Impact of Cardiovascular Failure in Intensive Care Unit-Acquired Pneumonia: A Single-Center, Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Definition of Cardiovascular Failure
2.4. Definition of Pneumonia, Microbiology Studies and Treatment
2.5. Data Analysis
2.6. Primary and Secondary Outcomes
3. Results
3.1. Study Sample
3.2. Characteristics upon ICUAP Diagnosis
3.3. Inflammatory Markers
3.4. Aetiology and Antibiotic Treatment
3.5. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin-Loeches, I.; Rodriguez, A.H.; Torres, A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr. Opin. Crit. Care 2018, 24, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Righy, C.; do Brasil, P.; Vallés, J.; Bozza, F.A.; Martin-Loeches, I. Systemic antibiotics for preventing ventilator-associated pneumonia in comatose patients: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 67. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and cardiovascular failure among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Brun-Buisson, C.; Meshaka, P.; Pinton, P.; Vallet, B. EPISEPSIS: A reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004, 30, 580–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Tomlanovich, M. Early goal-directed therapy in the treatment of severe sepsis and cardiovascular failure. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [Green Version]
- Brun-Buisson, C.; Doyon, F.; Carlet, J.; Dellamonica, P.; Gouin, F.; Lepoutre, A.; Régnier, B. Incidence, risk factors, and outcome of severe sepsis and cardiovascular failure in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA 1995, 274, 968–974. [Google Scholar] [CrossRef]
- Alberti, C.; Brun-Buisson, C.; Burchardi, H.; Martin, C.; Goodman, S.; Artigas, A.; Le Gall, J. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef]
- Leone, M.; Bouadma, L.; Bouhemad, B.; Brissaud, O.; Dauger, S.; Gibot, S.; Chanques, G. Hospital-acquired pneumonia in ICU. Anaesth. Crit. Care Pain Med. 2018, 37, 83–98. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.J.J.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet. Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Valles, J.; Mesalles, E.; Mariscal, D.; del Mar Fernández, M.; Peña, R.; Jiménez, J.L.; Rello, J. A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Intensive Care Med. 2003, 29, 1981–1988. [Google Scholar] [CrossRef]
