Abstract
Helicobacter pylori infection is commonly treated with a combination of antibiotics and proton pump inhibitors. However, since H. pylori is becoming increasingly resistant to standard antibiotic regimens, novel treatment strategies are needed. Previous studies have demonstrated that black and red berries may have antibacterial properties. Therefore, we analyzed the antibacterial effects of black and red raspberries and blackberries on H. pylori. Freeze-dried powders and organic extracts from black and red raspberries and blackberries were prepared, and high-performance liquid chromatography was used to measure the concentrations of anthocyanins, which are considered the major active ingredients. To monitor antibiotic effects of the berry preparations on H. pylori, a high-throughput metabolic growth assay based on the Biolog system was developed and validated with the antibiotic metronidazole. Biocompatibility was analyzed using human gastric organoids. All berry preparations tested had significant bactericidal effects in vitro, with MIC90 values ranging from 0.49 to 4.17%. Antimicrobial activity was higher for extracts than powders and appeared to be independent of the anthocyanin concentration. Importantly, human gastric epithelial cell viability was not negatively impacted by black raspberry extract applied at the concentration required for complete bacterial growth inhibition. Our data suggest that black and red raspberry and blackberry extracts may have potential applications in the treatment and prevention of H. pylori infection but differ widely in their MICs. Moreover, we demonstrate that the Biolog metabolic assay is suitable for high-throughput antimicrobial susceptibility screening of H. pylori.
1. Introduction
Helicobacter pylori is the major cause of human gastric disease worldwide [1,2]. H. pylori is an acid-resistant, Gram-negative bacterium that persistently infects the gastric mucosa of approximately half the world’s population, leading to chronic active gastritis [1]. A proportion of infected individuals also develop peptic ulcer disease, autoimmune gastritis or gastric adenocarcinoma, the second leading cause of cancer-related mortality [3]. In spite of decades of active research, no effective vaccine to prevent H. pylori-associated illnesses has been developed [4]. Once diagnosed, H. pylori infection is generally treated with a combination of antibiotics and proton pump inhibitors. However, increased resistance to two of the standard antibiotics included in H. pylori treatment regimens, clarithromycin and metronidazole, has been reported in multiple studies, with resistance rates ranging from 22 to 80% [5,6]. Recently, clarithromycin-resistant H. pylori was included in the WHO’s high-priority pathogens list for research and development of new antibiotics [7]. Moreover, poor patient compliance with complex medication regimens contributes to decreased treatment success [8,9]. Therefore, eradication rates of H. pylori have dropped below 75% in several countries [10,11]. The high failure rate of traditional H. pylori therapies points to an urgent need for novel alternative treatments or preventative strategies to combat H. pylori infection [12].
A significant body of research in recent years has shown that natural dietary components, especially plants, contain many bioactive compounds—neutraceuticals—with antibacterial effects [13,14,15]. Multiple different berries and their products show significant antimicrobial activity in vitro and in vivo, and some promising studies suggesting effectiveness against H. pylori have been published. Thus, data by Chatterjee et al. [16] showed significant inhibition of H. pylori growth in the presence of extracts from raspberry, strawberry, cranberry, elderberry, blueberry and bilberry. In another recent study, extracts from unripe Korean raspberries and elm tree bark used in combination significantly suppressed H. pylori growth both in vitro and in a mouse model [17]. Amongst the multiple bioactive natural compounds, anthocyanins in colored berries of the genus Rubus have attracted special attention. Anthocyanins are glycosylated, water-soluble phenolic compounds that are responsible for the red, purple and blue coloring of multiple berry species [14]. Anthocyanins are strong antioxidants that have been used successfully in cancer chemoprevention models [18] and that have been implicated in the antibacterial activities of berry preparations [19,20]. In an in vitro model of H. pylori infection, the anthocyanin cyanidin 3-O-glucoside significantly decreased H. pylori-induced cell death [21]. Since anthocyanin-containing berry products also have proven anti-inflammatory effects and are stable under acidic conditions [22,23], their potential application in gastric H. pylori infection is particularly attractive.
In our study, we developed a high-throughput metabolic assay to screen different black raspberry, red raspberry and blackberry preparations for their ability to prevent H. pylori growth in vitro. In addition, a gastric organoid model was used to evaluate the biocompatibility of black raspberry extract. Our results demonstrate that all berry powders and extracts tested caused a significant reduction in H. pylori growth in two different strains at concentrations between 0.5 and 3%. An optimum preparation of black raspberry extract used at 0.5% led to complete inhibition of H. pylori growth but did not affect the viability of primary gastric epithelial cells. These results suggest that preparations from black and red raspberries and blackberries have potential as novel antimicrobial agents to combat H. pylori infection.
2. Results
2.1. Analysis of Powders and Extracts of Black and Red Raspberries and Blackberries for Anthocyanin Content and Composition
In order to study the potential antibacterial effects of black raspberry (BRB), red raspberry (RRB) and blackberry (BB) compounds on H. pylori, freeze-dried berry powders were purchased from different suppliers or were prepared in our laboratory from fresh-frozen berries. Organic extracts of all berry powders were then prepared using hexane/ethanol extraction. The workflow for sample preparation is shown in Figure 1A, and the different starting materials used are listed in Table 1.
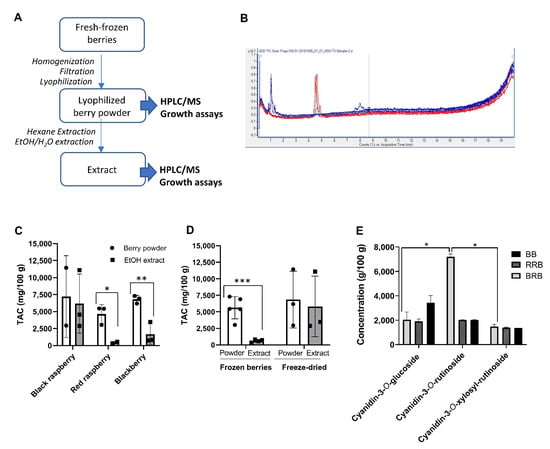
Figure 1.
Preparation and anthocyanin content analysis of black raspberries, red raspberries and blackberries. (A) Workflow for berry preparation and analysis. (B) Representative LC-MS spectrum of a berry preparation. Major peaks represent cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, and a combination of cyanidin-3-O-xylosylrutinoside and cyanidin-3-O-sambubioside. (C) Total anthocyanin content (TAC) in suspended powders and ethanol extracts of black raspberries (BRB), red raspberries (RRB) and blackberries (BB) determined by LC-MS. Individual data points, mean and SD are shown. (D) TAC in powders and extracts of BRB, RRB and BB purchased as fresh-frozen berries or as freeze-dried berry powder. Pooled data from all berries; individual data points, mean ± SD are shown. (E) Concentrations of major anthocyanins in suspended powders of BRB, RRB and BB. Statistically significant differences as determined by (C,D) Student’s t test (E) or two-way ANOVA are shown as * p < 0.05, ** p < 0.01 and *** p < 0.001.

Table 1.
Total concentrations of anthocyanins in black and red raspberry and blackberry powders and extracts determined by LC–MS.
