Abstract
Trimethoprim-sulfamethoxazole is a well-known antibiotic that inhibits folic acid synthesis, a topic of renewed interest. Since resistant strains are increasingly more common, an early and accurate discrimination of susceptibility may assure confident therapy. Two morphological assays were performed in Escherichia coli (n = 50; 27 non-susceptible) and Klebsiella pneumoniae (n = 52; 18 non-susceptible). First, the strains were incubated with the CLSI breakpoint of cotrimoxazole for 150 min, which induced cell lengthening in the susceptible strains. Second, the bacteria were incubated with mitomycin C (MMC) (0.5 mg/L) for 120 min to induce a SOS-linked cell enlargement higher than that obtained by cotrimoxazole alone. When cotrimoxazole was added 30 min before MMC, the inhibition of folic acid synthesis in the susceptible strain resulted in the suppression of MMC-induced extra elongation. In the non-susceptible strains, folic acid synthesis continued despite the antibiotic, so that the MMC-induced extra cell lengthening could not be impeded. Whereas the first assay resulted in five false negatives and four false positives of resistance, the results of the second assay matched those of the conventional antibiogram. This simple morphological procedure is performed in 2 h and 45 min and may allow a rapid selection of useful and relatively inexpensive therapy, thereby preserving the newer broad-spectrum antibiotics.
1. Introduction
Cotrimoxazole, the combination of trimethoprim and sulfamethoxazole, was introduced in the late 1960s due to their synergistic activity to block bacterial folic acid synthesis [1,2]. Sulfamethoxazole is a sulfonamide, structural analog of para-aminobenzoic acid that competitively inhibits dihydropteroate synthase, an enzyme that catalyzes the formation of dihydrofolate from para-aminobenzoic acid and pteridine. Trimethoprim is a diaminopyrimidine antibiotic, structural analog of the pteridine component of dihydrofolate, behaving as a competitive inhibitor of dihydrofolate reductase, thus blocking the reduction of dyhydrofolic acid to tetrahydrofolic acid [3]. Tetrahydrofolic acid is the physiologically active form of folic acid, being an essential cofactor for the synthesis of thymidine, purines and methionine. The depletion of tetrahydrofolate as a consequence of the sequential enzymatic inhibition in its metabolic pathway hinders bacterial DNA replication and transcription, as well as protein synthesis, leading to a bactericidal effect.
Cotrimoxazole is usually active against Gram-negative bacilli and Gram-positive cocci but, also, Pneumocystis jirovecii and Toxoplasma gondii infections [3,4]. Nevertheless, the prescription of cotrimoxazole has been progressively declining during the past decade due to the spread of strains expressing genotypic and phenotypic resistance. In fact, cotrimoxazole remains as a first option for the treatment of noncomplicated urinary tract infections and in diseases caused by Stenotrophomonas maltophilia [5,6]. It is also prescribed for the acute infectious exacerbation of chronic obstructive pulmonary disease, otitis media in pediatric population, traveler’s diarrhea, shigellosis, pneumocystis pneumonia and toxoplasmosis [3,4]. More recently, due to the global spread of multidrug-resistant (MDR) Gram-negative bacilli, interest in the use of cotrimoxazole has been renewed. This is a widely available and relatively inexpensive medication whose adequate prescription may help to prevent the advancement of dangerous resistant pathogens by the misuse of antibiotics and to preserve newer broad-spectrum antibiotics and last option antibacterial drugs [7,8,9]. Since cotrimoxazole resistant strains are usual, an accurate determination of the susceptibility or resistance should be performed [10]. It is evident that the earlier the testing, the more reliable and successful the therapy will be.
