 44, 677–689. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Liou, J.-M.; Gisbert, J.P.; O’Morain, C. Review: Treatment of Helicobacter pylori Infection 2019. Helicobacter 2019, 24, e12640. [Google Scholar] [CrossRef] [Green Version]
- Serrano, C.A.; Leon-Rios, M.; Palma, C.; Vera, M.; Hernandez, C.; Harris, P.R. Helicobacter pylori-Clarithromycin Resistance in Symptomatic Pediatric Patients in a High Prevalence Country. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e56–e60. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Al-Eidan, F.A.; McElnay, J.C.; Scott, M.G.; McConnell, J.B. Management of Helicobacter pylori eradication—The influence of structured counselling and follow-up. Br. J. Clin. Pharmacol. 2002, 53, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Cutler, A.F.; Schubert, T.T. Patient factors affecting Helicobacter pylori eradication with triple therapy. Am. J. Gastroenterol. 1993, 88, 505–509. [Google Scholar]
- Gatta, L.; Vakil, N.; Vaira, D.; Scarpignato, C. Global eradication rates for Helicobacter pylori infection: Systematic review and meta-analysis of sequential therapy. BMJ 2013, 347, f4587. [Google Scholar] [CrossRef] [Green Version]
- Liou, J.-M.; Chang, C.-Y.; Chen, M.-J.; Chen, C.-C.; Fang, Y.-J.; Lee, J.-Y.; Wu, J.-Y.; Luo, J.-C.; Liou, T.-C.; Chang, W.-H.; et al. The Primary Resistance of Helicobacter pylori in Taiwan after the National Policy to Restrict Antibiotic Consumption and Its Relation to Virulence Factors—A Nationwide Study. PLoS ONE 2015, 10, e0124199. [Google Scholar] [CrossRef]
- Graham, D.Y. Transitioning of Helicobacter pylori Therapy from Trial and Error to Antimicrobial Stewardship. Antibiotics 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.-L.; Oksman-Caldentey, K.-M. Bioactive berry compounds-novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005, 67, 8–18. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.K.; Padhi, L.; Leyssen, P.; Liu, M.; Neyts, J.; Luyten, W. Antimicrobial, Anthelmintic, and Antiviral Activity of Plants Traditionally Used for Treating Infectious Disease in the Similipal Biosphere Reserve, Odisha, India. Front. Pharmacol. 2017, 8, 658. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S.J. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Mol. Cell. Biochem. 2004, 265, 19–26. [Google Scholar] [CrossRef]
- Park, J.U.; Cho, J.S.; Kim, J.S.; Kim, H.K.; Jo, Y.H.; Rahman, A.A.; Lee, Y.I. Synergistic Effect of Rubus crataegifolius and Ulmus macrocarpa Against Helicobacter pylori Clinical Isolates and Gastritis. Front. Pharmacol. 2020, 11, 4. [Google Scholar] [CrossRef]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samad, M.A.; Hashim, S.H.; Simarani, K.; Yaacob, J.S. Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules 2016, 21, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Woo, H.; Park, M.; Rhee, K.