- Aydogdu, M.; Gursel, G. Predictive factors for cardiovascular failure in patients with ventilator-associated pneumonia. South Med. J. 2008, 101, 1222–1226. [Google Scholar] [CrossRef]
- von Dossow, V.; Rotard, K.; Redlich, U.; Hein, O.V.; Spies, C.D. Circulating immune parameters predicting the progression from hospital-acquired pneumonia to cardiovascular failure in surgical patients. Crit. Care 2005, 9, R662–R669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillas, G.; Vassilakopoulos, T.; Plantza, P.; Rasidakis, A.; Bakakos, P. C-reactive protein and procalcitonin as predictors of survival and cardiovascular failure in ventilator-associated pneumonia. Eur. Respir. J. 2010, 35, 805–811. [Google Scholar] [CrossRef]
- Esperatti, M.; Ferrer, M.; Theessen, A.; Liapikou, A.; Valencia, M.; Saucedo, L.M.; Torres, A. Nosocomial Pneumonia in the Intensive Care Unit Acquired during Mechanical Ventilation or Not. Am. J. Respir. Crit. Care Med. 2010, 182, 1533–1539. [Google Scholar] [CrossRef]
- Martin-Loeches, I. Current Concepts in Community and Ventilator Associated Lower Respiratory Tract Infections in ICU Patients. Antibiotics 2020, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Ioanas, M.; Ferrer, M.; Cavalcanti, M.; Ferrer, R.; Ewig, S.; Filella, X.; de la Bellacasa, J.P.; Torres, A. Causes and predictors of non-response to treatment of the ICU-acquired pneumonia. Crit. Care Med. 2004, 32, 938–945. [Google Scholar] [CrossRef]
- Esperatti, M.; Ferrer, M.; Giunta, V.; Ranzani, O.T.; Saucedo, L.M.; Bassi, G.L.; Torres, A. Validation of Predictors of Adverse Outcomes in Hospital-Acquired Pneumonia in the ICU. Crit. Care Med. 2013, 41, 2151–2161. [Google Scholar] [CrossRef]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Fabregas, N.; Ewig, S.; Torres, A.; El-Ebiary, M.; Ramirez, J.; de la Bellacasa, J.P.; Cabello, H. Clinical diagnosis of ventilator associated pneumonia revisited: Comparative