Trimethoprim has been shown to induce filamentation in the clinical strains of some Enterobacteriaceae, and it has been proposed as a rapid test for the discrimination of susceptibility or resistance [11]. In a previous report, we introduced a rapid assay for the identification of resistant strains of Gram-negative bacilli to antibiotic inhibitors of protein synthesis based on the simple evaluation of the bacterial cell length [12]. The cultures were incubated with mitomycin C (MMC), which induced significant cell enlargement as a rapid consequence of the triggering of the SOS response [13,14]. An addition of the antibiotic before MMC prevented the elongation when the strain was susceptible to the antibiotic, thereby inhibiting protein synthesis. However, cell enlargement was not prevented in the antibiotic-resistant strains where protein synthesis had not been successfully inhibited [12]. Here, we demonstrate that the MMC-induced elongation assay, in addition to the antibiotic inhibitors of protein synthesis, can also be successfully adapted for the rapid detection of susceptibility or resistance to cotrimoxazole in Enterobacteriaceae Escherichia coli and Klebsiella pneumoniae. The discriminative capacity of the MMC-induced elongation assay may be more accurate than the evaluation of filamentation induced by the antibiotic.
2. Results
2.1. Technical Assay
The technical bases of the assay are presented in Figure 1. Two strains of E. coli, one susceptible (S) to cotrimoxazole (MIC: 0.125 mg/L) and one resistant (R) (MIC: >32 mg/L), were incubated with the CLSI (Clinical and Laboratory Standards Institute) breakpoint concentration of the antibiotic (2 mg/L) for 150 min, with MMC (0.5 mg/L) for 120 min and with cotrimoxazole 150 min and MMC added during last 120 min. Cotrimoxazole alone increased the cell length in the susceptible strain but not in the resistant strain (basal-S: 2.18 ± 0.39 μm; basal-R: 2.16 ± 0.46 μm; cotrimoxazole-S: 3.61 ± 1.19 μm; cotrimoxazole-R: 2.33 ± 0.37 μm; mean ± SD). MMC alone induced cell lengthening in both strains, higher than that produced by cotrimoxazole alone (MMC-S: 4.57 ± 2.18 μm; MMC-R: 6.49 ± 4.13 μm). When cotrimoxazole was added 30 min before MMC, the susceptible strain could not enlarge more than that achieved by cotrimoxazole alone, whereas the resistant strain increased the cell length similarly to that from the culture incubated with MMC alone (cotrimoxazole + MMC-S: 3.68 ± 1.35 μm; cotrimoxazole + MMC-R: 6.35 ± 4.10 μm). According to these results, two morphological parameters may provide information about the susceptibility or resistance to cotrimoxazole (1): cotrimoxazole-induced lengthening and (2) cotrimoxazole-induced suppression of MMC-linked elongation.
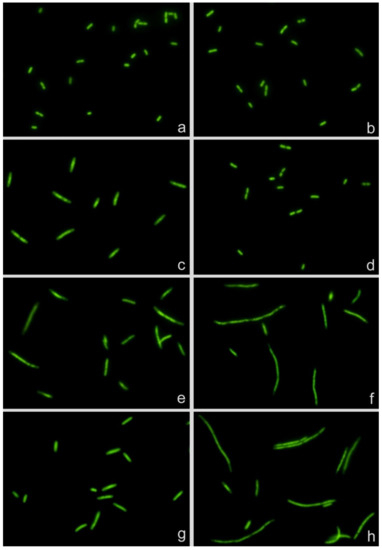
Figure 1.
Escherichia coli strains susceptible (left, MIC: 0.125 mg/L) and resistant (right, MIC: >32 mg/L) to cotrimoxazole. (a,b) Control without antibiotic. (c,d) Incubated with cotrimoxazole, 2 mg/L. (e,f) Treated with mitomycin C (MMC), 0.5 mg/L. (g,h) Incubated with cotrimoxazole and MMC. MMC induces cell elongation in both strains (e,f). Cotrimoxazole induces cell lengthening and prevents MMC-induced cell enlargement in the susceptible strain (c,g) but not in the resistant strain (d,h).
2.2. Dose Response
Three strains of E. coli and three of K. pneumoniae with increasing MICs of cotrimoxazole were incubated with increasing concentrations of the antibiotic, ranging from 0.06 to 32 mg/L, to evaluate both (1): the cotrimoxazole-induced lengthening (Figure 2) and (2) cotrimoxazole-induced suppression of MMC-linked elongation (Figure 3 and Figure 4). Given that the CLSI breakpoint concentration of susceptibility is 2/38 mg/L trimethoprim/sulfamethoxazole, two E. coli strains were categorized as susceptible (MICs 0.125 and 0.38 mg/L, respectively) and one resistant (MIC > 32 mg/L). Regarding K. pneumoniae, one was susceptible (MIC: 0.38 mg/L), one was at the susceptibility breakpoint (MIC: 2 mg/L) and one was resistant (MIC > 32/mg/L).