-J.; Moon, C.; Lee, D.; Seo, W.D.; Kim, J.B. Cyanidin 3-O-Glucoside Reduces Helicobacter pylori VacA-Induced Cell Death of Gastric KATO III Cells through Inhibition of the SecA Pathway. Int. J. Med. Sci. 2014, 11, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Markakis, P. Anthocyanins as Food Colors; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Levy, R.; Okun, Z.; Shpigelman, A. The Influence of Chemical Structure and the Presence of Ascorbic Acid on Anthocyanins Stability and Spectral Properties in Purified Model Systems. Foods 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulio, A.Z.; Reese, R.N.; Wyzgoski, F.J.; Rinaldi, P.L.; Fu, R.; Scheerens, J.C.; Miller, A.R. Cyanidin 3-Rutinoside and Cyanidin 3-Xylosylrutinoside as Primary Phenolic Antioxidants in Black Raspberry. J. Agric. Food Chem. 2008, 56, 1880–1888. [Google Scholar] [CrossRef]
- Bochner, B.R. Global phenotypic characterization of bacteria. FEMS Microbiol. Rev. 2008, 33, 191–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chey, W.D.; I Leontiadis, G.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, N.; Nam, R.H.; Lee, S.M.; Kwon, Y.H.; Sohn, S.D.; Kim, J.M.; Lee, D.H.; Jung, H.C. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter 2019, 24, e12561. [Google Scholar] [CrossRef]
- Tan, B.; Yang, J.-C.; Young, C.L.; Bishu, S.; Owyang, S.Y.; El-Zaatari, M.; Zhang, M.; Grasberger, H.; Qian, J.-M.; Kao, J.Y. Helicobacter pylori Antimicrobial Susceptibility Testing-Guided Salvage Therapy in the USA: A Real Life Experience. Dig. Dis. Sci. 2017, 63, 437–445. [Google Scholar] [CrossRef]
- Biernat, M.M.; Poniewierka, E.; Błaszczuk, J.; Czapla, L.; Kempiński, R.; Ksiądzyna, D.; Grabińska, J.; Bińkowska, A.; Mégraud, F.; Gościniak, G. Antimicrobial susceptibility of Helicobacter pylori isolates from Lower Silesia, Poland. Arch. Med. Sci. 2014, 10, 505–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastukh, N.; Peretz, A.; Brodsky, D.; Isakovich, N.; Azrad, M.; On, A. Antimicrobial susceptibility of Helicobacter pylori strains isolated from children in Israel. J. Glob. Antimicrob. Resist. 2018, 12, 175–178. [Google Scholar] [CrossRef]
- Lee, W.C.; Goh, K.L.; Loke, M.F.; Vadivelu, J. Elucidation of the Metabolic Network of Helicobacter pylori J99 and Malaysian Clinical Strains by Phenotype Microarray. Helicobacter 2017, 22, e12321. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.; Kamimoto, M.; Shimamoto, T.; Shimamoto, T. Antimicrobial Effects of Blueberry, Raspberry, and Strawberry Aqueous Extracts and their Effects on Virulence Gene Expression in Vibrio cholerae. Phytotherapy Res. 2015, 29, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Wojnicz, D.; Cisowska, A.; Walkowski, S.; Sroka, Z.; Gabrielska, J.; Hendrich, A.B. Biological Activity of the Methanol and Water Extracts of the Fruits of Anthocyanin-Rich Plants Grown in South-West Poland. Nat. Prod. Commun. 2015, 10, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Bakar, M.F.; Ismail, N.A.; Isha, A.; Ling, A.L.M. Phytochemical Composition and Biological Activities of Selected Wild Berries (Rubus moluccanus L., R. fraxinifolius Poir., and R. alpestris Blume). Evid. Based Complement. Altern. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A.; Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; et al. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Klesk, K.; Qian, M.; Martin, R.R. Aroma Extract Dilution Analysis of cv. Meeker (Rubus idaeus L.) Red Raspberries from Oregon and Washington. J. Agric. Food Chem. 2004, 52, 5155–5161. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. Effect of Processing Methods and Storage Time on the Content of Bioactive Compounds in Blue Honeysuckle Berry Purees. Agronomy 2019, 9, 860. [Google Scholar] [CrossRef] [Green Version]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef]
- Gurbuz, I.; Ozcelik, B.; Gunbatan, T.; Akkol, E.K.; Sahinoz, M.; Akaydin, G. Antibacterial, antifungal and enzyme inhibitory effects of selected plants from Turkey. Pak. J. Pharm. Sci. 2018, 34, 1011–1017. [Google Scholar]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Serrano, C.; Harris, P.R.; Smith, P.D.; Bimczok, D. Interactions between H. pylori and the gastric microbiome: Impact on gastric homeostasis and disease. Curr. Opin. Physiol. 2021, 21, 57–64. [Google Scholar] [CrossRef]
- Tabarki, S.; Aouadhi, C.; Mechergui, K.; Hammi, K.M.; Ksouri, R.; Raies, A.; Toumi, L. Comparison of Phytochemical Composition and Biological Activities of Rubus ulmifolius Extracts Originating from Four Regions of Tunisia. Chem. Biodivers 2016, 14, e1600168. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.S.; Jung, H.C.; Kim, N.; Kim, Y.-J.; Song, I.S. Distribution of Antibiotic MICs for Helicobacter pylori Strains over a 16-Year Period in Patients from Seoul, South Korea. Antimicrob. Agents Chemother. 2004, 48, 4843–4847. [Google Scholar] [CrossRef] [Green Version]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and Antibacterial Properties of Anthocyanins and Flavonols from Fruits of Black and Non-Black Mulberries. Molecules 2017, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.-P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-M.; Guttenplan, J.; Sun, Y.-W.; Cooper, T.; Shalaby, N.; Kosinska, W.; Benitez, G.; Aliaga, C.; Zhu, J.; Liao, J.; et al. Effects of Black Raspberry on Dibenzo[a,l]Pyrene Diol Epoxide Induced DNA Adducts, Mutagenesis, and Tumorigenesis in the Mouse Oral Cavity. Cancer Prev. Res. 2018, 11, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- God, J.; Tate, P.L.; Larcom, L.L. Red raspberries have antioxidant effects that play a minor role in the killing of stomach and colon cancer cells. Nutr. Res. 2010, 30, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.-S.; Ransom, B.W.; Carmella, S.G.; Kuo, C.-T.; Siddiqui, J.; Chen, J.-H.; Oshima, K.; Huang, Y.-W.; et al. Chemoprevention of Esophageal Cancer with Black Raspberries, Their Component Anthocyanins, and a Major Anthocyanin Metabolite, Protocatechuic Acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Ceci, C.; Graziani, G.; Faraoni, I.; Cacciotti, I. Strategies to improve ellagic acid bioavailability: From natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology 2020, 31, 382001. [Google Scholar] [CrossRef]