validation using immediate post-mortem lung biopsies. Thorax 1999, 54, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Woodhead, M.A.; Torres, A. Pneumonia. In Definition and Classification of Community-Acquired and Nosocomial Pneumonias; Torres, A., Woodhead, M., Eds.; European Respiratory Society Journals Ltd.: Sheffield, UK, 1997; pp. 1–12. [Google Scholar]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Brozek, J.L. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Torres, A.; Ewig, S.; Marcos, M.A.; Alcón, A.; Lledó, R.; Maldonaldo, A. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: Evaluation of outcome. Am. J. Respir. Crit. Care Med. 2000, 162, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Chastre, J. The standardization of bronchoscopic techniques for ventilator-associated pneumonia. Chest 1992, 102, 557S–564S. [Google Scholar] [CrossRef]
- Kollef, M.H.; Bock, K.R.; Richards, R.D.; Hearns, M.L. The safety and diagnosis accuracy of minibronchoalveolar lavage in patients with suspected ventilator associated pneumonia. Ann. Intern. Med. 1995, 122, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ioanas, M.; Cavalcanti, M.; Ferrer, M.; Valencia, M.; Agusti, C.; de la Bellacasa, J.P.; Torres, A. Hospital-acquired pneumonia: Coverage and treatment adequacy of current guidelines. Eur. Respir. J. 2003, 22, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia, A.M.; Torres, M.A.; Insausti, O.J.; Alvarez, L.F.; Carrasco, J.N.; Herranz, C.M.; Tirapu, L.J.P. Diagnostic value of quantitative cultures of endotracheal aspirate in ventilator-associated pneumonia: A multicenter study. Arch. Bronconeumol. 2003, 39, 394–399. [Google Scholar]
- Tablan, O.C.; Anderson, L.J.; Besser, R.; Bridges, C.; Hajjeh, R. Guidelines for preventing health-care--associated pneumonia, 2003, Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm. Rep. 2004, 53, 1–36. [Google Scholar]
- Murray, P.R.; Baron, E.J.; Joergensen, J.H.; Pfaller, M.A.; Yolken, R.H. Manual of Clinical Microbiology, 8th ed.; American Society for Microbiology: Washington, DC, USA, 2003. [Google Scholar]