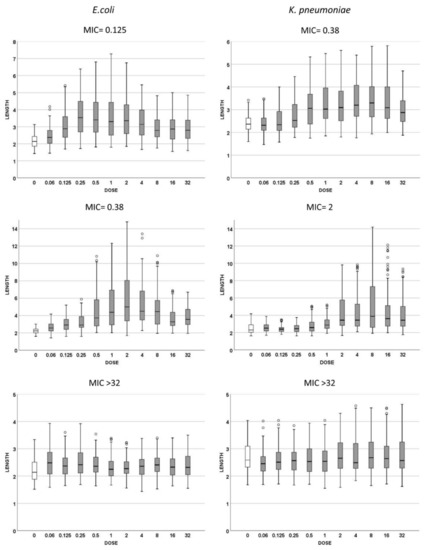
Figure 2.
Cell length measures (μm) of Escherichia coli (left) and Klebsiella pneumoniae (right) strains with different MICs for cotrimoxazole. The first row corresponds to susceptible strains with MIC 0.125 mg/L for E. coli and 0.38 mg/L for K. pneumoniae; the second row represents the strains with MIC 0.38 mg/L for E. coli and 2 mg/L for K. pneumoniae; the third row represents the resistant strains, both with MIC > 32 mg/L. Each strain shows the cell length (vertical axis) measured in cultures incubated with increasing doses of cotrimoxazole, from 0 to 32 mg/L. The data are presented as box and whisker plots. The horizontal line in the box indicates the median, the lower line of the box is the first quartile, the upper line of the box is the third quartile and the whiskers (the end of the vertical lines) are the maximum and minimum values. Abnormal values are presented as dots outside the box.
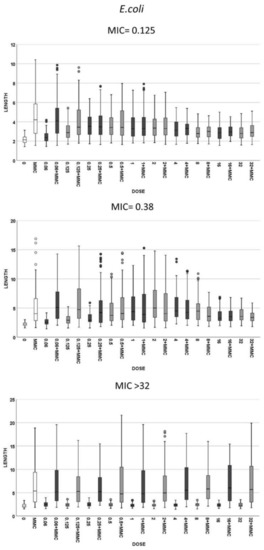
Figure 3.
Cell length measures (µm) of E. coli in the same strains that were presented in Figure 2. The first and second graphics correspond to the strains susceptible to cotrimoxazole, with MIC 0.125 and 0.38 mg/L, respectively, and the third is a resistant strain with MIC > 32 mg/L. Each strain shows the cell length (vertical axis) in, from left to right (horizontal axis), control cultures (0), cultures incubated with 0.5-mg/L mitomycin C (MMC) and cultures incubated with increasing doses of cotrimoxazole alone, each paired with incubation of cotrimoxazole together with 0.5-mg/L MMC.
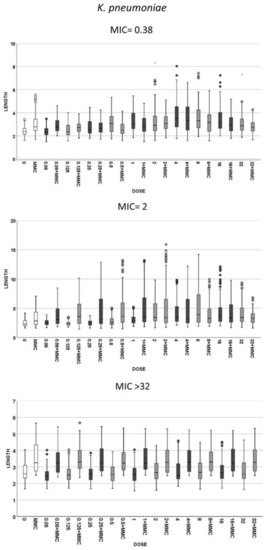
Figure 4.
Cell length measures (µm) in the same strains of K. pneumoniae that were shown in Figure 2. The first image corresponds to a strain susceptible to cotrimoxazole, with MIC 0.38 mg/L, the second graphic is a strain with MIC 2 mg/L and third image is a resistant strain with MIC ≥ 32 mg/L. Each strain shows the cell length (vertical axis) in, from left to right (horizontal axis), control cultures (0), cultures incubated with 0.5-mg/L mitomycin C (MMC) and cultures incubated with increasing doses of cotrimoxazole alone, each paired with incubation of cotrimoxazole together with 0.5-mg/L MMC.