To determine the concentration of anthocyanins, all samples were analyzed by LC–MS for the presence of keracyanin (cyanidin-3-O-rutinoside), kuromanin (cyanidin-3-O-glucoside) and cyanidin-3-O-xylosyrutinoside (Table 1 and Figure 1A,B). Because of overlapping peaks, the cyanidin-3-O-xylosyrutinoside may include cyanidin-3-O-sambubioside, another phenolic berry compound that has a similar composition and MW as the xylosylrutinoside, and that is known to be present in BRB at a low concentration [24].
Total anthocyanin content (TAC) was calculated by adding up the concentrations of all detected anthocyanins (Figure 1C and Table 1). Overall, large variations in anthocyanin concentrations were observed for berries from different sources and with different processing techniques. Interestingly, lyophilized but otherwise untreated berry powder from RRB and BB contained significantly higher amounts of anthocyanins than the water/ethanol extracts prepared in our laboratory (Figure 1C). This was likely due to an inefficient recovery of anthocyanins in extracts prepared from fresh-frozen berries that were lyophilized in-house (Figure 1D, p < 0.001, Student’s t test), because anthocyanin recovery was higher if extracts were prepared from commercial berry powders (Figure 1D). Individual data for cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, and cyanidin-3-O-xylosylrutinoside are presented in Figure 1E and Table 2. Notably, BRB, RRB and BB powders contained similar levels of cyanidin-3-O-sambubioside and cyanidin-3-O-glucoside, but cyanidin-3-O-rutinoside levels were significantly higher in BRBs than in RRBs and BBs, as previously described (Figure 1E, p < 0.05, mixed model ANOVA).

Table 2.
Anthocyanin composition within powdered berries and berry extracts determined by HPLC–MS.
2.2. Development and Validation of a High-Throughput Assay to Measure H. pylori Growth
A metabolic bacterial growth assay based on the Biolog system was developed to test a large number of different berry products at different concentrations. This system enables kinetic analysis of microbial growth in a 96-well format based on detection of a redox-sensitive dye by the OmniLog® incubator-reader [25]. Since optimal H. pylori growth requires microaerophilic conditions, the 96-well plates were sealed into a plastic sleeve with a CO2 Gen Compact sachet to reduce oxygen levels. As shown in Figure 2A and the Supplemental Video S1, addition of H. pylori bacteria to the plates at different dilutions resulted in a dose-dependent color change over 48 h. Growth curves had a typical appearance, with an exponential growth phase followed by a plateau phase (Figure 2B). Area under the curve measurements showed significant differences in the growth of H. pylori plated at different concentrations, which was confirmed by endpoint measurements at 590 nm in a standard ELISA reader (Figure 2C,D). These results show that H. pylori growth can be effectively analyzed in liquid cultures using a high-throughput metabolic growth assay.
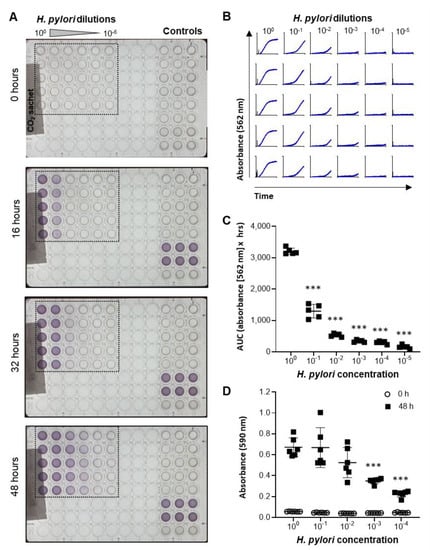
Figure 2.
Development of a high-throughput metabolic assay to measure H. pylori growth. (A) Images of 96-well plates containing various concentrations of H. pylori (100 = stock solution used at OD600 = 0.5) obtained by the OmniLog® incubator/reader at different time points after plating the bacteria. (B) Growth curves based on absorbance at 562 nm for the wells outlined in panel A. (C) Area under the curve was determined using GraphPad Prism and shows significant differences in H. pylori metabolism between cultures with different initial concentrations of bacteria. (D) Endpoint absorbance (48 h) of an H. pylori culture analyzed in a standard 96-well plate reader at 590 nm. Data are representative of n = 4 similar experiments with 5–6 technical replicates each. Individual datapoints, mean ± SD are shown. *** p< 0.01 compared to undiluted bacteria, one-way ANOVA with Dunnett’s multiple comparisons.
2.3. Analysis of the Antibacterial Effects of Black and Red Raspberry and Blackberry Powders and Extracts on H. pylori
The metabolic growth assay was utilized to determine whether anthocyanin-rich berry extracts would inhibit H. pylori growth. Metronidazole, a standard antibiotic commonly used in H. pylori treatment regimens [26], was utilized to confirm that the OmniLog® assay successfully detected antimicrobial growth inhibition of H. pylori. Metronidazole inhibited H. pylori growth in a concentration-dependent manner, with complete growth inhibition achieved at 136 µg/mL (Figure 3A,B). Next, we confirmed that the colored berry extracts did not interfere with dye detection in the OmniLog® assay. As shown in Figure 3C, BRB powder (8%) caused no significant signal over baseline after 48 h, whereas in the presence of both H. pylori PMSS1 and the metabolic dye, significant absorbance was measured (p ≤ 0.001). Absorbance was significantly decreased in the presence of BRB powder. Growth curves over 30 h revealed a concentration-dependent inhibition of H. pylori growth at BRB powder concentrations between 0.26% and 4.17% (Figure 3D). Area under the curve (AUC) calculations for the H. pylori growth curves similarly showed a significant, concentration-dependent decrease in bacterial growth beginning at 0.26% of berry powder (Figure 3E). To validate the metabolic growth data, 48 h liquid cultures from the growth experiments were re-plated on Brucella agar plates and analyzed for formation of H. pylori colonies. Consistent with the results from the metabolic assay, complete growth inhibition was seen at 2.08% and 4.17% of BRB powder, demonstrating strong antibacterial activity of the blackberries on H. pylori, whereas colony formation was observed in the absence of BRBs or with lower BRB concentrations (Figure 3E). These results demonstrate the ability of BRBs to inhibit H. pylori growth in vitro and the effectiveness of the OmniLog® assay for evaluating H. pylori growth and growth suppression by berry compounds.
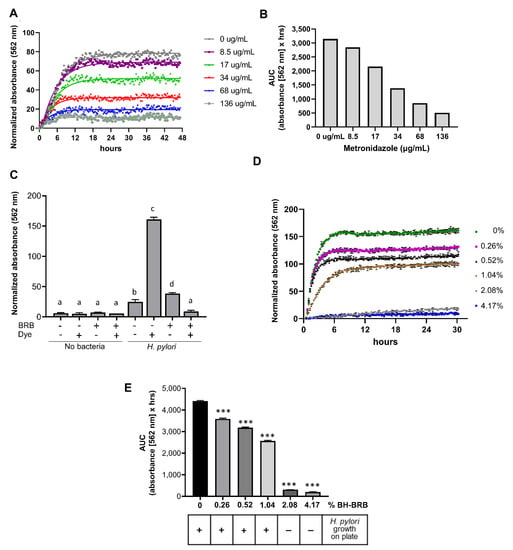
Figure 3.