- Heyland, D.K.; Dodek, P.; Muscedere, J.; Day, A.; Cook, D. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit. Care Med. 2008, 36, 737–744. [Google Scholar] [CrossRef]
- Cavalcanti, M.; Ferrer, M.; Ferrer, R.; Morforte, R.; Garnacho, A.; Torres, A. Risk and prognostic factors of ventilator-associated pneumonia in trauma patients. Crit. Care Med. 2006, 34, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Cook, D.J.; Christou, N.V.; Bernard, G.R.; Sprung, C.L.; Sibbald, W.J. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit. Care Med. 1995, 23, 1638–1652. [Google Scholar] [CrossRef] [PubMed]
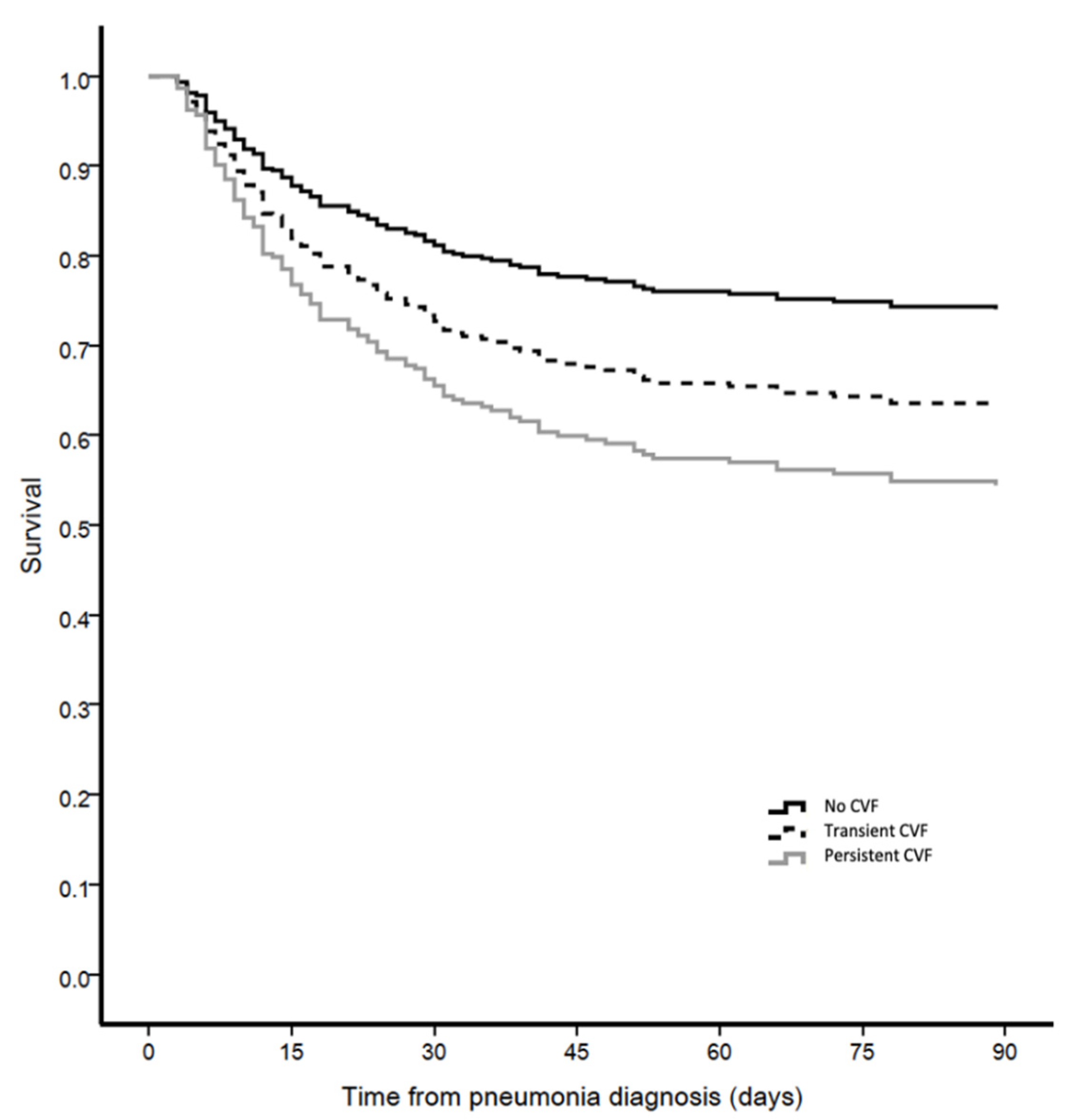
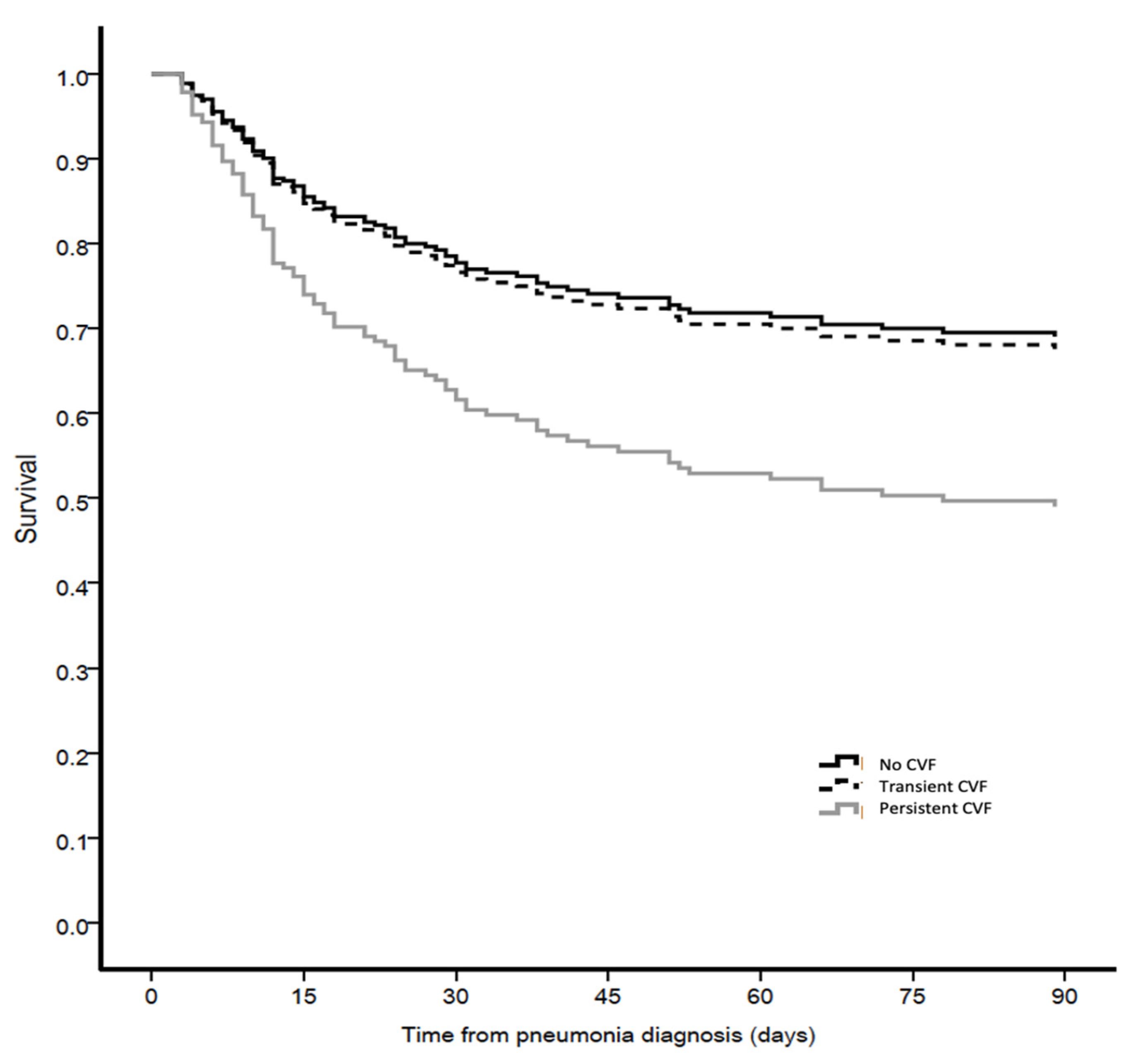
| Characteristic | No CVF (n = 203) | Transient CVF (n = 82) | Persistent CVF (n = 73) | p-Value | Post-Hoc Comparisons |
|---|---|---|---|---|---|
| Age (year) | 63.5 ± 16 | 62.9 ± 14 | 63.2 ± 13 | 0.95 | |
| Sex (female) | 53 (26%) | 24 (29%) | 29 (40%) | 0.091 | |
| Smoker (current or past) | 113 (56%) | 37 (45%) | 35 (48%) | 0.21 | |
| Alcohol abuse (current or past) | 57 (28%) | 22 (27%) | 15 (21%) | 0.45 | |
| Co-morbid conditions | |||||
| Chronic heart disease | 73 (36%) | 20 (24%) | 19 (26%) | 0.090 | |
| Chronic lung disease | 70 (35%) | 24 (29%) | 21 (29%) | 0.55 | |
| Solid cancer | 41 (20%) | 13 (16%) | 11 (15%) | 0.51 | |
| Diabetes | 48 (24%) | 17 (21%) | 17 (23%) | 0.87 | |
| Chronic liver disease | 28 (14%) | 23 (28%) | 21 (29%) | 0.003 | a |
| Chronic renal failure | 14 (7%) | 11 (13%) | 7 (10%) | 0.21 | |
| Chronic systemic steroid use | 26 (14%) | 8 (11%) | 11 (15%) | 0.72 | |
| Recent surgery | 115 (57%) | 35 (43%) | 26 (36%) | 0.004 | b |
| Previous hospitalization | 53 (26%) | 24 (29%) | 27 (37%) | 0.22 | |
| Severity at ICU admission | |||||
| APACHE-II at ICU admission | 15.8 ± 6 | 18.2 ± 6 | 18.3 ± 6 | 0.001 | a,b |
| SOFA at ICU admission | <0.001 | a,b | |||
| mean | 6.6 ± 3 | 8.3 ± 3 | 8.4 ± 3 | ||
| Median [IQR] | 6 [4–9] | 8 [6–10] | 8 [7–10] | ||
| Reason for ICU admission | 0.019 | ||||
| Hypercapnic respiratory failure | 25 (12%) | 10 (12%) | 7 (10%) | 0.82 | |
| Hypoxemic respiratory failure | 30 (15%) | 10 (12%) | 16 (22%) | 0.22 | |