(1) Cotrimoxazole induced statistically significant lengthening with respect to each respective control untreated culture when reaching a concentration similar or close to the MIC of the strain (Figure 2). The median length may increase even more with subsequent doses, but this was not statistically significant, thus remaining stable at the highest doses, although with a lower variability. No elongation was evidenced in the resistant strains (MIC > 32 mg/L) at any dose (Figure 2). (2) Regarding MMC-linked elongation, a decrease in the MMC-induced extra lengthening was evident at concentrations of cotrimoxazole close or similar to the MIC, so that the bacterial length was not significantly different to that produced by cotrimoxazole alone (Figure 3 and Figure 4). This absence of differences was maintained at higher doses. The resistant strains (MIC > 32 mg/L) never showed a decrease of cell length of MMC at any dose of cotrimoxazole assayed (Figure 3 and Figure 4).
Given that, according to the dose response, it is evidenced that the effect is significant at or close to the MIC of the strain, the incubation with the CLSI breakpoint concentration of susceptibility based on the criterion of cell growth inhibition, i.e., 2/38-mg/L trimethoprim/sulfamethoxazole, was chosen to be validated for the discrimination of susceptibility or not based on the two cell elongation assays.
2.3. Assay Validation
Clinical isolates of E. coli and K. pneumoniae were blindly processed following the protocol of the assay. Both parameters: (1) cotrimoxazole-induced lengthening and (2) cotrimoxazole-induced suppression of MMC-linked extra elongation were estimated and compared with the results of the standard antibiogram (Table 1). (1) Cotrimoxazole-induced lengthening resulted in five false negatives of the identification of resistance, two in E. coli and three in K. pneumoniae. These strains enlarged despite being non-susceptible, and a cotrimoxazole prescription would be ineffective, with threatening consequences in cases of severe infection. Moreover, four false positives of resistance were identified, one for E. coli and three for K. pneumoniae. These strains did not enlarge despite being susceptible but were not clinically relevant, since another effective antibiotic was selected. (2) Importantly, neither false positives nor false negatives were evidenced when the parameters of the cotrimoxazole-induced suppression of MMC-linked elongation were used; all the strains were correctly identified.

Table 1.
Analytical evaluation of the technical assays of cotrimoxazole-induced cell elongation (CTX) and cotrimoxazole prevention of mitomycin C-induced cell enlargement (CTX+MMC), in comparison with the categorization by the standard antibiogram and CLSI criteria of Escherichia coli and Klebsiella pneumoniae strains. S: susceptible; NS: non-susceptible; AUC: area under the receiver operating characteristic (ROC) curve.
3. Discussion
MMC mainly induces DNA interstrand crosslinks that trigger the bacterial SOS response, a complex regulatory network for DNA repair [15,16]. The response is possible through the short-term expression of inducible proteins. One of these proteins is SulA (or SfiA), which is an inhibitor of the protein FtsZ. The latter is necessary to constitute the contractile ring in the future septum required for cell division [14,17]. As a result, cytokinesis is prevented to allow DNA repair before segregation. However, the cell continues to grow, and the morphology lengthens [18]. In fact, given its effectiveness, MMC is commonly employed as a positive control in experiments dealing with the SOS response, including the cell elongation effect [19,20].
Overall, the SOS response requires a rapid RNA transcription and coupled ribosomal protein synthesis [21]. In a previous report, it was demonstrated that the antibiotic inhibitors of protein synthesis prevented MMC-SOS linked cell elongation when the strain was susceptible to the antibiotic [12]. Possibly, the general inhibition of the protein synthesis at the ribosomes by the antibiotic included the SOS-inducible SuIA. Nevertheless, in resistant strains, the antibiotic could not be effective in inhibiting protein synthesis, and therefore, the MMC-induced cell lengthening was maintained. The optimization of the procedure allowed a simple and rapid assay to determine the susceptibility or resistance to the antibiotic inhibitors of protein synthesis in E. coli, K. pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa [12].
