Clostridioides difficile Infection among Cirrhotic Patients with Variceal Bleeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Admission Protocol
2.3. CDI Diagnosis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Factors Associated with Mortality in Cirrhotic Patients with CDI
3.3. Risk Factors Associated with CDI in Cirrhotic Patients with Variceal Bleeding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eberly, M.D.; Susi, A.; Adams, D.J.; Love, C.S.; Nylund, C.M. Epidemiology and outcomes of patients with healthcare facility-onset clostridioides difficile infection. Mil. Med. 2021, usab116. [Google Scholar] [CrossRef]
- Davies, K.A.; Longshaw, C.M.; Davis, G.L.; Bouza, E.; Barbut, F.; Barna, Z.; Delmee, M.; Fitzpatrick, F.; Ivanova, K.; Kuijper, E.; et al. Underdiagnosis of Clostridium difficile across Europe: The European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalized patients with diarrhoea (EUCLID). Lancet Infect. Dis. 2014, 14, 1208–1219. [Google Scholar] [CrossRef]
- Bowman, A.J.; Utter, H.G. Evolving strategies to manage clostridium difficile colitis. J. Gastrointest. Surg. 2020, 24, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Chen, C.X.; Wang, M.; Liao, H.R.; Wang, M.X.; Hua, S.Z.; Huang, B.; Xiong, Y.; Zhang, J.Y.; Xu, Y.L. Updated meta-analysis of controlled observational studies: Proton-pump inhibitors and risk of Clostridium difficile infection. J. Hosp. Infect. 2018, 98, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Maseda, D.; Zackular, J.P.; Trindade, B.; Kirk, L.; Roxas, J.L.; Rogers, L.M.; Washington, M.K.; Du, L.; Koyama, T.; Viswanathan, V.K.; et al. Nonsteroidal anti-inflammatory drugs alter the microbiota and exacerbate Clostridium difficile colitis while dysregulating the inflammatory response. mBio 2019, 10, e02282-18. [Google Scholar] [CrossRef] [PubMed]
- Mendo-Lopez, R.; Villafuerte-Gálvez, J.; White, N.; Mahoney, M.V.; Kelly, C.P.; Alonso, C.D. Recent developments in the management of recurrent Clostridioides difficile infection. Anaerobe 2020, 62, 102108. [Google Scholar] [CrossRef] [PubMed]
- Lesmana, C.R.A.; Raharjo, M.; Gani, R.A. Managing liver cirrhotic complications: Overview of esophageal and gastric varices. Clin. Mol. Hepatol. 2020, 26, 444–460. [Google Scholar] [CrossRef]
