A Short-Course Antibiotic Prophylaxis Is Associated with Limited Antibiotic Resistance Emergence in Post-Operative Infection of Pelvic Primary Bone Tumor Resection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Participants
2.1.2. Antibiotic Prophylaxis and Preventive Strategy
2.2. SSI Management
2.3. Microbiological Analysis
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Study Population
3.2. Bacteriological Flora in SSI after Pelvic Tumor Bone Resection
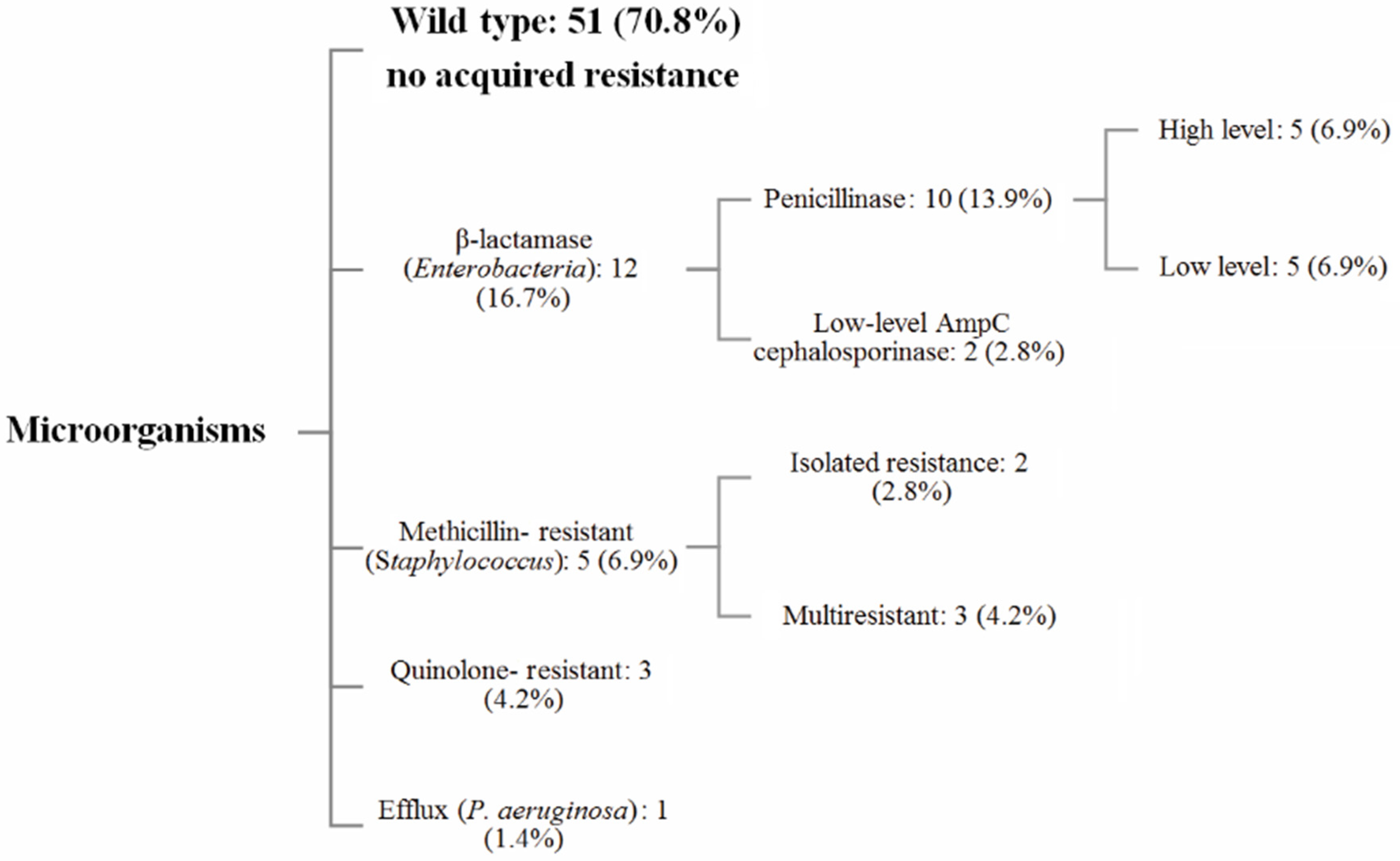
3.3. Antibiotic Resistance Analysis
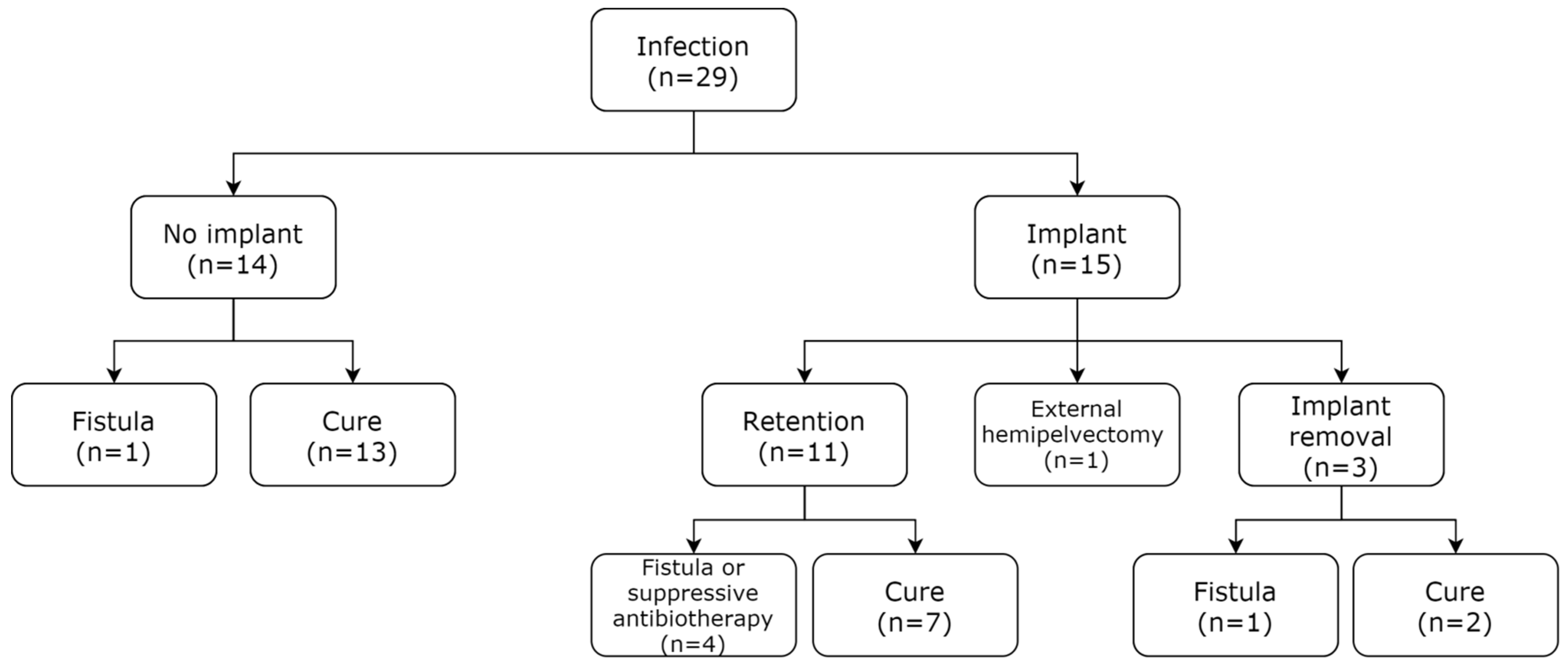
3.4. Patient Management and Healing Rate after a DA or DAIR Procedure
4. Discussion
4.1. Polymicrobial Infections in Pelvic Bone Resections
4.2. Impact of Antibiotic Prophylaxis on the SSI Ecology: Avoiding Selection Pressure
4.2.1. Prophylaxis Duration
4.2.2. Choice of Prophylaxis Drugs
4.2.3. Resistance Pattern Analysis
4.3. SSI Management: Healing and Implant Retention
4.3.1. SSI Healing Rate
4.3.2. Factors Influencing the SSI Healing Rate
4.3.3. Implant Retention and DAIR
4.4. Study Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puri, A.; Gulia, A.; Jambhekar, N.A.; Laskar, S. Results of surgical resection in pelvic Ewing’s sarcoma. J. Surg. Oncol. 2012, 106, 417–422. [Google Scholar] [CrossRef]
- Brookes, M.; Revell, W.J. Blood Supply of Bone: Scientific Aspects; Springer Science & Business Media: London, UK, 2012; ISBN 978-1-4471-1543-4. [Google Scholar]
- Sanders, P.; Bus, M.; Scheper, H.; Van Der Wal, R.; Van De Sande, M.; Bramer, J.; Schaap, G.; De Boer, M.; Dijkstra, P. Multiflora and gram-negative microorganisms predominate in infections affecting pelvic endoprostheses following tumor resection. J. Bone Joint Surg. Am. 2019, 101, 797–803. [Google Scholar] [CrossRef]
- Angelini, A.; Drago, G.; Trovarelli, G.; Calabrò, T.; Ruggieri, P. Infection after surgical resection for pelvic bone tumors: An analysis of 270 patients from one institution. Clin. Orthop. 2014, 472, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behnam, A.B.; Chen, C.M.; Pusic, A.L.; Mehrara, B.J.; Disa, J.J.; Athanasian, E.A.; Cordeiro, P.G. The pedicled latissimus dorsi flap for shoulder reconstruction after sarcoma resection. Ann. Surg. Oncol. 2007, 14, 1591–1595. [Google Scholar] [CrossRef]
- López, J.F.; Hietanen, K.E.; Kaartinen, I.S.; Kääriäinen, M.T.; Pakarinen, T.-K.; Laitinen, M.; Kuokkanen, H. Primary flap reconstruction of tissue defects after sarcoma surgery enables curative treatment with acceptable functional results: A 7-year review. BMC Surg. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, S.; Shirai, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Tada, K.; Kajino, Y.; Higuchi, T.; Abe, K.; Aiba, H.; et al. Risk factors for surgical site infection after malignant bone tumor resection and reconstruction. BMC Cancer 2019, 19, 33. [Google Scholar] [CrossRef] [Green Version]