The essence of the assay is the prevention or nonprevention of the synthesis of the proteins necessary for cell division, like SuIA. However, this prevention could take place not only in the final step of protein synthesis in the ribosome but, also, in the previous step of RNA transcription. Cotrimoxazole produces a sequential inhibition of the enzymes of the tetrahydrofolate synthesis pathway. The depletion of tetrahydrofolate impedes the synthesis of thymidine essential for DNA replication, the synthesis of purines required for DNA replication and RNA transcription and the synthesis of methionine necessary for protein synthesis. Although the influence of the other physiological processes cannot be discarded, the deficiency of purines would affect the RNA transcription necessary for some of the outcomes of the induced SOS response, including the inhibition of bacterial division.
Intriguingly, trimethoprim and cotrimoxazole induce bacterial enlargement in the susceptible strains of many Enterobacteriaceae, and the induction of the SOS response has been suggested as responsible, as with the MMC [11,22]. It seems paradoxical that trimethoprim, an inducer of bacterial enlargement, prevents extra bacterial lengthening by the MMC. When combined cotrimoxazole and MMC, on one side, the bacteria is enlarged by cotrimoxazole, but on the other side, the bacteria cannot go on enlarging at the level achieved by MMC alone. Nevertheless, this response is not universal, and some susceptible strains do not enlarge with trimethoprim but show lengthening after incubation with MMC [11]. In fact, our study identified four strains that did not enlarge after cotrimoxazole despite being susceptible, i.e., false positives, according to this parameter. Nevertheless, these strains were enlarged by MMC, and cotrimoxazole prevented this MMC-induced lengthening. Although trimethoprim may induce a SOS response, this differential behavior suggests that the SOS response is questionable as the main factor responsible for cell elongation by this antibiotic. Alternatively, the impaired RNA transcription due to deficiency of purines may compromise the ribosomal translation, resulting in the depletion of some proteins necessary for bacterial division, so the cell lengthens. Otherwise, this same mechanism could impede the extra lengthening linked to SOS when MMC is later added to the culture, as previously discussed.
In the previous report about cell lengthening by trimethoprim, the effect was evaluated after 4 to 5 h and 16 h of incubation [11]. Although some more species from Enterobacteriaceae were tested, only very scarce strains were scored, e.g., 14 E. coli strains, including six resistant and three susceptible K. pneumonia strains. Here, we optimized the procedure for cotrimoxazole in E. coli and K. pneumoniae, obtaining the results in 2 h and 45 min and testing many more strains. Although the induced lengthening was revealed as a reliable parameter for the discrimination of susceptibility or resistance to cotrimoxazole, the accuracy of the evaluation of the induced suppression of MMC-linked elongation was complete in our clinical samples, demonstrating its superiority.
This is a rapid and simple phenotypic assay that detects resistance without the need to previously know the causal mechanism, as usually required when using molecular genetics or proteomic procedures. Evaluation of the cell length is a simple parameter that may be easily determined with a standard automatized microscopic or flow cytometry platforms. The present work elucidated that the evaluation of the prevention or nonprevention of MMC-induced lengthening, successfully applied for the rapid determination of resistance to antibiotic inhibitors of protein synthesis, may also be extended to cotrimoxazole, thus helping to preserve the newer broad-spectrum antibiotics and last option antibacterial drugs.
4. Materials and Methods
4.1. Bacterial Strains and Minimum Inhibitory Concentrations (MICs) Determination
Clinical isolates of E. coli (n = 50) and K. pneumoniae (n = 52) were collected at the University Hospital A Coruña between 2001 and 2018. MICs to cotrimoxazole were ascertained by automated microdilution (MicroScan Walkaway, Siemens, Munich, Germany) and corroborated by a Liofilmchem MIC Test Strip according to the manufacturer’s instructions, with susceptibility defined using CLSI criteria [23].
4.2. Determining Susceptibility-Resistance Using Cell Elongation
Bacteria cultured on solid media plates were used to inoculate a 2-mL Mueller–Hinton broth culture and incubated at 37 °C for 90 min. Afterwards, the bacteria were diluted to an OD600 of 0.1 in Mueller–Hinton broth and incubated at 37 °C in 200-μL tubes. Cotrimoxazole (6 μL) was added to the culture 30 min before the addition of the MMC (0.5 mg/L), and both were maintained for 2 h of incubation in a final volume of 30 μL. The concentration and incubation time of MMC was optimized previously in E. coli and K. pneumoniae [10], corresponding to the minimum dose and time that provided the highest lengthening without decreasing the cell number.