- Plaz Torres, M.C.; Best, L.M.; Freeman, S.C.; Roberts, D.; Cooper, N.J.; Sutton, A.J.; Roccarina, D.; Benmassaoud, A.; Iogna Prat, L.; Williams, N.R.; et al. Secondary prevention of variceal bleeding in adults with previous oesophageal variceal bleeding due to decompensated liver cirrhosis: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 3, CD013122. [Google Scholar]
- Trifan, A.; Stoica, O.; Stanciu, C.; Cojocariu, C.; Singeap, A.M.; Girleanu, I.; Miftode, E. Clostridium difficile infection in patients with liver disease: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2313–2324. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed]
- Negrut, N.; Nistor-Cseppento, D.C.; Khan, S.A.; Pantis, C.; Maghiar, T.A.; Maghiar, O.; Aleya, S.; Rus, M.; Tit, D.M.; Aleya, L.; et al. Clostridium difficile Infection Epidemiology over a Period of 8 Years—A Single Centre Study. Sustainability 2020, 12, 4439. [Google Scholar] [CrossRef]
- Tschudin-Sutter, S.; Kuijper, E.J.; Durovic, A.; Vehreschild, M.J.G.T.; Barbut, F.; Eckert, C.; Fitzpatrick, F.; Hell, M.; Norèn, T.; O’Driscoll, J.; et al. Guidance document for prevention of Clostridium difficile infection in acute healthcare settings. Clin. Microbiol. Infect. 2018, 24, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Adelman, M.W.; Goodenough, D.; Sefton, S.; Mackey, C.; Thomas, S.; Fridkin, S.K.; Woodworth, M.H. Changes in treatment of community-onset Clostridioides difficile infection after release of updated guidelines, Atlanta, Georgia, 2018. Anaerobe 2021, 13, 102364. [Google Scholar]
- Skinner, A.M.; Phillips, S.T.; Merrigan, M.M.; O’Leary, K.J.; Sambol, S.P.; Siddiqui, F.; Peterson, L.R.; Gerding, D.N.; Johnson, S. The Relative Role of Toxins A and B in the Virulence of Clotridioides difficile. J. Clin. Med. 2020, 10, 96. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Chalmers, J.D.; Nord, C.E.; Freeman, J.; Bouza, E. Role of cephalosporins in the era of Clostridium difficile infection. J. Antimicrob. Chemother. 2017, 72, 1–18. [Google Scholar] [CrossRef]
- Abdalla, A.O.; Pisipati, S.; Elnaggar, M.; Rishi, M.; Doshi, R.; Gullapalli, N. Outcomes of Clostridioides difficile Infection in Patients with Liver Cirrhosis: A Nationwide Study. Gastroenterol. Res. 2020, 13, 53–57. [Google Scholar] [CrossRef]