| CVF | 15 (7%) | 17 (21%) | 9 (12%) | 0.006 | a |
| Acute coronary syndrome | 11 (5%) | 4 (5%) | 1 (1%) | 0.35 | |
| Multiple trauma | 22(11%) | 2 (2%) | 3 (4%) | 0.024 | |
| Postoperative | 46 (23%) | 12 (15%) | 12 (16%) | 0.22 | |
| Cardiac arrest | 7 (3%) | 4 (5%) | 7 (10%) | 0.12 | |
| Decreased consciousness | 29 (14%) | 8 (10%) | 12 (17%) | 0.45 | |
| Non-surgical abdominal condition | 11 (5%) | 6 (7%) | 4 (6%) | 0.82 | |
| Cardiogenic/hypovolemic shock | 4 (2%) | 4 (5%) | 1 (1%) | 0.29 | |
| Other | 3 (2%) | 5 (6%) | 1 (1%) | 0.062 | |
| CVF (Septic, Cardio, Hypo) | 19 (9%) | 21 (26%) | 10 (14%) | 0.002 | a |
| Laboratory Parameters | No CVF (n = 203) | Transient CVF (n = 82) | Persistent CVF (n = 73) | p-Value | Post-Hoc Comparisons |
|---|---|---|---|---|---|
| Temperature | 37.0 ± 1.3 | 36.6 ± 1.5 | 36.3 ± 1.6 | <0.001 | b |
| Leukocytes | 13.64 ± 6.43 | 15.37 ± 7.80 | 15.20 ± 7.46 | 0.087 | |
| PaO2/FiO2 | 201 ± 80 | 180 ± 80 | 184 ± 77 | 0.076 | |
| Bilateral infiltrates | 45 (22%) | 28 (34%) | 34 (47%) | <0.001 | b |
| Multilobar infiltrates | 77 (38%) | 41 (51%) | 44 (60%) | 0.003 | b |
| Pleural effusion | 60 (30%) | 27 (33%) | 32 (45%) | 0.074 | |
| ARDS criteria | 14 (7%) | 13 (16%) | 22 (30%) | <0.001 | b |
| CPIS at onset of ICUAP | 6.4 ± 1.5 | 6.7 ± 1.6 | 7.0 ± 1.6 | 0.019 | b |
| Corticosteroids at diagnosis | 86 (42%) | 30 (37%) | 30 (41%) | 0.67 | |
| Intubation | |||||
| VAP | 118 (58%) | 52 (63%) | 47 (64%) | 0.54 | |
| NV-ICUAP | 85 (42%) | 30 (37%) | 26 (36%) | ||
| Need for intubation among NV-ICUAP | 35/85 (41%) | 23/30 (77%) | 23/26 (89%) | <0.001 | a,b |
| Severity Scores | |||||
| APACHE II score at ICUAP | 14.9 ± 5 | 18.0 ± 5 | 18.0 ± 5 | <0.001 | a,b |
| SOFA score at ICUAP | <0.001 | a,b | |||
| mean | 5.6 ± 3 | 10.2 ± 3 | 10.3 ± 3 | ||
| Median [IQR] | 5 [4–7] | 10 [8–12] | 9 [8–12] | ||
| SOFA Score at ICUAP (no cardiovascular) | <0.001 | a,b | |||
| mean | 5.0 ± 2 | 6.5 ± 3 | 6.6 ± 3 | ||
| Median [IQR] | 5 [4–6] | 6 [5–8] | 6 [5–8] |
| Biomarkers | No CVF (n = 203) | Transient CVF (n = 82) | Persistent CVF (n = 73) | p-Value | Post-Hoc Comparisons |
|---|---|---|---|---|---|
| CRP (n = 340) | 0.087 | ||||