- Nagano, S.; Yokouchi, M.; Setoguchi, T.; Sasaki, H.; Shimada, H.; Kawamura, I.; Ishidou, Y.; Kamizono, J.; Yamamoto, T.; Kawamura, H.; et al. Analysis of Surgical Site Infection after Musculoskeletal Tumor Surgery: Risk Assessment Using a New Scoring System. Available online: https://www.hindawi.com/journals/sarcoma/2014/645496/ (accessed on 25 February 2021).
- Zeifang, F.; Buchner, M.; Zahlten-Hinguranage, A.; Bernd, L.; Sabo, D. Complications following operative treatment of primary malignant bone tumours in the pelvis. Eur. J. Surg. Oncol. 2004, 30, 893–899. [Google Scholar] [CrossRef]
- Hillmann, A.; Hoffmann, C.; Gosheger, G.; Rödl, R.; Winkelmann, W.; Ozaki, T. Tumors of the pelvis: Complications after reconstruction. Arch. Orthop. Trauma Surg. 2003, 123, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Bus, M.P.; Campanacci, D.A.; Albergo, J.I.; Leithner, A.; van de Sande, M.A.; Gaston, C.L.; Caff, G.; Mettelsiefen, J.; Capanna, R.; Tunn, P.-U.; et al. Conventional primary central chondrosarcoma of the pelvis: Prognostic factors and outcome of surgical treatment in 162 patients. J. Bone Joint Surg. Am. 2018, 100, 316–325. [Google Scholar] [CrossRef]
- Puchner, S.E.; Funovics, P.T.; Böhler, C.; Kaider, A.; Stihsen, C.; Hobusch, G.M.; Panotopoulos, J.; Windhager, R. Oncological and surgical outcome after treatment of pelvic sarcomas. PLoS ONE 2017, 12, e0172203. [Google Scholar] [CrossRef]
- Vos, L.M.; Morand, P.C.; Biau, D.; Archambeau, D.; Eyrolle, L.-J.; Loubinoux, J.; Perut, V.; Leclerc, P.; Arends, J.E.; Anract, P.; et al. High frequency of polymicrobial infections after surgical resection of malignant bone and soft tissue tumors: A retrospective cohort study. Infect. Dis. Ther. 2015, 4, 307–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rod-Fleury, T.; Uçkay, I. Microbiological particularities of surgical site infections in oncologic orthopedic surgery compared to non-oncologic surgery-single center experience and literature review. Clin. Surg. 2019, 4, 2443. [Google Scholar] [CrossRef]
- Trouillet-Assant, S.; Valour, F.; Mouton, W.; Martins-Simões, P.; Lustig, S.; Laurent, F.; Ferry, T.; Lyon Bji Study Group. Methicillin-susceptible strains responsible for postoperative orthopedic infection are not selected by the use of cefazolin in prophylaxis. Diagn. Microbiol. Infect. Dis. 2016, 84, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.P. Preoperative antibiotic prophylaxis. N. Engl. J. Med. 1992, 326, 337–339. [Google Scholar] [CrossRef]