- Rosenblatt, R.; Mehta, A.; Cohen-Mekelburg, S.; Shen, N.; Snell, D.; Lucero, C.; Jesudian, A.; Fortune, B.; Crawford, C.V.; Kumar, S. The rise of Clostridioides difficile infections and fall of associated mortality in hospitalized advanced cirrhotics. Liver Int. 2019, 39, 1263–1270. [Google Scholar] [CrossRef]
- Roberts, D.; Best, L.M.; Freeman, S.C.; Sutton, A.J.; Cooper, N.J.; Arunan, S.; Begum, T.; Williams, N.R.; Walshaw, D.; Milne, E.J.; et al. Treatment for bleeding oesophageal varices in people with decompensated liver cirrhosis: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 1, CD013155. [Google Scholar] [CrossRef][Green Version]
- Tandon, P.; Garcia-Tsao, G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin. Liver Dis. 2008, 28, 26–42. [Google Scholar] [CrossRef]
- Soares-Weiser, K.; Brezis, M.; Tur-Kaspa, R.; Leibovici, L. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding. Cochrane Database Syst. Rev. 2002, 2, CD002907. [Google Scholar]
- Brown, M.R.; Jones, G.; Nash, K.L.; Wright, M.; Guha, I.N. Antibiotic prophylaxis in variceal hemorrhage: Timing, effectiveness and Clostridium difficile rates. World J. Gastroenterol. 2010, 16, 5317–5323. [Google Scholar] [CrossRef]
- Slimings, C.; Riley, T.V. Antibiotics and hospital-acquired Clostridium difficile infection: Update of systematic review and meta-analysis. J. Antimicrob. Chemother. 2014, 69, 881–891. [Google Scholar] [CrossRef]
- Brogard, J.M.; Blickle, J.F.; Jehl, F.; Arnaud, J.P.; Paris-Bockel, D.; Monteil, H. High biliary elimination of ceftriaxone in man. Int. J. Clin. Pharmacol. Ther. Toxicol. 1988, 26, 167–172. [Google Scholar]
- Chavez-Tapia, N.C.; Tellez-Avila, F.I.; Garcia-Leiva, J.; Valdovinos, M.A. Use and overuse of proton pump inhibitors in cirrhotic patients. Med. Sci. Monit. 2008, 14, CR468–CR472. [Google Scholar]
- Khan, M.A.; Kamal, S.; Khan, S.; Lee, W.M.; Howden, C.W. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1327–1336. [Google Scholar] [CrossRef]