- De, R.; Sarkar, A.; Ghosh, P.; Ganguly, M.; Karmakar, B.C.; Saha, D.R.; Halder, A.; Chowdhury, A.; Mukhopadhyay, A.K. Antimicrobial activity of ellagic acid against Helicobacter pylori isolates from India and during infections in mice. J. Antimicrob. Chemother. 2018, 73, 1595–1603. [Google Scholar] [CrossRef]
- Lengsfeld, C.; Deters, A.; Faller, G.; Hensel, A. High Molecular Weight Polysaccharides from Black Currant Seeds Inhibit Adhesion of Helicobacter pylori to Human Gastric Mucosa. Planta Med. 2004, 70, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T.T.H.; Jin, J.; Septiana, I.; Son, G.-M.; Lee, G.-H.; Jung, Y.-J.; Qureshi, D.; Mok, I.K.; Pal, K.; et al. Anti-cariogenic Characteristics of Rubusoside. Biotechnol. Bioprocess Eng. 2019, 24, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Blutt, S.E.; Crawford, S.E.; Ramani, S.; Zou, W.Y.; Estes, M.K. Engineered Human Gastrointestinal Cultures to Study the Microbiome and Infectious Diseases. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.R.; Spence, J.R. Gastrointestinal Organoids: Understanding the Molecular Basis of the Host–Microbe Interface. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Kula, M.; Krauze-Baranowska, M. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutr. Cancer 2015, 68, 18–28. [Google Scholar] [CrossRef]
- Stoner, G.D.; Wang, L.-S.; Casto, B.C. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [Google Scholar] [CrossRef] [Green Version]
- Stoner, G.D.; Wang, L.-S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple Berry Types Prevent N-nitrosomethylbenzylamine-Induced Esophageal Cancer in Rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Skaer, C.W.; Wang, H.-T.; Stirdivant, S.M.; Young, M.R.; Oshima, K.; Stoner, G.D.; Lechner, J.F.; Huang, Y.-W.; Wang, L.-S. Black raspberries suppress colonic adenoma development in ApcMin/+mice: Relation to metabolite profiles. Carcinogenesis 2015, 36, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-S.; Arnold, M.; Huang, Y.-W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.-M.; Riedl, K.; Schwartz, S.; Frankel, W.; et al. Modulation of Genetic and Epigenetic Biomarkers of Colorectal Cancer in Humans by Black Raspberries: A Phase I Pilot Study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef] [Green Version]