Concentration-dependent growth suppression of H. pylori by metronidazole and black raspberries measured using the OmniLog® assay. (A) Absorbance of H. pylori strain 60190 measured over time in the presence of different concentrations of metronidazole, one representative experiment of three experiments is shown. (B) Area under the curve (AUC) values for the experiment shown in (A). (C) Endpoint absorbance values measured by the OmniLog® after 48 h of culture with and without H. pylori strain PMSS1, 8% BH-BRB powder, and/or tetrazolium dye. One representative experiment of three independent experiments with triplicate wells is shown. One-way ANOVA; different letters indicate statistically significant differences at p ≤ 0.001. (D) Absorbance of H. pylori cultures measured over time in the presence of different concentrations of BH-BRB powder. Mean ± SD of triplicate wells, representative of one out of three experiments. (E) AUC values for the experiment shown in (D). One-way ANOVA; *** indicates statistically significant differences from the untreated control at p ≤ 0.001. Bottom panel: matched H. pylori growth data on plates, representative of four independent experiments.
As shown in Figure 1 and Table 1 and Table 2, the composition of berry preparations was highly variable, depending on berry species, source and processing method. Therefore, the different BRB, RRB and BB whole berry powders and the extracts described above were dissolved/suspended in culture media and then compared for their ability to inhibit the growth of two well-characterized H. pylori strains, 60190 and PMSS1, in the OmniLog® microarray assay. All berry preparations tested had significant antibacterial activity (Figure 4 and Figure 5), with complete inhibition of H. pylori growth generally achieved at a concentration of about 4%. However, the different berry preparations showed a great variability in their ability to suppress H. pylori growth, with significant effects of the specific preparation identified for extracts from BRB and BB for both H. pylori strains (p ≤ 0.001) for the RRB extract and the BRB powders for strain 60190 (p ≤ 0.05) identified by two-way ANOVA The UMN BRB extract had the strongest antibacterial activity of all the preparations tested, with an MIC90 of <0.5%. Conversely, powdered berries generally were less effective than extracts (Figure 5). Both H. pylori strains had similar responses to the different berry extracts.
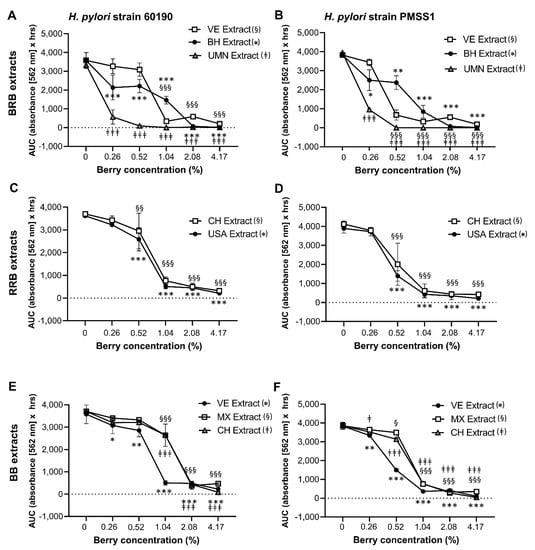
Figure 4.
Growth inhibition of H. pylori by extracts from BRB, RRB and BB. Growth of H. pylori in the presence of different concentrations of the BRB, RRB and BB extracts described in Table 1 and Table 2. Area under the curve (AUC) for metabolic culture activity was measured over 30 h for strain 60190 with (A) BRB extracts, (C) RRB extracts and (E) BB extracts and for strain PMSS1 with (B) BRB extracts, (D) RRB extracts and (F) BB extracts using the OmniLog® assay. Pooled data from n = 2–4 independent experiment with 3 technical replicates are shown. Data were analyzed by 2-factorial ANOVA. Tukey’s multiple comparisons test was used to identify differences between a specific concentration of a berry extract and the matching untreated control. Significant differences at p ≤ 0.05/p ≤ 0.01/p ≤ 0.001, respectively, are indicated by */**/***, §/§§/§§§ and ‡/‡‡/‡‡‡ as defined in the panel labels on the right.
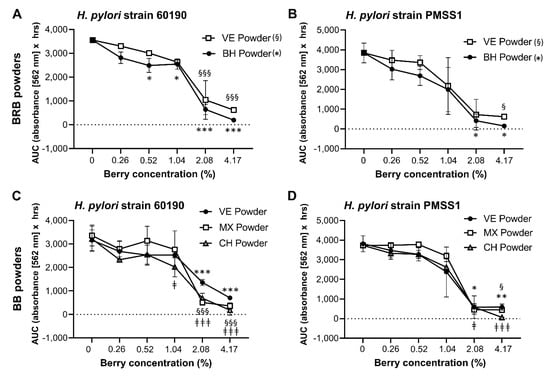
Figure 5.
Growth inhibition of H. pylori by lyophilized BRB and BB powder. (A) Growth of H. pylori strains 60190 and PMSS1 in the presence of different concentrations of the BRB and BB powders described in Table 1 and Table 2. Area under the curve (AUC) for metabolic culture activity was measured over 30 h for strain 60190 with (A) BRB extracts and (C) BB extracts and for strain PMSS1 with (B) BRB extracts and (D) BB extracts using the OmniLog® assay. Pooled data from n = 2–4 independent experiment with 3 technical replicates are shown. Data were analyzed by 2-factorial ANOVA. Tukey’s multiple comparisons test was used to identify differences between a specific concentration of a berry extract and the matching untreated control. Significant differences at p ≤ 0.05/p ≤ 0.01/p ≤ 0.001, respectively, are indicated by */**/***, §/§§/§§§ and ‡/‡‡/‡‡‡ as defined in the panel labels on the right.
To better understand the large variability in the antibacterial effects of the different berry preparations, the minimum inhibitory concentrations (MICs) required to suppress H. pylori growth were analyzed by multifactorial analysis of variance (ANOVA) of (Figure 6A). Overall, berry extracts exhibited a stronger antibacterial response, as evidenced by significantly lower MICs (p ≤ 0.001). For BRB and BB, the type of berry preparation used (powdered lyophilized powder vs. extract) was responsible for 23% of the variation in MIC. The type of berry (BRB, RRB or BB) and the strain of bacteria also significantly impacted MIC, with BRB associated with lower MICs than BB (p = 0.019), and strain PMSS1 exhibiting a slightly higher sensitivity to the antibacterial effects of berries than 60190 (p = 0.039). Notably, concentrations of berry products required to achieve antibacterial effects were almost two orders of magnitude higher than those observed for metronidazole (Figure 6B). Surprisingly, no significant relationship between the MIC and the total anthocyanin contents or the concentration of cyanidin-3-O-rutinoside or cyanidin-3-O-xylosylrutinoside was detected based on Pearson’s correlation coefficient (Figure 6C,E,F). In contrast, there was a significant positive correlation between cyanidin-3-O-glucoside concentrations and the MICs (Figure 6D, p = 0.01, R2 = 0.45), indicating that high concentrations of cyanidin-3-O-glucoside prevented antibacterial activities. These findings suggest that berry components other than the three anthocyanins analyzed contribute to antibacterial activities of BRB, BB and RRB against H. pylori.