- Trifan, A.; Stanciu, C.; Girleanu, I.; Stoica, O.C.; Singeap, A.M.; Maxim, R.; Chiruac, S.A.; Ciobica, A.; Boiculese, L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 6500–6515. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, D.; Yu, Y.; Lou, L.; Li, X. Proton pump inhibitor use and mortality in patients with cirrhosis: A meta-analysis of cohort studies. Biosci. Rep. 2020, 40, BSR20193890. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Bi, Q.; Zheng, X.; Zhang, J.; Huang, W. Adverse outcomes of proton pump inhibitors in chronic liver disease: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 385–398. [Google Scholar] [CrossRef]
- Howell, M.D.; Novack, V.; Grgurich, P.; Soulliard, D.; Novack, L.; Pencina, M.; Talmor, D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch. Intern. Med. 2010, 170, 784–790. [Google Scholar] [CrossRef]
- Hegarty, J.P.; Sangster, W.; Harris, L.R., 3rd; Stewart, D.B. Proton pump inhibitors induce changes in colonocyte gene expression that may affect Clostridium difficile infection. Surgery 2014, 156, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Seto, C.T.; Jeraldo, P.; Orenstein, R.; Chia, N.; DiBaise, J.K. Prolonged use of a proton pump inhibitor reduces microbial diversity: Implications for Clostridium difficile susceptibility. Microbiome 2014, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Arthur, A.K.; Anibueze, C.I.; Singh, S.; Cavallazzi, R.; Loke, Y.K. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: Meta-analysis. Am. J. Gastroenterol. 2012, 107, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Ananthakrishnan, A.N.; Hafeezullah, M.; Zadvornova, Y.; Dye, A.; McGinley, E.L.; Saeian, K.; Heuman, D.; Sanyal, A.J.; Hoffmann, R.G. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am. J. Gastroenterol. 2010, 105, 106–113. [Google Scholar] [CrossRef]
- Saab, S.; Alper, T.; Sernas, E.; Pruthi, P.; Alper, M.A.; Sundaram, V. Hospitalized Patients with Cirrhosis Should Be Screened for Clostridium difficile Colitis. Dig. Dis. Sci. 2015, 60, 3124–3129. [Google Scholar] [CrossRef]
- Sbeit, W.; Kadah, A.; Shahin, A.; Abed, N.; Haddad, H.; Jabbour, A.; Said Ahmad, H.; Pellicano, R.; Khoury, T.; Mari, A. Predictors of in-hospital mortality among patients with clostridium difficile infection: A multicenter study. Minerva Med. 2021, 112, 124–129. [Google Scholar] [CrossRef]