- Severyns, M.; Briand, S.; Waast, D.; Touchais, S.; Hamel, A.; Gouin, F. Postoperative infections after limb-sparing surgery for primary bone tumors of the pelvis: Incidence, characterization and functional impact. Surg. Oncol. 2017, 26, 171–177. [Google Scholar] [CrossRef]
- Gebert, C.; Wessling, M.; Gosheger, G.; Aach, M.; Streitbürger, A.; Henrichs, M.P.; Dirksen, U.; Hardes, J. Pelvic reconstruction with compound osteosynthesis following hemipelvectomy. Bone Jt. J. 2013, 95-B, 1410–1416. [Google Scholar] [CrossRef]
- Enzler, M.J.; Berbari, E.; Osmon, D.R. Antimicrobial prophylaxis in adults. Mayo Clin. Proc. 2011, 86, 686–701. [Google Scholar] [CrossRef] [Green Version]
- Angelini, A.; Calabrò, T.; Pala, E.; Trovarelli, G.; Maraldi, M.; Ruggieri, P. Resection and reconstruction of pelvic bone tumors. Orthopedics 2015, 38, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Hawn, M.T.; Richman, J.S.; Vick, C.C.; Deierhoi, R.J.; Graham, L.A.; Henderson, W.G.; Itani, K.M.F. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. J. Am. Med. Assoc. Surg. 2013, 148, 649–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, A.J.; Roberts, S.A.; Grae, N.; Frampton, C.M. Surgical site infection rate is higher following hip and knee arthroplasty when cefazolin is underdosed. Am. J. Health-Syst. Pharm. 2020, 77, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Xu, W. Risk Factors affect success rate of Debridement, Antibiotics and Implant Retention (DAIR) in Periprosthetic joint infection. Arthroplasty 2020, 2, 37. [Google Scholar] [CrossRef]
- Müller, D.; Kaiser, D.; Sairanen, K.; Studhalter, T.; Uçkay, İ. Antimicrobial prophylaxis for the prevention of surgical site infections in orthopaedic oncology—A narrative review of current concepts. J. Bone Jt. Infect. 2019, 4, 254–263. [Google Scholar] [CrossRef]
- Bémer, P.; Plouzeau, C.; Tande, D.; Léger, J.; Giraudeau, B.; Valentin, A.S.; Jolivet-Gougeon, A.; Vincent, P.; Corvec, S.; Gibaud, S.; et al. Evaluation of 16S RRNA Gene PCR sensitivity and specificity for diagnosis of prosthetic joint infection: A prospective multicenter cross-sectional study. J. Clin. Microbiol. 2014, 52, 3583–3589. [Google Scholar] [CrossRef] [Green Version]
- Dhanoa, A.; Ajit Singh, V.; Elbahri, H. Deep infections after endoprosthetic replacement operations in orthopedic oncology patients. Surg. Infect. 2015, 16, 323–332. [Google Scholar] [CrossRef]