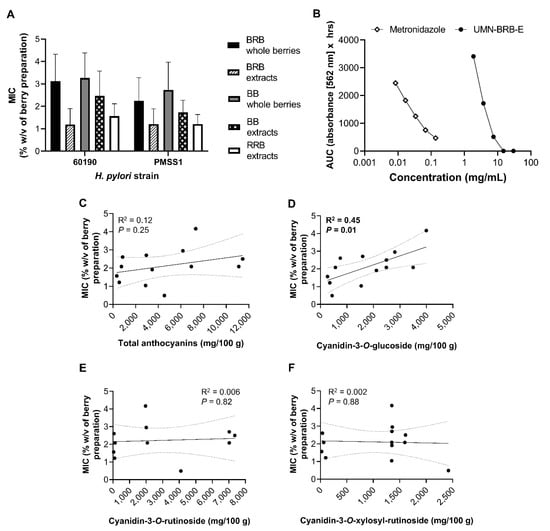
Figure 6.
Minimum inhibitory concentrations (MICs) of berry preparations for antibacterial activities against H. pylori. (A) MIC means ± SD for different berry types and preparations against H. pylori strains 60190 and PMSS1. (B) Side-by-side comparison of inhibitory effects of metronidazole and H. pylori strain 60190; data representative of n = 2 independent experiments. (C–F) Correlation between mean MIC observed with both H. pylori strains and concentrations of (C) total anthocyanins, (D) cyanidin-3-O-glucoside, (E) cyanidin-3-O-rutinoside and (F) cyanidin-3-O-xylosylrutinoside for all berry preparations analyzed. R2 = Pearson’s correlation coefficient.
2.4. Effect of BRB Extract on Gastric Epithelial Cell Viability in a Human Gastric Organoid Model
Having shown significant antibacterial effects of multiple different berry preparations against H. pylori in vitro, we next sought to confirm that the berries were not toxic to the gastric epithelium, where H. pylori bacteria generally reside. Five gastroid lines derived from non-H. pylori-infected human gastric biopsies or surgical material were cultured in the presence of UMN-BRB extract, which had the greatest antibacterial activity of all berry preparations tested (Figure 4). As shown in Figure 7, the organoids tolerated the BRB extract over a wide range of concentrations up to 5 mg/mL (0.5%) without any significant impact on organoid viability, as determined by flow cytometric analysis of 7-AAD staining and phase contrast microscopy.
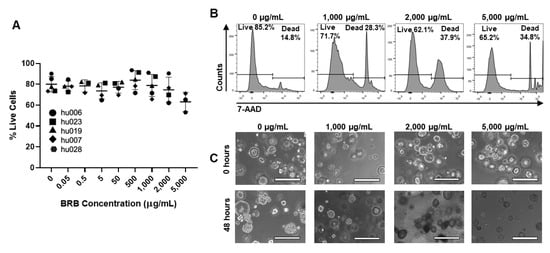
Figure 7.
Toxicity analysis of BRB extract in primary human gastric epithelial cell cultures. (A) Gastric organoid viability following 48 h of culture in the presence of UMN-BRB extract at different concentrations. Following BRB exposure, cells were harvested by trypsinization and analyzed for 7-AAD dye exclusion by flow cytometry. Pooled data from n = 3–5 independent experiments; individual data point, mean ± SD. Differences between treated organoids and untreated controls were analyzed by ANOVA. (B) Representative FACS histograms for the data in (A) showing 7-AAD staining of gastric organoid cells. (C) Representative images of organoid cultures treated with UMN-BRB extract after 0 and 48 h. Bar = 400 µm.
3. Discussion
In this study, significant antimicrobial activity of black and red raspberry and blackberry preparations against H. pylori was demonstrated in a high-throughput bacterial growth assay. Interestingly, our analyses showed that both the chemical composition and antimicrobial activity were highly variable depending on berry type, origin and processing method.
One major advancement of our study was the development of a high-throughput metabolic microarray assay compatible with the growth requirements of the microaerophilic bacterium H. pylori, which enabled us to test a large number of different berry preparations at a wide range of concentrations. The efficacy of antimicrobial treatments for H. pylori is typically still analyzed using the agar dilution or E strip diffusion methods [27,28,29,30], which are work intensive and not easy to scale up. In a previous study, Lee et al. utilized proprietary Biolog Phenotype Microarray plates to evaluate the ability of H. pylori to metabolize different carbon sources [31]. Here, Biolog’s tetrazolium dye and media were used together with metronidazole and various berry dilutions on standard 96- well plates to dynamically analyze H. pylori growth inhibition. By sealing liquid H. pylori cultures in transparent, gas-impermeable plastic sleeves with small CO2 sachets, microaerophilic growth conditions were maintained. Importantly, microarray culture results matched growth profiles on standard agar plates, as demonstrated by re-culturing H. pylori samples from the microarray plates. The presence of colored berry preparations did not interfere with dye detection, suggesting that our analysis method is suitable for use with other colored natural products as well as a wide range of other chemical compounds.
The metabolic growth assay revealed that black and red raspberries and blackberries have the capacity to block H. pylori growth in vitro. Antimicrobial properties of Rubus berries have been described in multiple previous studies [15,32,33,34,35]. Our analysis of powders and extracts from BRB, RRB and BB from various suppliers and geographical regions revealed that all berry preparations had significant antimicrobial activity against H. pylori in vitro, regardless of the H. pylori strain used. However, our analyses showed that both the chemical composition and antimicrobial activity (MIC90) were highly variable depending on berry type, origin and processing method, corroborating with data from previous studies. Concentrations of active ingredients in Rubus berries are known to vary based on geographical location, environmental conditions and berry type [34,36]. In addition, post-harvest processing and storage may affect active ingredients [37,38]. Moreover, different bacterial species vary in their susceptibility to the antibacterial effects of berries [39]. One additional consideration that was not tested here is that consumption of berries or berry products can impact other bacteria in the gastrointestinal tract [40], which in turn might impact H. pylori growth [41]. Our data indicate that organic extracts were more potent antimicrobials than powdered berries. Overall, these previous studies and our data indicate that each berry product needs to be carefully tested for specific biological activity and applications prior to use as a neutraceutical. The top-performing preparation in our study was a black raspberry extract from the University of Minnesota (UMB-BRB-E), which achieved complete H. pylori growth inhibition at (>90%) at 0.5% (5 mg/mL). This inhibitory concentration is similar to or lower than those of Rubus extracts described in other studies [16,32,42], but several log folds higher than standard antibiotics such as amoxicillin and clarithromycin [43] or the metronidazole used in our study, which completely blocked H. pylori growth at 136 µg/mL.