- Bednarska, A.; Bursa, D.; Podlasin, R.; Paciorek, M.; Skrzat-Klapaczyńska, A.; Porowski, D.; Raczyńska, J.; Puła, J.; Krogulec, D.; Makowiecki, M.; et al. Advanced age and increased CRP concentration are independent risk factors associated with Clostridioides difficile infection mortality. Sci. Rep. 2020, 10, 14681. [Google Scholar] [CrossRef]
- Kruger, A.J.; Durkin, C.; Mumtaz, K.; Hinton, A.; Krishna, S.G. Early Readmission Predicts Increased Mortality in Cirrhosis Patients after Clostridium difficile Infection. J. Clin. Gastroenterol. 2019, 53, e322–e327. [Google Scholar] [CrossRef]
- Czepiel, J.; Krutova, M.; Mizrahi, A.; Khanafer, N.; Enoch, D.A.; Patyi, M.; Deptuła, A.; Agodi, A.; Nuvials, X.; Pituch, H.; et al. Mortality Following Clostridioides difficile Infection in Europe: A Retrospective Multicenter Case-Control Study. Antibiotics 2021, 10, 299. [Google Scholar] [CrossRef]
- Hong, S.J.; Feuerstadt, P.; Brandt, L.J. MELD is the only predictor of short-term mortality in cirrhotic patients with C. difficile infection. Dig. Liver Dis. 2019, 51, 275–280. [Google Scholar] [CrossRef]
- Ungureanu, B.S.; Vladut, C.; Bende, F.; Sandru, V.; Tocia, C.; Turcu-Stiolica, R.A.; Groza, A.; Balan, G.G.; Turcu-Stiolica, A. Impact of the COVID-19 Pandemic on Health-Related Quality of Life, Anxiety, and Training among Young Gastroenterologists in Romania. Front. Psychol. 2020, 11, 579177. [Google Scholar] [CrossRef]
- Spigaglia, P. Clostridioides difficile infection in the COVID-19 era: Old and new problems. Pol. Arch. Intern. Med. 2021, 131, 118–120. [Google Scholar] [CrossRef]
- Hazel, K.; Skally, M.; Glynn, E.; Foley, M.; Burns, K.; O’Toole, A.; Boland, K.; Fitzpatrick, F. The other ‘C’: Hospital-acquired Clostridioides difficile infection during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2021. [Google Scholar] [CrossRef]
- Ochoa-Hein, E.; Rajme-López, S.; Rodríguez-Aldama, J.C.; Huertas-Jiménez, M.A.; Chávez-Ríos, A.R.; de Paz-García, R.; Haro-Osnaya, A.; González-Colín, K.K.; González-González, R.; González-Lara, M.F.; et al. Substantial reduction of healthcare facility-onset Clostridioides difficile infection (HO-CDI) rates after conversion of a hospital for exclusive treatment of COVID-19 patients. Am. J. Infect. Control 2020. [Google Scholar] [CrossRef]