In order to assess the cell length, the bacteria were diluted to a concentration of 1 × 107 microorganisms/mL in Mueller–Hinton broth, included in a thin agarose microgel on a slide, dehydrated in increasing ethanol baths, dried, stained with the fluorochrome SYBR Gold and examined under an epifluorescence microscope (Nikon E800). Images were captured using a high-sensitive CCD camera (KX32ME, Apogee Instruments, Roseville, CA, USA). Image analysis was performed using NIS-Elements software (Nikon Instruments Inc., Tokyo, Japan), measuring the length of 100 cells per assay.
Cultures with neither cotrimoxazole nor MMC, with cotrimoxazole only and with MMC only were always processed simultaneously. Controls without antibiotic and MMC provided the basal cell length of each specific strain. The culture with MMC established the elongation size. The culture with cotrimoxazole co-incubated with MMC revealed if there was prevention or not of MMC-induced cell enlargement.
The relationship between the prevention of MMC-induced cell elongation and the CLSI breakpoint concentrations of cotrimoxazole were empirically established after processing a subset of strains comprising a wide range of MICs, including susceptible and resistant strains exposed to progressively increasing antibiotic doses. Several strains with MICs close to the CLSI breakpoints for susceptibility and resistance were employed. Antibiotic concentrations that suppressed MMC-induced cell enlargement in the susceptible, but not in the resistant strains, were determined. It should be noted that the suppression of MMC-induced cell elongation was considered when the bacterial size was not significantly different from that of the cotrimoxazole-only treated culture.
The concentrations of antibiotics were optimized such that a strain categorized as susceptible following the CLSI criteria demonstrated the abolition of cellular elongation after incubation with the lowest concentration of antibiotic established as the susceptibility breakpoint when using the parameter of elongation suppression. Notably, a non-susceptible strain should not show any abolition of MMC-induced elongation with the established breakpoint concentration of the antibiotic.
Once implemented, the breakpoint concentration for the elongation suppression criterion, which correlates with the CLSI susceptibility breakpoint for each antibiotic and bacterium, the collected isolates were blindly processed. For each isolate, four cultures were established, as indicated above: (1) a control culture with neither cotrimoxazole nor MMC, (2) a culture with the established breakpoint dose of antibiotic only, (3) a culture with MMC only and (4) a culture with cotrimoxazole (the established breakpoint dose) and MMC.
4.3. Data Analysis
Data were analyzed using the SPSS Statistics 21 software package for Windows (IBM). Friedman test and Wilcoxon test with Bonferroni correction were employed for statistical comparisons. Significance was defined as p < 0.05. Receiver operating characteristic (ROC) curves were constructed with the results obtained during the validation assay. The area under the curve (AUC) values indicated the global classification ability of the test. Validation indexes were estimated using the Epidat 3.1 software package (Consellería de Sanidade, Xunta de Galicia, Spain and Panamerican Health Organization, Washington, DC, USA).
Author Contributions
Conceptualization: J.L.F. and G.B.; study design, J.L.F. and G.B.; methodology, I.L., F.O., R.G. and M.d.C.F.; software, I.L., F.O. and R.G.; validation, I.L., F.O. and R.G.; statistical analysis, F.O., G.B. and J.G.; writing—original draft preparation, J.L.F. and G.B.; writing—review and editing, J.L.F., G.B. and J.G.; supervision, J.L.F. and funding acquisition, J.L.F. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Fondo de Investigaciones Sanitarias (PI20/01263) and REIPI, Spanish Network for Research in Infectious Diseases (Instituto de Salud Carlos III, RD16/0016/0006). This work was also funded by IMI 6th call COMBACTE New Drugs 4 Bad Bugs (ND4BB).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
We are grateful to Michael E. Kjelland for revision of the manuscript.
Conflicts of Interest
J.G. and J.L.F. are advisers of Halotech DNA SL. All other authors declare no conflict of interest.