| Mean ± SD | 13.6 ± 10 | 15.8 ± 11 | 16.5 ± 11 | ||
| Median [IQR] | 12.5 [5.0–19.8] | 14.5 [6.5–24.4] | 15.0 [8.0–26.2] | ||
| PCT (n = 189) | <0.001 | a,b | |||
| Mean ± SD | 0.75 ± 1 | 2.9 ± 6 | 5.1 ± 15 | ||
| Median [IQR] | 0.24 [0.11–0.87] | 0.72 [0.36–3.29] | 0.73 [0.16–2.44] | ||
| IL-6 (n = 185) | <0.001 | a,b | |||
| Mean ± SD | 219 ± 433 | 722 ± 1155 | 570 ± 868 | ||
| Median [IQR] | 94 [39–194] | 240 [57–739] | 216 [109–511] | ||
| IL-8 (n = 185) | 0.070 | ||||
| Mean ± SD | 540 ± 3226 | 419 ± 1322 | 842 ± 3424 | ||
| Median [IQR] | 89 [52–149] | 99 [66–214] | 114 [73–283] | ||
| TNF-alpha (n = 185) | 0.21 | ||||
| Mean ± SD | 10.6 ± 12 | 16.1 ± 31 | 14.0 ± 17 | ||
| Median [IQR] | 7 [5–11] | 9 [5–18] | 9 [5–17] | ||
| Pro-adrenomedullin (n = 205) | 0.004 | a | |||
| Mean ± SD | 1.54 ± 2 | 3.27 ± 4 | 3.03 ± 4 | ||
| Median [IQR] | 1.10 [0.33–1.91] | 1.71 [0.94–3.50] | 1.36 [0.75–4.04] | ||
| suPAR (n = 176) | 0.012 | b | |||
| Mean ± SD | 6.93 ± 5 | 10.67 ± 9 | 10.76 ± 8 | ||
| Median [IQR] | 5.5 [3.6–9.0] | 6.9 [4.4–15.5] | 10.0 [4.8–15.5] |
| Biomarkers | No CVF (n = 203) | Transient CVF (n = 82) | Persistent CVF (n = 73) | p-Value | Post-Hoc Comparisons |
|---|---|---|---|---|---|
| CRP (n = 320) | 0.002 | b | |||
| Mean ± SD | 10.7 ± 8 | 12.2 ± 10 | 16.4 ± 11 | ||
| Median [IQR] | 9.18 [4.5–15.8] | 8.6 [4.0–17.6] | 16.7 [5.3–25.9] | ||
| PCT (n = 163) | 0.001 | a,b | |||
| Mean ± SD | 0.93 ± 4 | 2.2 ± 5 | 3.0 ± 10 | ||
| Median [IQR] | 0.16 [0.09–0.47] | 0.64 [0.14–2.21] | 0.58 [0.15–1.80] | ||
| IL-6 (n = 155) | 0.001 | b | |||
| Mean ± SD | 137 ± 319 | 208 ± 333 | 325 ± 552 | ||
| Median [IQR] | 66 [15–141] | 100 [41–224] | 163 [52–306] | ||
| IL-8 (n = 155) | 0.63 | ||||
| Mean ± SD | 675 ± 3029 | 472 ± 2117 | 128 ± 102 | ||
| Median [IQR] | 71 [43–137] | 80 [41–145] | 82 [59–160] | ||
| TNF-alpha (n = 155) | 0.219 | ||||
| Mean ± SD | 11.5 ± 22 | 9.7 ± 7 | 11.2 ± 7 | ||
| Median [IQR] | 7 [5–10] | 7 [5–13] | 11 [5–15] | ||
| Pro-adrenomedullin (n = 179) | 0.003 | b | |||
| Mean ± SD | 1.52 ± 2 | 2.36 ± 3 | 2.92 ± 3 | ||
| Median [IQR] | 0.94 [0.35–1.75] | 1.51 [0.58–2.67] | 1.73 [0.91–3.26] | ||