- Kabała, M.; Aptekorz, M.; Martirosian, G. Rola środowiska szpitalnego i rąk personelu medycznego w szerzeniu się zakażeń Clostridioides (Clostridium) difficile [The role of hospital environment and the hands of medical staff in the transmission of the Clostridioides (Clostridium) difficile infection]. Med. Pract. 2019, 70, 739–745. (In Polish) [Google Scholar]
- Ragusa, R.; Giorgianni, G.; Lupo, L.; Sciacca, A.; Rametta, S.; La Verde, M.; Mulè, S.; Marranzano, M. Healthcare-associated Clostridium difficile infection: Role of correct hand hygiene in cross-infection control. J. Prev. Med. Hyg. 2018, 59, E145–E152. [Google Scholar]
- Marra, A.R.; Perencevich, E.N.; Nelson, R.E.; Samore, M.; Khader, K.; Chiang, H.Y.; Chorazy, M.L.; Herwaldt, L.A.; Diekema, D.J.; Kuxhausen, M.F.; et al. Incidence and Outcomes Associated With Clostridium difficile Infections: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e1917597. [Google Scholar] [CrossRef]

| Cirrhotic with CDI Patients Mean (±S.D.)/Number of Patients (%) Total n = 25 | |
|---|---|
| Age (years) | 65.8 (±8.06) |
| Alcohol | 20 (80%) |
| Death (yes) | 11 (44%) |
| Time between admission and CDI diagnosis | 4.16 (±1.4) |
| Viral | |
| HBV | 3 (12%) |
| HCV | 5 (20%) |
| No | 17 (68%) |
| Hepatic cancer | 7 (28%) |
| ICU | 15 (60%) |
| Atlas score | 4.32 (2.3) |
| PPI | 23 (92%) |
| Rifaximin | 5 (20%) |
| Encephalopathy | 20 (80%) |
| Ascites | 25 (100%) |
| SBP | 5 (20%) |
| CRP (mg/mL) | 50.4 (±23.82) |
| Leukocytes (cells/μL) | 15,610.68 (±6900.72) |
| Neutrophils (%) | 79.61 (±9.78) |
| Erythrocytes (cells/μL) | 5305.44 (±6422.44) |
| Haematocrit (%) | 27.61 (±7.67) |
| Glomerular filtration rate (mL/min/1.73 m2) | 73.42 (±45.55) |
| Na (mEq/L) | 129.96 (±4.84) |
| K (mEq/L) | 4.45 (±0.85) |
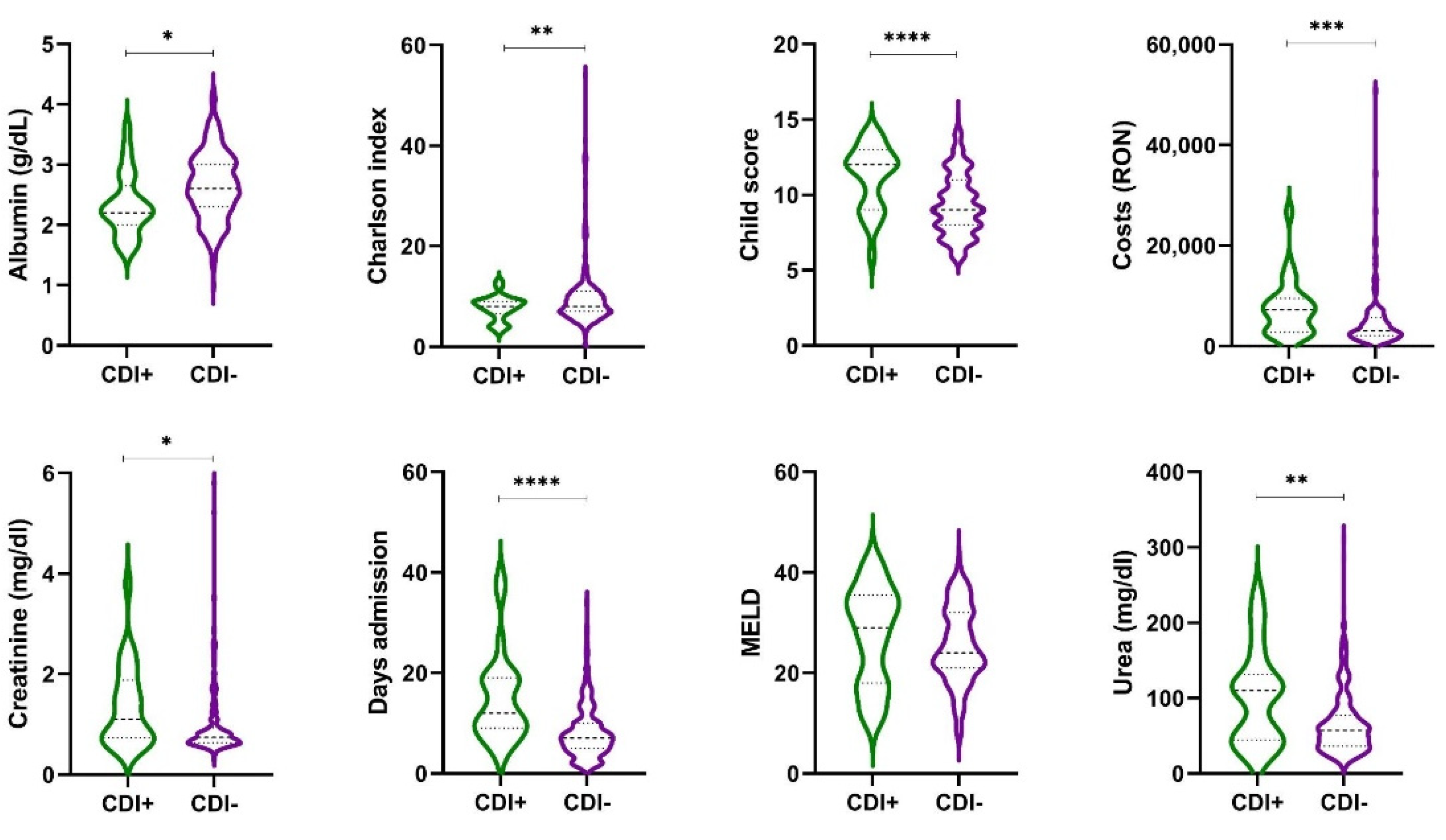
| Characteristics | Cirr+CDI+ n = 25 | Cirr+CDI− n = 342 | p-Value |
|---|---|---|---|
| Death (yes) | 11 (44%) | 89 (25.6%) | 0.0446 * |
| Rebleeding rate | 1 (4%) | 23 (6.73%) | 0.5321 |
| Proton pump inhibitor (yes) | 23 (92%) | 164 (47.95%) | <0.0001 * |
| Days admission | 14.84 (8.87) 12 (9–19) | 8.08 (5.22) 7 (5–10) | <0.0001 * |