- Abdul-Jabbar, A.; Berven, S.H.; Hu, S.S.; Chou, D.; Mummaneni, P.V.; Takemoto, S.; Ames, C.; Deviren, V.; Tay, B.; Weinstein, P.; et al. Surgical site infections in spine surgery: Identification of microbiologic and surgical characteristics in 239 cases. Spine 2013, 38, 1425–1431. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilchmann, T.; Zimmerli, W.; Bolliger, L.; Graber, P.; Clauss, M. Risk of infection in primary, elective total hip arthroplasty with direct anterior approach or lateral transgluteal approach: A prospective cohort study of 1104 hips. BMC Musculoskelet. Disord. 2016, 17, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedi, M.; King, D.M.; DeVries, J.; Hackbarth, D.A.; Neilson, J.C. Does vacuum-assisted closure reduce the risk of wound complications in patients with lower extremity sarcomas treated with preoperative radiation? Clin. Orthop. 2019, 477, 768–774. [Google Scholar] [CrossRef]
- Wittekamp, B.H.J.; Oostdijk, E.A.N.; Cuthbertson, B.H.; Brun-Buisson, C.; Bonten, M.J.M. Selective decontamination of the digestive tract (SDD) in critically ill patients: A narrative review. Intensive Care Med. 2020, 46, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Racano, A.; Pazionis, T.; Farrokhyar, F.; Deheshi, B.; Ghert, M. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: A systematic review. Clin. Orthop. 2013, 471, 2017–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renz, N.; Trampuz, A.; Zimmerli, W. Controversy about the role of rifampin in biofilm infections: Is it justified? Antibiot. Basel Switz. 2021, 10, 165. [Google Scholar] [CrossRef]
- Wyles, C.C.; Hevesi, M.; Osmon, D.R.; Park, M.A.; Habermann, E.B.; Lewallen, D.G.; Berry, D.J.; Sierra, R.J. John Charnley Award: Increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin: The value of allergy testing for antibiotic prophylaxis. Bone Jt. J. 2019, 101-B, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.L.; Glenny, A.M.; Song, F. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst. Rev. 2009, CD001181. [Google Scholar] [CrossRef]
- Cartelle Gestal, M.; Dedloff, M.R.; Torres-Sangiao, E. Computational health engineering applied to model infectious diseases and antimicrobial resistance spread. Appl. Sci. 2019, 9, 2486. [Google Scholar] [CrossRef] [Green Version]