Since anthocyanins are considered the major active ingredients of black and red berries, we hypothesized that anthocyanins would also be responsible for the antibacterial activities observed in our experiments. It was expected that correlation analysis would show a significant inverse relationship between the anthocyanin concentration and the MIC of the berry preparations. Surprisingly, no significant correlation between the concentration of any anthocyanin analyzed in our preparations and increased antibacterial activity was detected, indicating that antimicrobial activity was largely independent of anthocyanins. Anthocyanins exhibit antimicrobial activity against Gram-negative bacteria by causing damage to the cell walls, membranes, and intercellular matrix [44]. Importantly, anthocyanins have been linked to the antimicrobial effects of berry preparations in previous studies [45,46,47] and are responsible for the major chemopreventive effects of blackberries and black and red raspberries [18,24,48,49,50]. However, our correlation analysis suggests that the antibacterial effects against H. pylori were independent of anthocyanins and thus must be caused by other active compounds. Indeed, ellagic acid, another polyphenol present in red and black berries, is known to exert antibacterial activity against H. pylori as well as other bacteria [17,51,52]. In addition, Lengsfeld et al. demonstrated that berry-derived polysaccharides can combat H. pylori infection in vivo by preventing bacterial binding to the gastric mucosa [53]. Additional studies have shown antibacterial effects for berry-derived sanguiin H-6 [35] and rubusoside [54]. Further experiments are needed to identify the raspberry and blackberry compounds that mediate antimicrobial activity against H. pylori.
It remains to be tested whether BRB extract can be used to successfully treat H. pylori infection in vivo. Berry compounds have been investigated in many studies, and their pharmacokinetics and pharmacodynamics have previously been characterized [51,55]. In our study, human gastric organoids were used as model of primary human gastric epithelial cells to analyze compatibility of BRB extract with gastric epithelial cells, since they closely represent the architecture and cellular complexity of the human gastric mucosa [56,57]. Organoids are three-dimensional long-term cultures of primary cells maintained in a gelatinous extracellular matrix in the presence of specific growth factors. Importantly, exposure of the organoids to BRB did not negatively impact cell viability at the concentrations tested. Moreover, previous animal studies aimed at characterizing the chemopreventive properties of berries have demonstrated that berry products including powdered BRBs are well tolerated at dietary concentrations of up to 10% [58] and that supplementation of the diet with 5% BRB powder, equivalent to 45 g/day in humans [59], prevented esophageal, oral and colon cancer in rats and colonic polyps in mice [48,60,61]. In a phase I clinical trial, administration of 60 g of BRB powder per day had beneficial effects in colorectal cancer patients with no significant side effects except transient diarrhea or constipation [62]. Other studies have demonstrated that berry extracts have anti-Helicobacter activity in animal models. Thus, Park et al. [17] recently showed that extracts prepared from dried, unripened Korean raspberry (Rubus crataegifolius) decreased H. pylori colonization by about 4 log-fold in a murine model of infection with H. pylori strain SS1. Notably, the berry preparation used in the study by Park et al. was highly potent, with an in vitro MIC90 of 150 µg/mL [17].
In summary, we have established a high-throughput metabolic growth assay to analyze antimicrobial effects of berry preparations against H. pylori. Both freeze-dried powders and ethanol extracts from BRBs, RRBs and BBs obtained from various sources significantly suppressed growth of multiple H. pylori strains in vitro. Toxicity studies with human gastric organoids demonstrated good biocompatibility over a wide range of concentrations, including the MIC90 determined in the growth assay. Together, our findings confirm the potential of berry products as antimicrobial agents but highlight the importance of carefully selecting specific preparations with high antibacterial activity.
4. Materials and Methods
4.1. Berry Powders and Preparation of Extracts
Commercially available black raspberry (Rubus occidentalis; BRB), blackberry (Rubus fruticosus; BB) and red raspberry (Rubus idaeus; RRB) samples were either purchased as freeze-dried powders or fresh-frozen whole berries. One additional BRB extract prepared as described in previous studies in Dr. S. Hecht’ laboratory at the University of Minnesota was included for comparison [50,63,64] Suppliers and countries of origin for the berries are listed in Table 1. Fresh-frozen whole berries were processed into freeze-dried powder using a food processor (Cuisinart® Elemental, Stamfort, CT, USA) and lyophilizer (SP VirTis Genesis Pilot Lyophilizer, Warminster, PA, USA) (Figure 1A). Berry powders were either used directly in experiments after mixing the material with IF-10a media plus dye D, both Biolog, Hayward, CA, USA at a final concentration of 0.26–4.17% (w/v; powders) or were processed for hexane/ethanol extraction (extracts). Notably, berry powders contain both water soluble and insoluble materials, so that mixing of the powders with aqueous media results in a colloidal suspension. For hexane/ethanol extraction, nonpolar compounds were extracted using 3 × 100 mL hexane per 10 g powder. Samples were filtered between each extraction, and the hexane filtrate discarded. Anthocyanins and all water-soluble compounds were then extracted using an 80:20 ethanol:water mixture (3 × 100 mL per 10 g sample). This extract was dried to a syrup under reduced pressure at 30 °C then lyophilized to yield between 1 and 4 g per 20 g powder. All berry preparations were stored in airtight containers at −20 °C until use.
4.2. Analysis of Anthocyanin Content
Concentrations of major active compounds, i.e., cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, cyanidin-3-O-xylosyl-rutinoside and cyanidin-3-sambubioside, for both powder and extract samples were measured by HPLC–MS. The lyophilized samples were mixed with 80:20 (water: sample). The samples were filtered and then injected into an LC–MS system (Agilent 6538 UHD-QTOF equipped with Agilent 1290 infinity UPLC). Upon extracting the chromatograms based on the reported m/z, calculations were performed by integrating the peak to obtain the area. Anthocyanin standards were purchased from Extrasynthese S.A.S. (Lyon, France).
4.3. Helicobacter pylori Strains and Culture Conditions
Two well-characterized cagA+, vacA s1/m1 H. pylori strains, originally isolated from human patients, were used in our experiments: the reference strain 60190 (kind gift from Dr. G. Perez-Perez, New York University, ATCC #49503 [65]), and strain PMSS1 (kind gift from Dr. K. Wilson, Vanderbilt University), which is widely used in murine infection experiments [66]. H. pylori strain 60190 was shown to be susceptible to metronidazole, amoxicillin, clarithromycin, levofloxacin, rifampicin and tetracycline [67], and strain PMSS1 was confirmed to be susceptible to metronidazole, amoxicillin, clarithromycin, and tetracycline in previous studies [68,69]. For the experiments, bacteria were grown at 37 °C under microaerophilic conditions on Brucella agar plates, 5% sheep blood (Becton Dickinson) for 3 days. Colonies were harvested into warm Brucella broth supplemented with 10% FBS and were then cultured in a shaking incubator for a further 18 h period prior to use in the experiments.