| Child–Pugh score | 11.20 (2.14) 12 (9–13) | 7.9 (3.83) 9 (7–10) | <0.0001 * |
| MELD | 10.9 (15.64) 8.32 (6.54–9.25) | 14.79 (69.35) 8.3 (6.66–10.5) | 0.98 |
| Albumin (g/dL) | 2.33 (0.52) 2.2 (2–2.65) | 2.45 (0.88) 2.6 (2.1–3) | 0.013 * |
| Haemoglobin (g/dL) | 8.73 (2.55) 8.8 (7.03–10.15) | 13.7 (69.37) 8.09 (6.48–10) | 0.368 |
| Platelet count (cells/μL) | 136,661.6 (111,271.56) 114,000 (80,500–168,000) | 110,425.07 (77,579.62) 93,000 (64,112.5–137,000) | 0.095 |
| Creatinine (mg/dL) | 1.35 (0.81) 1.1 (0.73–1.88) | 0.96 (0.75) 0.74 (0.63–0.92) | 0.037 * |
| Urea (mg/dL) | 101.68 (62.79) 110 (44.5–131.5) | 64.03 (42.24) 55.5 (36–77) | 0.368 |
| Costs (EUR) | 1502.45 (1125.94) 1448.47 (558.99–1898.34) | 998.31 (1253.4) 618.41 (412.38–1142.08) | 0.0006 * |
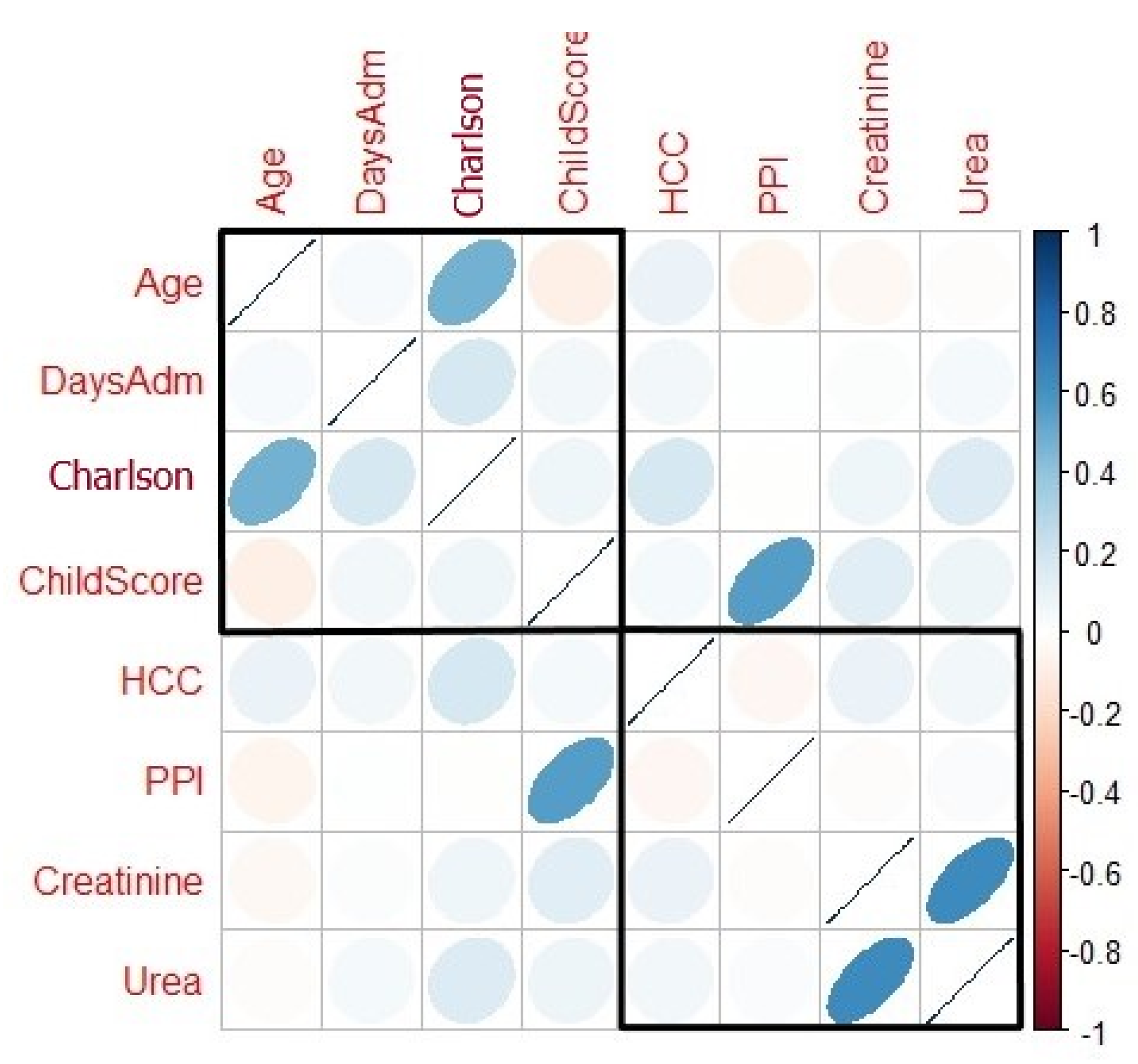
| Charlson index | 8.64 (3.46) 8.0 (5.50–12.00) | 6.31 (1.83) 6.0 (5.0–8.0) | 0.001 * |
| Factors | COR | 95%CI | p-Value |
|---|---|---|---|
| Age | 1.03 | 0.929–1.142 | 0.57 |
| Days admission | 0.919 | 0.816–1.035 | 0.163 |
| Child–Pugh score | 3.787 | 1.174–12.208 | 0.026 * |
| Alcohol (yes) | 2.25 | 0.304–16.632 | 0.427 |
| Virala HBV HCV | 0.364 1.333 | 0.047–2.817 0.067–26.618 | 0.333 0.851 |
| Diabetes (yes) | 2.0 | 0.366–10.919 | 0.423 |
| Hepatic cancer (yes) | 5.0 | 0.74–33.777 | 0.099 |
| Rifaximin (yes) | 7.429 | 0.69–79.957 | 0.098 |
| HDS (yes) | 0.205 | 0.018–2.327 | 0.201 |
| SBP (yes) | 4.875 | 0.43–55.292 | 0.201 |
| Proton pump inhibitor (yes) | 0.00 | - | 0.99 |
| Albumin (g/dL) | 0.167 | 0.021–1.315 | 0.089 |
| Platelet (cells/μL) | 1 | 1–1 | 0.413 |
| Leukocytes (cells/μL) | 1 | 1–1.001 | 0.023 * |
| CRP | 1.139 | 1.029–1.261 | 0.012 * |
| Atlas | 4.22 | 1.387–12.837 | 0.011 * |
| MELD | 1.48 | 1.058–2.07 | 0.022 * |
| Charlson index | 63.32 | 0.0–96.1 | 0.996 |
| Factors | AOR | 95%CI | p-Value |
|---|---|---|---|
| Child–Pugh score | 1.409 | 0.883–2.25 | 0.787 |
| Liver cancer (yes) | 14.082 | 0.245–89.231 | 0.201 |
| Rifaximin (yes) | 10.039 | 0.005–19.27 | 0.55 |
| Leukocytes | 1.0 | 1.0–1.001 | 0.147 |
| CRP | 1.016 | 0.032–31.933 | 0.993 |
| Atlas | 3.704 | 0.622–22.072 | 0.15 |
| MELD | 1.281 | 0.098–1.643 | 0.042 * |
| Factors | COR | 95%CI | p-Value |
|---|---|---|---|
| Age | 1.062 | 1.017–1.109 | 0.006 * |