- Nocedo-Mena, D.; Cornelio, C.; Camacho-Corona, M.D.R.; Garza-González, E.; Waksman de Torres, N.; Arrasate, S.; Sotomayor, N.; Lete, E.; González-Díaz, H. Modeling antibacterial activity with machine learning and fusion of chemical structure information with microorganism metabolic networks. J. Chem. Inf. Model. 2019, 59, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Arepyeva, M.A.; Kolbin, A.S.; Sidorenko, S.V.; Lawson, R.; Kurylev, A.A.; Balykina, Y.E.; Mukhina, N.V.; Spiridonova, A.A. A Mathematical model for predicting the development of bacterial resistance based on the relationship between the level of antimicrobial resistance and the volume of antibiotic consumption. J. Glob. Antimicrob. Resist. 2017, 8, 148–156. [Google Scholar] [CrossRef]
- Deroche, L.; Bémer, P.; Valentin, A.-S.; Jolivet-Gougeon, A.; Tandé, D.; Héry-Arnaud, G.; Lemarié, C.; Kempf, M.; Bret, L.; Burucoa, C.; et al. The right time to safely re-evaluate empirical antimicrobial treatment of hip or knee prosthetic joint infections. J. Clin. Med. 2019, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Sciubba, D.M.; Nelson, C.; Gok, B.; McGirt, M.J.; McLoughlin, G.S.; Noggle, J.C.; Wolinsky, J.P.; Witham, T.F.; Bydon, A.; Gokaslan, Z.L. Evaluation of factors associated with postoperative infection following sacral tumor resection. J. Neurosurg. Spine 2008, 9, 593–599. [Google Scholar] [CrossRef]
- Desta, K.; Woldeamanuel, Y.; Azazh, A.; Mohammod, H.; Desalegn, D.; Shimelis, D.; Gulilat, D.; Lamisso, B.; Makonnen, E.; Worku, A.; et al. High gastrointestinal colonization rate with extended-spectrum β-lactamase-producing enterobacteriaceae in hospitalized patients: Emergence of carbapenemase-producing k. pneumoniae in ethiopia. PLoS ONE 2016, 11, e0161685. [Google Scholar] [CrossRef]
- Agostinho, A.; Renzi, G.; Haustein, T.; Jourdan, G.; Bonfillon, C.; Rougemont, M.; Hoffmeyer, P.; Harbarth, S.; Uçkay, I. Epidemiology and acquisition of extended-spectrum beta-lactamase-producing enterobacteriaceae in a septic orthopedic ward. SpringerPlus 2013, 2, 91. [Google Scholar] [CrossRef] [Green Version]
- Idowu, O.J.; Onipede, A.O.; Orimolade, A.E.; Akinyoola, L.A.; Babalola, G.O. Extended-spectrum beta-lactamase orthopedic wound infections in nigeria. J. Glob. Infect. Dis. 2011, 3, 211–215. [Google Scholar] [CrossRef]
- Takoudju, E.; Bémer, P.; Touchais, S.; Asseray, N.; Corvec, S.; Khatchatourian, L.; Serandour, N.; Boutoille, D.; Nantes Bone and Joint Infections Study Group. Bacteriological relevance of linezolid vs. vancomycin in postoperative empirical treatment of osteoarticular infections: A retrospective single-center study. Int. J. Antimicrob. Agents 2018, 52, 663–666. [Google Scholar] [CrossRef]