4.4. High-Throughput Helicobacter pylori Growth Assay
High-throughput bacterial growth assays were performed in 96-well plates using an OmniLog (Biolog, Hayward, CA, USA) plate reader-incubator. Bacterial growth was visualized with a proprietary redox-sensitive tetrazolium dye [70] (dye D, Biolog). Serial dilutions of berry suspensions or extracts (0.26–4.17% w/v) or of metronidazole (8.5–136 µg/mL; Acros Organics, Fair Lawn, NJ, USA) prepared in IF-10a were added to the plates as indicated together with dye D (0.01%) and PM additive (0.05% BSA, 0.01% NaHCO3 and 0.045% glucose w/v final concentrations). Live H. pylori was resuspended in IF-10a (Biolog) to a final OD600 = 0.5, which corresponds to 3.4 × 108 bacteria/mL [71], and 20 µL of the bacterial suspension were added to the plates together with berry preparations at appropriate dilutions, for a total volume of 120 µL. For analysis, loaded plates were sealed in a gas-impermeable bag with a CO2 Compact sachet (Oxoid, Nepean, ON, Canada), following the manufacturer’s instructions for culturing of microaerophilic bacteria within the OmniLog incubator-reader. Using the bags and sachets was necessary to create microaerophilic conditions, since the OmniLog does not have a controlled CO2 atmosphere. Plates then were incubated at 37 °C in the OmniLog incubator for 30–48 h. Absorbance values were recorded at 562 nm every 15 min. In some instances, 10-fold serial dilutions of the H. pylori cultures were recovered from the plates and were re-streaked on Brucella agar plates to confirm growth and growth suppression.
4.5. Data Analysis for Bacterial Growth Assays
To analyze H. pylori growth inhibition by berry compounds, OmniLog data were exported to Excel using the Biolog Data Converter (version 1.0) and PM Analysis Software (Microbe, version 1.20.02, all Biolog, Hayward, CA, USA). Absorption data for each sample and time point were normalized to baseline by subtracting the average of the first four absorption values from each data point. To quantitate bacterial growth over time, peak area under the curve (AUC; absorbance [562 nm] × h) was determined using GraphPad version 8.3.1 (San Diego, CA, USA). The minimum inhibitory concentration 90 (MIC90) of a berry preparation was defined as the concentration at which the AUC values for the growth curves were decreased to ≤10% of the maximum.
4.6. Human Gastric Organoid Culture and Viability Assay
Human gastric organoid cultures (gastroids) were established and maintained as previously described [72,73]. Briefly, human gastric tissue samples were obtained with informed consent and IRB approval from patients undergoing endoscopy and biopsy at the Bozeman Health Deaconess Hospital (protocol DB050718-FC). Alternatively, tissue samples from sleeve gastrectomy surgeries were provided by the National Disease Research Interchange (protocol DB062615-EX). None of the donors were positive for active H. pylori infection, as determined by rapid urease CLO test (Halyard Health, Alpharetta, GA, USA). Gastric glands were prepared by collagenase digestion and then were plated in Matrigel. Following polymerization, matrigel was overlaid with L-WRN medium which includes Advanced DMEM/F12 (Gibco by Life Technologies, Grand Island, NY, USA) and 50% supernatant from murine L-WRN cells—which secrete Wnt3a, noggin, and R-spondin 3—and supplemented with 10% FBS (Rocky Mountain Bio, Missoula, MT, USA), 1% L-Glutamine, 10µM Y-27632 (Tocris Biosciences, Bristol, UK), 10 µM SB-431542 (Tocris Biosciences, Bristol, UK), and 10 mM HEPES buffer. Black raspberry extract prepared in 90% DMSO and 10% HCl was externally administered to the organoids for a 48 h treatment at 37 °C with 5% CO2. Control cultures were treated with dilutions of 90% DMSO/10% HCl alone. To determine cell viability, organoids were harvested by trypsinization, and single-cell suspensions stained with 7-aminoactinomycin D (7-AAD; ThermoFisher Scientific, Waltham, MA, USA) were analyzed on an LSR II flow cytometer (Becton Dickinson).
4.7. Statistical Analysis
Data shown are representative of three or more replicate experiments. For OmniLog assays, 3–6 technical replicates were prepared. All data were analyzed using GraphPad version 8.3.1 (San Diego, CA, USA). Data are shown as the mean ± SD. Student’s t test or a one- or two-way ANOVA with Tukey’s or Dunnett’s multiple comparisons test were used to determine statistical significance. Differences were considered significant at p ≤ 0.05.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10070845/s1, Video S1: Time lapse series of plate images of the H. pylori culture in Figure 2 obtained on the OmniLog™ incubator-reader shows color development consistent with H. pylori metabolism and growth over 48 h.
Author Contributions
Conceptualization and methodology, C.G., G.D.S. and D.B.; investigation, C.G., K.N.L., A.S., C.S., T.A.S., A.B.G., G.B. and D.B.; formal analysis, C.G., K.N.L., A.S., G.B., G.D.S. and D.B.; writing—original draft preparation, C.G., K.N.L., G.D.S. and D.B.; writing—review and editing, all authors; funding acquisition, G.D.S., C.G. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grant funding from the Oregon Raspberry and Blackberry Commission (to GS and CG; https://oregon-berries.com/, accessed on 5 June 2021); Montana INBRE (NIH award P20GM103474, to DB, AS and KL; https://inbre.montana.edu/, accessed on 5 June 2021); the Montana Agricultural Experiment Station (project #1015768/MONB00450, to DB; https://agresearch.montana.edu/maes.html, accessed on 5 June 2021) and an equipment grant from the M. J. Murdock Charitable Trust (#2016028, to DB; https://murdocktrust.org/grant-opportunities/, accessed on 5 June 2021). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Human tissue sample collection was approved by the Institutional Review Board of Montana State University, protocols DB050718-FC and DB062615-EX.
Informed Consent Statement
Written consent was obtained from study participants unless collected materials were de-identified surgical discard materials covered under exempt protocol DB062615-EX.
Data Availability Statement
Original data will be made available by the authors upon reasonable request.
Acknowledgments
The collaborative support of Bozeman Health Deaconess Hospital for collecting human tissue samples is greatly appreciated. We also would like to thank Stephen Hecht, University of Minnesota, for providing BRB extract.
Conflicts of Interest
G.S and G.C. have received research grants from the Oregon Raspberry and Blackberry Commission. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. G.S. is the owner of Berristone Supplements, which sells berry-based nutritional supplements. All other authors declare no conflicts of interest.