- Peiffer, D.S.; Wang, L.-S.; Zimmerman, N.P.; Ransom, B.W.; Carmella, S.G.; Kuo, C.-T.; Chen, J.-H.; Oshima, K.; Huang, Y.-W.; Hecht, S.S.; et al. Dietary Consumption of Black Raspberries or Their Anthocyanin Constituents Alters Innate Immune Cell Trafficking in Esophageal Cancer. Cancer Immunol. Res. 2016, 4, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-S.; Hecht, S.; Carmella, S.G.; Yu, N.; LaRue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in Black Raspberries Prevent Esophageal Tumors in Rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peek, R.M.; Blaser, M.J.; Mays, D.J.; Forsyth, M.H.; Cover, T.; Song, S.Y.; Krishna, U.; A Pietenpol, J. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999, 59, 6124–6131. [Google Scholar] [PubMed]
- Dyer, V.; Brüggemann, H.; Sörensen, M.; Kühl, A.A.; Hoffman, K.; Brinkmann, V.; Reines, M.D.M.; Zimmerman, S.; Meyer, T.F.; Koch, M. Genomic features of the Helicobacter pylori strain PMSS1 and its virulence attributes as deduced from its in vivo colonisation patterns. Mol. Microbiol. 2018, 110, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J. High Throughput Genomic Analysis of Helicobacter pylori Within-Host Diversity. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, 2019. [Google Scholar]
- Arnold, I.C.; Lee, J.Y.; Amieva, M.R.; Roers, A.; Flavell, R.A.; Sparwasser, T.; Müller, A. Tolerance Rather Than Immunity Protects from Helicobacter pylori–Induced Gastric Preneoplasia. Gastroenterology 2011, 140, 199–209.e8. [Google Scholar] [CrossRef] [Green Version]
- Sierra, J.C.; Asim, M.; Verriere, T.G.; Piazuelo, M.B.; Suarez, G.; Romero-Gallo, J.; Delgado, A.G.; Wroblewski, L.E.; Barry, D.P.; Peek, R.M., Jr.; et al. Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut 2018, 67, 1247–1260. [Google Scholar] [CrossRef]
- Bochner, B.R.; A Savageau, M. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl. Environ. Microbiol. 1977, 33, 434–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimczok, D.; Clements, R.H.; Waites, K.B.; Novak, L.; E Eckhoff, D.; Mannon, P.J.; Smith, P.D.; Smythies, L.E. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Mucosal Immunol. 2010, 3, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Sebrell, T.A.; Sidar, B.; Bruns, R.; Wilkinson, R.A.; Wiedenheft, B.; Taylor, P.J.; Perrino, B.A.; Samuelson, L.C.; Wilking, J.N.; Bimczok, D. Live imaging analysis of human gastric epithelial spheroids reveals spontaneous rupture, rotation and fusion events. Cell Tissue Res. 2017, 371, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebrell, T.A.; Hashimi, M.; Sidar, B.; Wilkinson, R.A.; Kirpotina, L.; Quinn, M.; Malkoç, Z.; Taylor, P.J.; Wilking, J.N.; Bimczok, D. A Novel Gastric Spheroid Co-culture Model Reveals Chemokine-Dependent Recruitment of Human Dendritic Cells to the Gastric Epithelium. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 157–171.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
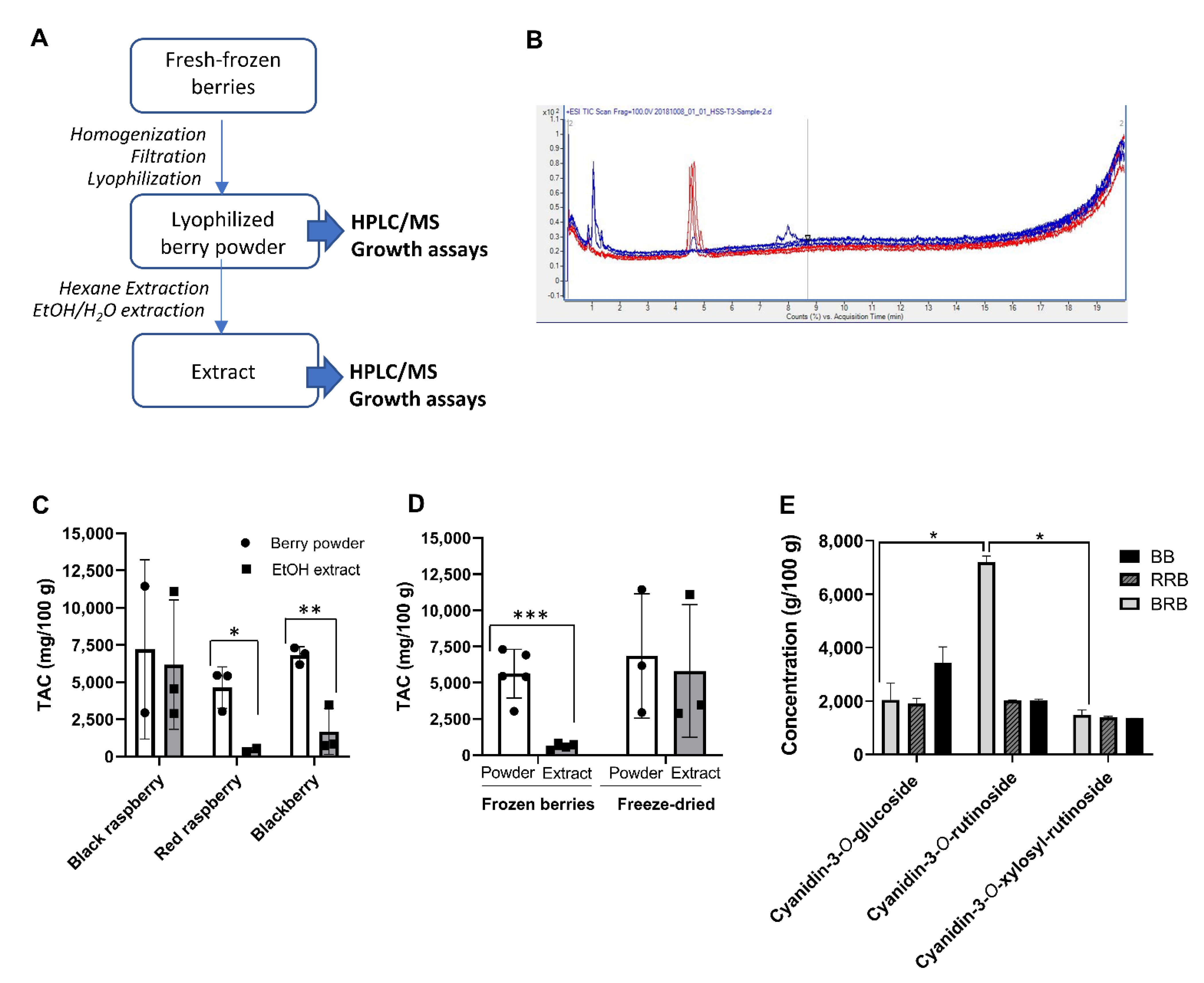


| Sample | Source | Country of Origin | Material Type | HPLC–MS * | |
|---|---|---|---|---|---|
| Berry Powder TAC (mg/100 g) | Extract TAC (mg/100 g) * | ||||
| VE-BRB | VirginExtracts, Foods Super, Bradford, PA, USA | Unknown | Freeze-dried powder | 2945 | 2885 |
| BH-BRB | BerriHealth, Berri Products LLC, Corbett, OR, USA | USA | Freeze-dried powder | 11,455 | 11,109 |