- Cobo, J.; Miguel, L.G.S.; Euba, G.; Rodríguez, D.; García-Lechuz, J.M.; Riera, M.; Falgueras, L.; Palomino, J.; Benito, N.; del Toro, M.D.; et al. Early prosthetic joint infection: Outcomes with debridement and implant retention followed by antibiotic therapy. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2011, 17, 1632–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, L.; Arvieux, C.; Brunschweiler, B.; Touchais, S.; Ansart, S.; Bru, J.-P.; Oziol, E.; Boeri, C.; Gras, G.; Druon, J.; et al. Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N. Engl. J. Med. 2021, 384, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Guo, W.; Yang, R.L.; Tang, X.D.; Wang, Y.F. Modular hemipelvic endoprosthesis reconstruction-experience in 100 patients with mid-term follow-up results. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2013, 39, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, J.; Pei, G.-X.; Li, X.-D.; Wang, Z. Pelvic reconstruction with a combined hemipelvic prostheses after resection of primary malignant tumor. Surg. Oncol. 2010, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
| SSI Cohort (n = 29) | ||
|---|---|---|
| Characteristics | ||
| Age (years) | 49.8 (±19.7) | |
| Male | 22 (75.9%) | |
| BMI (kg/m2) | 26.8 (±4.8) | |
| Follow-up (months) | 56 (±38) | |
| Localization | ||
| Iliac bone | 20 (69.0%) | |
| Sacrum | 9 (31.0%) | |
| Histology | ||
| Chondrosarcoma | 15 (51.7%) | |
| Osteosarcoma | 5 (17.2%) | |
| Ewing | 4 (13.8%) | |
| Chordoma | 4 (13.8%) | |
| Others | 1 (3.4%) | |
| Co-morbidities | ||
| Diabetes | 4 (13.8%) | |
| Active smoking | 3 (10.3%) | |
| Immunosuppressive treatment | 2 (6.9%) | |
| Inflammatory disease | 1 (3.4%) | |
| Albumin (g/L) | 28.0 (±8.1) | |
| Scores | ||
| ASA | 1.8 (±0.5) | |
| CCI | 2.6 (±1.2) |
| SSI Cohort (n = 29) | ||
|---|---|---|
| 24 h Antibiotic prophylaxis | ||
| Monotherapy (1GC *) | 21 (72.4%) | |
| Dual therapy (1GC * + nitroimidazole) | 8 (27.6%) | |
| Adjuvant treatment (global) | 10 (34.5%) | |
| Chemotherapy | 9 (31.0%) | |
| Radiotherapy | 3 (10.3%) | |
| Surgical margins | ||
| R0 | 16 (55.2%) | |
| R1 | 11 (34.5%) | |
| R2 | 2 (6.9%) | |
| Cutting planning (global) | 12 (41.4%) | |
| CT Navigation | 1 (3.4%) | |
| PSI guide | 11 (37.9%) | |
| Reconstruction, implant | ||
| Prosthesis/Fixation | 15 (51.7%) | |
| Associated procedure | ||
| Combined visceral surgery | 10 (34.5%) | |
| Pedicled flap | 3 (10.3%) | |
| Perioperative data | ||
| Surgical specimen volume (cm3) | 750 (±725) | |
| Duration (min) | 479 (±172) | |
| Blood loss (mL) | 2690 (±1.786) | |
| Packed red blood cells | 17.55 (±8.89) |
| Gram-Negative Bacilli (GNB): 26 (36.1%) | Escherichia coli | 12 (16.7%) |
| Proteus mirabilis | 5 (6.9%) | |
| Enterobacter cloacae | 3 (4.2%) | |