References
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017, 22, e12403. [Google Scholar] [CrossRef] [PubMed]
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori–Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, T.G.; Czinn, S.J. Current Status and Prospects for a Helicobacter pylori Vaccine. Gastroenterol. Clin. N. Am. 2015, 44, 677–689. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Liou, J.-M.; Gisbert, J.P.; O’Morain, C. Review: Treatment of Helicobacter pylori Infection 2019. Helicobacter 2019, 24, e12640. [Google Scholar] [CrossRef] [Green Version]
- Serrano, C.A.; Leon-Rios, M.; Palma, C.; Vera, M.; Hernandez, C.; Harris, P.R. Helicobacter pylori-Clarithromycin Resistance in Symptomatic Pediatric Patients in a High Prevalence Country. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e56–e60. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Al-Eidan, F.A.; McElnay, J.C.; Scott, M.G.; McConnell, J.B. Management of Helicobacter pylori eradication—The influence of structured counselling and follow-up. Br. J. Clin. Pharmacol. 2002, 53, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Cutler, A.F.; Schubert, T.T. Patient factors affecting Helicobacter pylori eradication with triple therapy. Am. J. Gastroenterol. 1993, 88, 505–509. [Google Scholar]
- Gatta, L.; Vakil, N.; Vaira, D.; Scarpignato, C. Global eradication rates for Helicobacter pylori infection: Systematic review and meta-analysis of sequential therapy. BMJ 2013, 347, f4587. [Google Scholar] [CrossRef] [Green Version]
- Liou, J.-M.; Chang, C.-Y.; Chen, M.-J.; Chen, C.-C.; Fang, Y.-J.; Lee, J.-Y.; Wu, J.-Y.; Luo, J.-C.; Liou, T.-C.; Chang, W.-H.; et al. The Primary Resistance of Helicobacter pylori in Taiwan after the National Policy to Restrict Antibiotic Consumption and Its Relation to Virulence Factors—A Nationwide Study. PLoS ONE 2015, 10, e0124199. [Google Scholar] [CrossRef]
- Graham, D.Y. Transitioning of Helicobacter pylori Therapy from Trial and Error to Antimicrobial Stewardship. Antibiotics 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.-L.; Oksman-Caldentey, K.-M. Bioactive berry compounds-novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005, 67, 8–18. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.K.; Padhi, L.; Leyssen, P.; Liu, M.; Neyts, J.; Luyten, W. Antimicrobial, Anthelmintic, and Antiviral Activity of Plants Traditionally Used for Treating Infectious Disease in the Similipal Biosphere Reserve, Odisha, India. Front. Pharmacol. 2017, 8, 658. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S.J. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Mol. Cell. Biochem. 2004, 265, 19–26. [Google Scholar] [CrossRef]
- Park, J.U.; Cho, J.S.; Kim, J.S.; Kim, H.K.; Jo, Y.H.; Rahman, A.A.; Lee, Y.I. Synergistic Effect of Rubus crataegifolius and Ulmus macrocarpa Against Helicobacter pylori Clinical Isolates and Gastritis. Front. Pharmacol. 2020, 11, 4. [Google Scholar] [CrossRef]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samad, M.A.; Hashim, S.H.; Simarani, K.; Yaacob, J.S. Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules 2016, 21, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Woo, H.; Park, M.; Rhee, K.-J.; Moon, C.; Lee, D.; Seo, W.D.; Kim, J.B. Cyanidin 3-O-Glucoside Reduces Helicobacter pylori VacA-Induced Cell Death of Gastric KATO III Cells through Inhibition of the SecA Pathway. Int. J. Med. Sci. 2014, 11, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Markakis, P. Anthocyanins as Food Colors; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Levy, R.; Okun, Z.; Shpigelman, A. The Influence of Chemical Structure and the Presence of Ascorbic Acid on Anthocyanins Stability and Spectral Properties in Purified Model Systems. Foods 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulio, A.Z.; Reese, R.N.; Wyzgoski, F.J.; Rinaldi, P.L.; Fu, R.; Scheerens, J.C.; Miller, A.R. Cyanidin 3-Rutinoside and Cyanidin 3-Xylosylrutinoside as Primary Phenolic Antioxidants in Black Raspberry. J. Agric. Food Chem. 2008, 56, 1880–1888. [Google Scholar] [CrossRef]
- Bochner, B.R. Global phenotypic characterization of bacteria. FEMS Microbiol. Rev. 2008, 33, 191–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chey, W.D.; I Leontiadis, G.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, N.; Nam, R.H.; Lee, S.M.; Kwon, Y.H.; Sohn, S.D.; Kim, J.M.; Lee, D.H.; Jung, H.C. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter 2019, 24, e12561. [Google Scholar] [CrossRef]
- Tan, B.; Yang, J.-C.; Young, C.L.; Bishu, S.; Owyang, S.Y.; El-Zaatari, M.; Zhang, M.; Grasberger, H.; Qian, J.-M.; Kao, J.Y. Helicobacter pylori Antimicrobial Susceptibility Testing-Guided Salvage Therapy in the USA: A Real Life Experience. Dig. Dis. Sci. 2017, 63, 437–445. [Google Scholar] [CrossRef]
- Biernat, M.M.; Poniewierka, E.; Błaszczuk, J.; Czapla, L.; Kempiński, R.; Ksiądzyna, D.; Grabińska, J.; Bińkowska, A.; Mégraud, F.; Gościniak, G. Antimicrobial susceptibility of Helicobacter pylori isolates from Lower Silesia, Poland. Arch. Med. Sci. 2014, 10, 505–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastukh, N.; Peretz, A.; Brodsky, D.; Isakovich, N.; Azrad, M.; On, A. Antimicrobial susceptibility of Helicobacter pylori strains isolated from children in Israel. J. Glob. Antimicrob. Resist. 2018, 12, 175–178. [Google Scholar] [CrossRef]
- Lee, W.C.; Goh, K.L.; Loke, M.F.; Vadivelu, J. Elucidation of the Metabolic Network of Helicobacter pylori J99 and Malaysian Clinical Strains by Phenotype Microarray. Helicobacter 2017, 22, e12321. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.; Kamimoto, M.; Shimamoto, T.; Shimamoto, T. Antimicrobial Effects of Blueberry, Raspberry, and Strawberry Aqueous Extracts and their Effects on Virulence Gene Expression in Vibrio cholerae. Phytotherapy Res. 2015, 29, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Wojnicz, D.; Cisowska, A.; Walkowski, S.; Sroka, Z.; Gabrielska, J.; Hendrich, A.B. Biological Activity of the Methanol and Water Extracts of the Fruits of Anthocyanin-Rich Plants Grown in South-West Poland. Nat. Prod. Commun. 2015, 10, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Bakar, M.F.; Ismail, N.A.; Isha, A.; Ling, A.L.M. Phytochemical Composition and Biological Activities of Selected Wild Berries (Rubus moluccanus L., R. fraxinifolius Poir., and R. alpestris Blume). Evid. Based Complement. Altern. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A.; Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; et al. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Klesk, K.; Qian, M.; Martin, R.R. Aroma Extract Dilution Analysis of cv. Meeker (Rubus idaeus L.) Red Raspberries from Oregon and Washington. J. Agric. Food Chem. 2004, 52, 5155–5161. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. Effect of Processing Methods and Storage Time on the Content of Bioactive Compounds in Blue Honeysuckle Berry Purees. Agronomy 2019, 9, 860. [Google Scholar] [CrossRef] [Green Version]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef]
- Gurbuz, I.; Ozcelik, B.; Gunbatan, T.; Akkol, E.K.; Sahinoz, M.; Akaydin, G. Antibacterial, antifungal and enzyme inhibitory effects of selected plants from Turkey. Pak. J. Pharm. Sci. 2018, 34, 1011–1017. [Google Scholar]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Serrano, C.; Harris, P.R.; Smith, P.D.; Bimczok, D. Interactions between H. pylori and the gastric microbiome: Impact on gastric homeostasis and disease. Curr. Opin. Physiol. 2021, 21, 57–64. [Google Scholar] [CrossRef]