| Days admission | 1.115 | 1.062–1.171 | <0.0001 * |
| Child–Pugh score | 1.543 | 1.262–1.887 | <0.0001 * |
| Alcohol (yes) | 1.5 | 0.52–4.3 | 0.451 |
| Hepatic cancer (yes) | 3.173 | 1.245–8.087 | 0.016 * |
| Creatinine | 1.376 | 1.0–1.892 | 0.050 |
| Urea | 1.013 | 1.006–1.020 | <0.0001 * |
| Proton pump inhibitor (yes) | 12.902 | 2.996–55.566 | 0.001 * |
| Charlson index | 1.609 | 1.330–1.947 | <0.0001 * |
| Factors | AOR | 95%CI | p-Value |
|---|---|---|---|
| Age | 1.067 | 1.004–1.134 | 0.037 * |
| Days admission | 1.159 | 1.086–1.238 | <0.0001 **** |
| Child–Pugh score | 1.224 | 0.916–1.636 | 0.171 |
| Liver cancer (yes) | 2.829 | 0.81–9.879 | 0.103 |
| Creatinine | 0.784 | 0.305–2.016 | 0.614 |
| Urea | 1.013 | 1.002–1.023 | 0.020 * |
| Proton pump inhibitor (yes) | 23.015 | 4.311–52.854 | <0.0001 **** |
| Charlson index | 1.671 | 1.326–2.106 | <0.0001 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, M.N.; Popescu, F.; Florescu, D.N.; Rogoveanu, I.; Turcu-Stiolica, A.; Gheonea, D.I.; Iovanescu, V.F.; Iordache, S.; Cazacu, S.M.; Ungureanu, B.S. Clostridioides difficile Infection among Cirrhotic Patients with Variceal Bleeding. Antibiotics 2021, 10, 731. https://doi.org/10.3390/antibiotics10060731
Voicu MN, Popescu F, Florescu DN, Rogoveanu I, Turcu-Stiolica A, Gheonea DI, Iovanescu VF, Iordache S, Cazacu SM, Ungureanu BS. Clostridioides difficile Infection among Cirrhotic Patients with Variceal Bleeding. Antibiotics. 2021; 10(6):731. https://doi.org/10.3390/antibiotics10060731
Chicago/Turabian StyleVoicu, Mirela Nicoleta, Florica Popescu, Dan Nicolae Florescu, Ion Rogoveanu, Adina Turcu-Stiolica, Dan Ionut Gheonea, Vlad Florin Iovanescu, Sevastita Iordache, Sergiu Marian Cazacu, and Bogdan Silviu Ungureanu. 2021. "Clostridioides difficile Infection among Cirrhotic Patients with Variceal Bleeding" Antibiotics 10, no. 6: 731. https://doi.org/10.3390/antibiotics10060731
APA StyleVoicu, M. N., Popescu, F., Florescu, D. N., Rogoveanu, I., Turcu-Stiolica, A., Gheonea, D. I., Iovanescu, V. F., Iordache, S., Cazacu, S. M., & Ungureanu, B. S. (2021). Clostridioides difficile Infection among Cirrhotic Patients with Variceal Bleeding. Antibiotics, 10(6), 731. https://doi.org/10.3390/antibiotics10060731