| Morganella morganii | 1 (1.4%) | |
| Citrobacter koseri | 1 (1.4%) | |
| Klebsiella oxytoca | 1 (1.4%) | |
| Proteus vulgaris | 1 (1.4%) | |
| Citrobacter freundii | 1 (1.4%) | |
| Pseudomonas aeruginosa | 1 (1.4%) | |
| Anaerobes: 10 (13.9%), except Cutibacterium acnes | Bacteroides fragilis | 4 (5.6%) |
| Bacteroides ovatus | 1 (1.4%) | |
| Bacteroides vulgatus | 1 (1.4%) | |
| Bacteroides uniformis | 1 (1.4%) | |
| Bacteroides sp. | 1 (1.4%) | |
| Actinomyces turicensis | 1 (1.4%) | |
| Prevotella sp. | 1 (1.4%) | |
| Enterococcus faecalis: | 15 (20.8%) | |
| Cutibacterium acnes: | 4 (5.6%) | |
| Gram-Positive Cocci (GPC): 17 (23.6%), except Enterococci | Staphylococcus aureus | 7 (9.7%) |
| Staphylococcus epidermidis | 6 (8.3%) | |
| Other coagulase-negative staphylococci | 3 (4.2%) | |
| Streptococcus mitis | 1 (1.4%) | |
| Total: 72 |
| Age | Gender | Antibiotic Prophylaxis | Microorganisms Involved in the SSI | Early Infection | Healing Status | ||||
|---|---|---|---|---|---|---|---|---|---|
| 57 | F | 1GC | P. mirabilis | C. freundii | Yes | Healed, material removal | |||
| 62 | M | 1GC | S. lugdunensis | Yes | Healed | ||||
| 71 | M | 1GC | E. coli3 | S. aureus | E. faecalis | P. vulgaris | Yes | Healed | |
| 55 | M | 1GC | E. coli4 | E. faecalis | Streptococcus mitis | Yes | Healed | ||
| 66 | M | 1GC | E. coli3 | No | Fistula, material retention | ||||
| 48 | M | 1GC | E. cloacae | E. faecalis | Yes | Healed | |||
| 20 | M | 1GC | S. aureus | No | Healed | ||||
| 47 | M | 1GC | E. coli3 | S. epidermidis | S. warnerii | No | Healed, material removal | ||
| 18 | M | 1GC | E. faecalis | S. epidermidis2 | No | External Hemipelvectomy | |||
| 18 | M | 1GC | E. coli3 | E. faecalis | Bacteroides sp. | Prevotella sp. | Yes | Healed | |
| 51 | M | 1GC | P. aeruginosa6 | S. epidermidis1 | C. acnes | Yes | Healed | ||
| 76 | F | 1GC | P. mirabilis3 | E. faecalis | E. coli4 | B. fragilis | M. morganii3 | Yes | Fistula, material removal |
| 26 | F | 1GC | E. coli | E. faecalis | Yes | Healed | |||
| 58 | F | 1GC | E. coli5 | E. faecalis | Yes | Healed | |||
| 76 | M | 1GC | S. aureus | C. koseri | C. acnes | Yes | Healed | ||
| 61 | M | 1GC | S. epidermidis2 | E. faecalis | E. cloacae | Yes | Fistula, material retention | ||
| 56 | M | 1GC | P. mirabilis | A. turicensis | B. ovatus5 | B. fragilis5 | No | Fistula, no initial reconstruction | |
| 84 | F | 1GC | S. aureus | No | Healed | ||||
| 41 | F | 1GC + NI | P. mirabilis | E. coli3 | E. faecalis | No | Healed | ||
| 64 | M | 1GC + NI | S. capitis | C. acnes | No | Healed | |||