| SuPAR (n = 148) | 0.022 | b | |||
| Mean ± SD | 7.16 ± 5 | 9.95 ± 7 | 12.01 b ± 9 | ||
| Median [IQR] | 6.2 [4.2–8.9] | 7.5 [4.5–13.3] | 10.6 [4.4–16.0] |
| Aetiology and Antibiotic Treatment | No CVF (n = 203) | Transient CVF (n = 82) | Persistent CVF (n = 73) | p-Value | Post-Hoc Comparisons |
|---|---|---|---|---|---|
| Defined causative pathogen | 120 (59%) | 56 (68%) | 52 (71%) | 0.11 | |
| Multiple causative pathogens (polymicrobial) | 29 (14%) | 12 (15%) | 16 (22%) | 0.29 | |
| Multi-drug resistant pathogen | 28/120 (23%) | 16/56 (29%) | 12/52 (23%) | 0.72 | |
| Empiric antibiotic treatment according to ATS guidelines | 133 (67%) | 51 (65%) | 49 (68%) | 0.89 | |
| Inadequate initial antibiotic treatment | 18/120 (15%) | 7/56 (13%) | 18/52 (35%) | 0.004 | b,c |
| Treatment failure | 91 (45%) | 44 (54%) | 57 (78%) | <0.001 | b,c |
| Antibiotic change | 134 (66%) | 46 (56%) | 52 (71%) | 0.12 | |
| Superinfection at day 3 | 11 (5%) | 9 (11%) | 13 (18%) | 0.006 | b |
| Causative Pathogens | No CVF (n = 120) | Early Transient CVF (n = 56) | Early Persistent CVF (n = 52) | p-Value | Post-Hoc Comparisons |
|---|---|---|---|---|---|
| Gram negative non-fermenting bacteria | 41 (34%) | 23 (41%) | 25 (48%) | 0.21 | |
| P. aeruginosa | 36 (30%) | 20 (36%) | 22 (42%) | 0.28 | |
| S. aureus | 38 (32%) | 12 (21%) | 14 (27%) | 0.36 | |
| MSSA | 26 (22%) | 7 (13%) | 11 (21%) | 0.33 | |
| MRSA | 12 (10%) | 5 (9%) | 3 (6%) | 0.67 | |
| Gram negative enteric bacteria | 37 (31%) | 17 (30%) | 14 (27%) | 0.87 | |
| Community-acquired pathogens (S. pneumoniae, H. influenza, etc.) | 12 (10%) | 7 (13%) | 4 (8%) | 0.71 | |
| Aspergillus spp. | 7 (6%) | 1 (2%) | 4 (7%) | 0.36 | |
| Other | 8 (7%) | 5 (9%) | 4 (8%) | 0.87 |
| Primary and Secondary Outcomes | No CVF (n = 120) | Transient CVF (n = 56) | Persistent CVF (n = 52) | p-Value | Post-Hoc Comparisons |
|---|---|---|---|---|---|
| 90-day mortality n (%) | 62 (31%) | 39 (48%) | 45 (62%) | <0.001 | b |
| ICU stay, days | 0.005 | b,c | |||
| Mean ± SD | 19.6 ± 18 | 18.6 ± 14 | 29.0 ± 26 | ||
| Median [IQR] | 14 [8–24] | 14 [9–22] | 24 [10–40] | ||
| Hospital stay, days | a,c | ||||