- Tabarki, S.; Aouadhi, C.; Mechergui, K.; Hammi, K.M.; Ksouri, R.; Raies, A.; Toumi, L. Comparison of Phytochemical Composition and Biological Activities of Rubus ulmifolius Extracts Originating from Four Regions of Tunisia. Chem. Biodivers 2016, 14, e1600168. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.S.; Jung, H.C.; Kim, N.; Kim, Y.-J.; Song, I.S. Distribution of Antibiotic MICs for Helicobacter pylori Strains over a 16-Year Period in Patients from Seoul, South Korea. Antimicrob. Agents Chemother. 2004, 48, 4843–4847. [Google Scholar] [CrossRef] [Green Version]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and Antibacterial Properties of Anthocyanins and Flavonols from Fruits of Black and Non-Black Mulberries. Molecules 2017, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.-P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-M.; Guttenplan, J.; Sun, Y.-W.; Cooper, T.; Shalaby, N.; Kosinska, W.; Benitez, G.; Aliaga, C.; Zhu, J.; Liao, J.; et al. Effects of Black Raspberry on Dibenzo[a,l]Pyrene Diol Epoxide Induced DNA Adducts, Mutagenesis, and Tumorigenesis in the Mouse Oral Cavity. Cancer Prev. Res. 2018, 11, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- God, J.; Tate, P.L.; Larcom, L.L. Red raspberries have antioxidant effects that play a minor role in the killing of stomach and colon cancer cells. Nutr. Res. 2010, 30, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.-S.; Ransom, B.W.; Carmella, S.G.; Kuo, C.-T.; Siddiqui, J.; Chen, J.-H.; Oshima, K.; Huang, Y.-W.; et al. Chemoprevention of Esophageal Cancer with Black Raspberries, Their Component Anthocyanins, and a Major Anthocyanin Metabolite, Protocatechuic Acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Ceci, C.; Graziani, G.; Faraoni, I.; Cacciotti, I. Strategies to improve ellagic acid bioavailability: From natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology 2020, 31, 382001. [Google Scholar] [CrossRef]
- De, R.; Sarkar, A.; Ghosh, P.; Ganguly, M.; Karmakar, B.C.; Saha, D.R.; Halder, A.; Chowdhury, A.; Mukhopadhyay, A.K. Antimicrobial activity of ellagic acid against Helicobacter pylori isolates from India and during infections in mice. J. Antimicrob. Chemother. 2018, 73, 1595–1603. [Google Scholar] [CrossRef]
- Lengsfeld, C.; Deters, A.; Faller, G.; Hensel, A. High Molecular Weight Polysaccharides from Black Currant Seeds Inhibit Adhesion of Helicobacter pylori to Human Gastric Mucosa. Planta Med. 2004, 70, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T.T.H.; Jin, J.; Septiana, I.; Son, G.-M.; Lee, G.-H.; Jung, Y.-J.; Qureshi, D.; Mok, I.K.; Pal, K.; et al. Anti-cariogenic Characteristics of Rubusoside. Biotechnol. Bioprocess Eng. 2019, 24, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Blutt, S.E.; Crawford, S.E.; Ramani, S.; Zou, W.Y.; Estes, M.K. Engineered Human Gastrointestinal Cultures to Study the Microbiome and Infectious Diseases. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.R.; Spence, J.R. Gastrointestinal Organoids: Understanding the Molecular Basis of the Host–Microbe Interface. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Kula, M.; Krauze-Baranowska, M. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutr. Cancer 2015, 68, 18–28. [Google Scholar] [CrossRef]
- Stoner, G.D.; Wang, L.-S.; Casto, B.C. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [Google Scholar] [CrossRef] [Green Version]
- Stoner, G.D.; Wang, L.-S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple Berry Types Prevent N-nitrosomethylbenzylamine-Induced Esophageal Cancer in Rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Skaer, C.W.; Wang, H.-T.; Stirdivant, S.M.; Young, M.R.; Oshima, K.; Stoner, G.D.; Lechner, J.F.; Huang, Y.-W.; Wang, L.-S. Black raspberries suppress colonic adenoma development in ApcMin/+mice: Relation to metabolite profiles. Carcinogenesis 2015, 36, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-S.; Arnold, M.; Huang, Y.-W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.-M.; Riedl, K.; Schwartz, S.; Frankel, W.; et al. Modulation of Genetic and Epigenetic Biomarkers of Colorectal Cancer in Humans by Black Raspberries: A Phase I Pilot Study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef] [Green Version]
- Peiffer, D.S.; Wang, L.-S.; Zimmerman, N.P.; Ransom, B.W.; Carmella, S.G.; Kuo, C.-T.; Chen, J.-H.; Oshima, K.; Huang, Y.-W.; Hecht, S.S.; et al. Dietary Consumption of Black Raspberries or Their Anthocyanin Constituents Alters Innate Immune Cell Trafficking in Esophageal Cancer. Cancer Immunol. Res. 2016, 4, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-S.; Hecht, S.; Carmella, S.G.; Yu, N.; LaRue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in Black Raspberries Prevent Esophageal Tumors in Rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peek, R.M.; Blaser, M.J.; Mays, D.J.; Forsyth, M.H.; Cover, T.; Song, S.Y.; Krishna, U.; A Pietenpol, J. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999, 59, 6124–6131. [Google Scholar] [PubMed]
- Dyer, V.; Brüggemann, H.; Sörensen, M.; Kühl, A.A.; Hoffman, K.; Brinkmann, V.; Reines, M.D.M.; Zimmerman, S.; Meyer, T.F.; Koch, M. Genomic features of the Helicobacter pylori strain PMSS1 and its virulence attributes as deduced from its in vivo colonisation patterns. Mol. Microbiol. 2018, 110, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J. High Throughput Genomic Analysis of Helicobacter pylori Within-Host Diversity. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, 2019. [Google Scholar]
- Arnold, I.C.; Lee, J.Y.; Amieva, M.R.; Roers, A.; Flavell, R.A.; Sparwasser, T.; Müller, A. Tolerance Rather Than Immunity Protects from Helicobacter pylori–Induced Gastric Preneoplasia. Gastroenterology 2011, 140, 199–209.e8. [Google Scholar] [CrossRef] [Green Version]
- Sierra, J.C.; Asim, M.; Verriere, T.G.; Piazuelo, M.B.; Suarez, G.; Romero-Gallo, J.; Delgado, A.G.; Wroblewski, L.E.; Barry, D.P.; Peek, R.M., Jr.; et al. Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut 2018, 67, 1247–1260. [Google Scholar] [CrossRef]
- Bochner, B.R.; A Savageau, M. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl. Environ. Microbiol. 1977, 33, 434–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimczok, D.; Clements, R.H.; Waites, K.B.; Novak, L.; E Eckhoff, D.; Mannon, P.J.; Smith, P.D.; Smythies, L.E. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Mucosal Immunol. 2010, 3, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Sebrell, T.A.; Sidar, B.; Bruns, R.; Wilkinson, R.A.; Wiedenheft, B.; Taylor, P.J.; Perrino, B.A.; Samuelson, L.C.; Wilking, J.N.; Bimczok, D. Live imaging analysis of human gastric epithelial spheroids reveals spontaneous rupture, rotation and fusion events. Cell Tissue Res. 2017, 371, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebrell, T.A.; Hashimi, M.; Sidar, B.; Wilkinson, R.A.; Kirpotina, L.; Quinn, M.; Malkoç, Z.; Taylor, P.J.; Wilking, J.N.; Bimczok, D. A Novel Gastric Spheroid Co-culture Model Reveals Chemokine-Dependent Recruitment of Human Dendritic Cells to the Gastric Epithelium. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 157–171.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).