References
- Bushby, S.R.M.; Hitchings, G.H. Thrimethoprim, a sulphonamide potentiator. Br. J. Pharmac. Chemother 1968, 33, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Darrell, J.H.; Garrod, L.P.; Waterworth, P.M. Trimethoprim: Laboratory and clinical studies. J. Clin. Pathol. 1968, 21, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.A.; O’Bryan, T.A.; Zurlo, J.; Miller, D.Q.; Joshi, N. Trimethoprim-sulfamethoxazole revisited. Arch. Intern. Med. 2003, 163, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Smilack, J.D. Trimethoprim-sulfamethoxazole. Mayo Clin. Proc. 1999, 74, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Bátori, Z.; Ábrók, M.; Lázár, A.; Burián, K. Characterization of Resistance in Gram-Negative Urinary Isolates Using Existing and Novel Indicators of Clinical Relevance: A 10-Year Data Analysis. Life 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.S. Stenotrophomonas maltophilia: An Emerging Global Opportunistic Pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Grammatikos, A.P.; Michalopoulos, A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev. Anti-Infect. Ther. 2008, 6, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Cassir, N.; Rolain, J.M.; Brouqui, P. A new strategy to fight antimicrobial resistance: The revival of old antibiotics. Front. Microbiol. 2014, 5, 551. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Gales, A.C.; Dubourg, G. Old antibiotics for multidrug-resistant pathogens: From in vitro activity to clinical outcomes. Int. J. Antimicrob. Agents 2017, 49, 542–548. [Google Scholar] [CrossRef]
- Huovinen, P. Resistance to trimethoprim–sulfamethoxazole. Clin. Infect. Dis. 2001, 32, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Ingham, C.J.; van den Ende, M.; Wever, P.C.; Schneeberger, P.M. Rapid antibiotic sensitivity testing and trimethoprim-mediated filamentation of clinical isolates of the Enterobacteriaceae assayed on a novel porous culture support. J. Med. Microbiol. 2006, 55, 1511–1519. [Google Scholar] [CrossRef][Green Version]
- Otero, F.; Gosálvez, J.; Bou, G.; Fernández, J.L. Simple and fast detection of resistance to antibiotic inhibitors of protein synthesis in Gram-negative pathogens through evaluation of mitomycin C-induced cell elongation. Microb. Drug Resist. 2017, 23, 973–981. [Google Scholar] [CrossRef]
- Suzuki, H.; Kilgore, W.W. Effects of mitomycin C on macromolecular synthesis in Escherichia coli. J. Bacteriol. 1967, 93, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Huisman, O.; D’Ari, R.; Gottesman, S. Cell-division control in Escherichia coli: Specific induction of the SOS function SfiA protein is sufficient to block septation. Proc. Natl. Acad. Sci. USA 1984, 81, 4490–4494. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, M.; Lipman, R.; Chowdary, D. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science 1987, 235, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Baharoglu, Z.; Mazel, D. SOS, the formidable strategy of bacteria against aggressions. FEMS Microbiol. 2014, 38, 1126–1145. [Google Scholar] [CrossRef]
- Chen, Y.; Milan, S.L.; Erickson, H.P. SulA inhibits assembly of FtsZ by a simple sequestration mechanism. Biochemistry 2012, 51, 3100–3109. [Google Scholar] [CrossRef]
- Margolin, W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell. Biol. 2005, 6, 862–871. [Google Scholar] [CrossRef]
- Gunderson, C.W.; Segall, A.M. DNA repair, a novel antibacterial target: Holliday junction-trapping peptides induce DNA damage and chromosome segregation defects. Mol. Microbiol. 2006, 59, 1129–1148. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 10, e13745. [Google Scholar] [CrossRef]
- Singh, S.B.; Young, K.; Silver, L.L. What is an “ideal” antibiotic? Discovery challenges and path forward. Biochem Pharmacol. 2017, 133, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lewin, C.S.; Amyes, S.G. The role of the SOS response in bacteria exposed to zidovudine or trimethoprim. J. Med. Microbiol. 1991, 34, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Document M100, 30th ed.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).