| Mean ± SD | 44.9 ± 37 | 35.8 ± 23 | 49.3 ± 43 | 0.17 | |
| Median [IQR] | 38 [20–56] | 29 [19–46] | 39 [19–64] | ||
| 28-day mortality, n (%) | 39 (19%) | 29 (35%) | 30 (41%) | <0.001 | b |
| ICU mortality, n (%) | 38 (19%) | 31 (38%) | 44 (60%) | <0.001 | a,b,c |
| (a) | |||
| Exposure * | Category | Adjusted HR (95% CI) * | p-Value |
| 0.008 | |||
| No CVF | Reference | 1 | |
| Transient CVF | Yes/No | 1.53 (0.95–2.46) | 0.082 |
| Persistent CVF | Yes/No | 2.02 (1.29–3.17) | 0.002 |
| (b) | |||
| Exposure * | Category | Adjusted HR (95% CI) * | p-Value |
| 0.044 | |||
| No CVF | Reference | 1 | |
| Transient CVF | Yes/No | 1.06 (0.59–1.91) | 0.85 |
| Persistent CVF | Yes/No | 1.93 (1.11–3.33) | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Loeches, I.; Ceccato, A.; Carbonara, M.; li Bassi, G.; di Natale, P.; Nogas, S.; Ranzani, O.; Speziale, C.; Senussi, T.; Idone, F.; et al. Impact of Cardiovascular Failure in Intensive Care Unit-Acquired Pneumonia: A Single-Center, Prospective Study. Antibiotics 2021, 10, 798. https://doi.org/10.3390/antibiotics10070798
Martin-Loeches I, Ceccato A, Carbonara M, li Bassi G, di Natale P, Nogas S, Ranzani O, Speziale C, Senussi T, Idone F, et al. Impact of Cardiovascular Failure in Intensive Care Unit-Acquired Pneumonia: A Single-Center, Prospective Study. Antibiotics. 2021; 10(7):798. https://doi.org/10.3390/antibiotics10070798
Chicago/Turabian StyleMartin-Loeches, Ignacio, Adrian Ceccato, Marco Carbonara, Gianluigi li Bassi, Pierluigi di Natale, Stefano Nogas, Otavio Ranzani, Carla Speziale, Tarek Senussi, Francesco Idone, and et al. 2021. "Impact of Cardiovascular Failure in Intensive Care Unit-Acquired Pneumonia: A Single-Center, Prospective Study" Antibiotics 10, no. 7: 798. https://doi.org/10.3390/antibiotics10070798
APA StyleMartin-Loeches, I., Ceccato, A., Carbonara, M., li Bassi, G., di Natale, P., Nogas, S., Ranzani, O., Speziale, C., Senussi, T., Idone, F., Motos, A., Ferrer, M., & Torres, A. (2021). Impact of Cardiovascular Failure in Intensive Care Unit-Acquired Pneumonia: A Single-Center, Prospective Study. Antibiotics, 10(7), 798. https://doi.org/10.3390/antibiotics10070798