| UMN-BRB | Dr. S. Hecht, University of Minnesota, Minneapolis, MN, USA | USA | Ethanol extract | N/A | 4540 |
| USA-RRB | Great Value. Wal-Mart Stores Inc. Bentonville, AR, USA | USA | Whole frozen berries | 5424 | 554 |
| CH-RRB | Cascadian Farms, Small Planet Foods, Inc., Sedro-Woolley, WA, USA | Chile | Whole frozen berries | 3027 | 336 |
| VE-BB | VirginExtracts, Foods Super, Bradford, PA, USA | Unknown | Freeze-dried powder | 6175 | 3465 |
| MX-BB | Western Family Foods, Inc., Portland, OR, USA | Mexico | Whole frozen berries | 6924 | 748 |
| CH-BB | Cascadian Farms, Small Planet Foods, Inc., Sedro-Woolley, WA, USA | Chile | Whole frozen berries | 7312 | 847 |
| Sample | HPLC–MS * (mg/100 g) | |||
|---|---|---|---|---|
| Cyanidin-3-O-glucoside | Cyanidin-3-O-rutinoside | Cyanidin-3-O-xylosylrutinoside | ||
| VE-BRB | Powder | 1593 | 7025 | 1352 |
| Extract | 1537 | No data | 1348 | |
| BH-BRB | Powder | 2490 | 7356 | 1609 |
| Extract | 2487 | 7025 | 1597 | |
| UMN-BRB | Powder | No data | No data | No data |
| Extract | 440 | 4100 | 2420 | |
| USA-RRB | Powder | 1999 | 1993 | 1432 |
| Extract | 355 | 98 | 101 | |
| CH-RRB | Powder | 1678 | No data | 1349 |
| Extract | 267 | 43 | 26 | |
| VE-BB | Powder | 2806 | 2010 | 1359 |
| Extract | 2113 | No data | 1352 | |
| MX-BB | Powder | 3499 | 2076 | 1349 |
| Extract | 564 | 121 | 63 | |
| CH-BB | Powder | 3995 | 1966 | 1351 |
| Extract | 748 | 60 | 39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodman, C.; Lyon, K.N.; Scotto, A.; Smith, C.; Sebrell, T.A.; Gentry, A.B.; Bala, G.; Stoner, G.D.; Bimczok, D. A High-Throughput Metabolic Microarray Assay Reveals Antibacterial Effects of Black and Red Raspberries and Blackberries against Helicobacter pylori Infection. Antibiotics 2021, 10, 845. https://doi.org/10.3390/antibiotics10070845
Goodman C, Lyon KN, Scotto A, Smith C, Sebrell TA, Gentry AB, Bala G, Stoner GD, Bimczok D. A High-Throughput Metabolic Microarray Assay Reveals Antibacterial Effects of Black and Red Raspberries and Blackberries against Helicobacter pylori Infection. Antibiotics. 2021; 10(7):845. https://doi.org/10.3390/antibiotics10070845
Chicago/Turabian StyleGoodman, Candace, Katrina N. Lyon, Aitana Scotto, Cyra Smith, Thomas A. Sebrell, Andrew B. Gentry, Ganesh Bala, Gary D. Stoner, and Diane Bimczok. 2021. "A High-Throughput Metabolic Microarray Assay Reveals Antibacterial Effects of Black and Red Raspberries and Blackberries against Helicobacter pylori Infection" Antibiotics 10, no. 7: 845. https://doi.org/10.3390/antibiotics10070845
APA StyleGoodman, C., Lyon, K. N., Scotto, A., Smith, C., Sebrell, T. A., Gentry, A. B., Bala, G., Stoner, G. D., & Bimczok, D. (2021). A High-Throughput Metabolic Microarray Assay Reveals Antibacterial Effects of Black and Red Raspberries and Blackberries against Helicobacter pylori Infection. Antibiotics, 10(7), 845. https://doi.org/10.3390/antibiotics10070845