| 16 | F | 1GC + NI | E. coli3 | E. faecalis | B. ovatus | B. fragilis | B. uniformis | Yes | Healed |
| 27 | M | 1GC + NI | S. epidermidis1 | Yes | Healed | ||||
| 70 | M | 1GC + NI | E. coli3 | E. faecalis | Yes | Healed | |||
| 30 | M | 1GC + NI | E. coli3 | E. faecalis | S. aureus | B. fragilis | Yes | Healed | |
| 54 | M | 1GC + NI | K. oxytoca | E. faecalis | S. aureus | Yes | Healed | ||
| 58 | M | Clindamycin + NI | P. mirabilis | Yes | Healed | ||||
| 22 | M | Cotrimoxazole | E. faecalis | S. epidermidis2 | Yes | Suppressive antibiotics | |||
| 54 | M | Vancomycin | S. aureus | C. acnes | Yes | Healed | |||
| 59 | M | Vancomycin | E. cloacae | Yes | Fistula, prosthesis retention | ||||
| Infection Healing Probability * | ||
|---|---|---|
| Model p-Value = 0.006 | ||
| Multivariate analysis | Coefficient (95.0% CI) | p Value |
| Early infection (<1 month) | 4.94 (0.39–62.41) | p = 0.217 |
| Material implantation | 0.49 (0.04–0.66) | p = 0.023 |
| Number of resistant bacteria† | 0.43 (0.12–1.52) | p = 0.189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varenne, Y.; Corvec, S.; Leroy, A.-G.; Boutoille, D.; Nguyễn, M.-V.; Touchais, S.; Bémer, P.; Hamel, A.; Waast, D.; Nich, C.; et al. A Short-Course Antibiotic Prophylaxis Is Associated with Limited Antibiotic Resistance Emergence in Post-Operative Infection of Pelvic Primary Bone Tumor Resection. Antibiotics 2021, 10, 768. https://doi.org/10.3390/antibiotics10070768
Varenne Y, Corvec S, Leroy A-G, Boutoille D, Nguyễn M-V, Touchais S, Bémer P, Hamel A, Waast D, Nich C, et al. A Short-Course Antibiotic Prophylaxis Is Associated with Limited Antibiotic Resistance Emergence in Post-Operative Infection of Pelvic Primary Bone Tumor Resection. Antibiotics. 2021; 10(7):768. https://doi.org/10.3390/antibiotics10070768
Chicago/Turabian StyleVarenne, Yoann, Stéphane Corvec, Anne-Gaëlle Leroy, David Boutoille, Mỹ-Vân Nguyễn, Sophie Touchais, Pascale Bémer, Antoine Hamel, Denis Waast, Christophe Nich, and et al. 2021. "A Short-Course Antibiotic Prophylaxis Is Associated with Limited Antibiotic Resistance Emergence in Post-Operative Infection of Pelvic Primary Bone Tumor Resection" Antibiotics 10, no. 7: 768. https://doi.org/10.3390/antibiotics10070768
APA StyleVarenne, Y., Corvec, S., Leroy, A.-G., Boutoille, D., Nguyễn, M.-V., Touchais, S., Bémer, P., Hamel, A., Waast, D., Nich, C., Gouin, F., & Crenn, V. (2021). A Short-Course Antibiotic Prophylaxis Is Associated with Limited Antibiotic Resistance Emergence in Post-Operative Infection of Pelvic Primary Bone Tumor Resection. Antibiotics, 10(7), 768. https://doi.org/10.3390/antibiotics10070768






