Temporal Variations in Patterns of Clostridioides difficile Strain Diversity and Antibiotic Resistance in Thailand
Abstract
1. Introduction
2. Results
2.1. Toxin Gene Profiles of C. difficile Isolates
2.2. Ribotypes of C. difficile Isolates
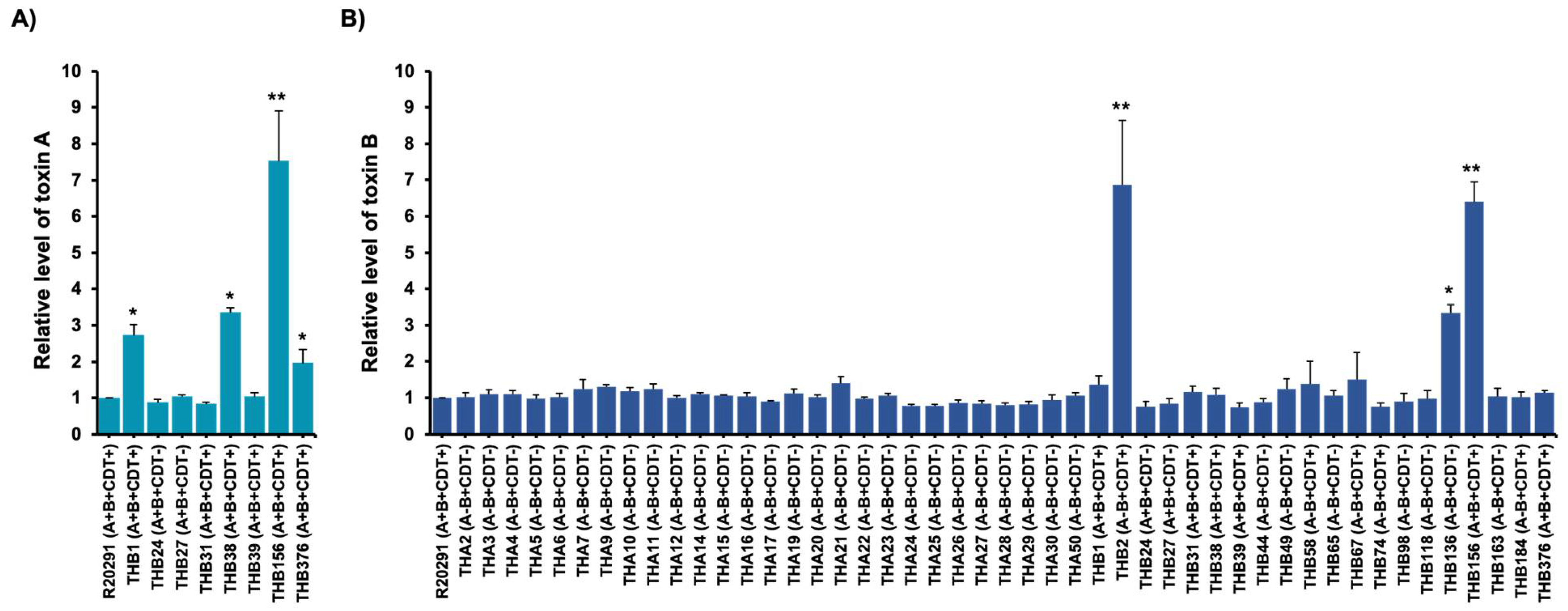
2.3. Toxin Production of C. difficile Isolates
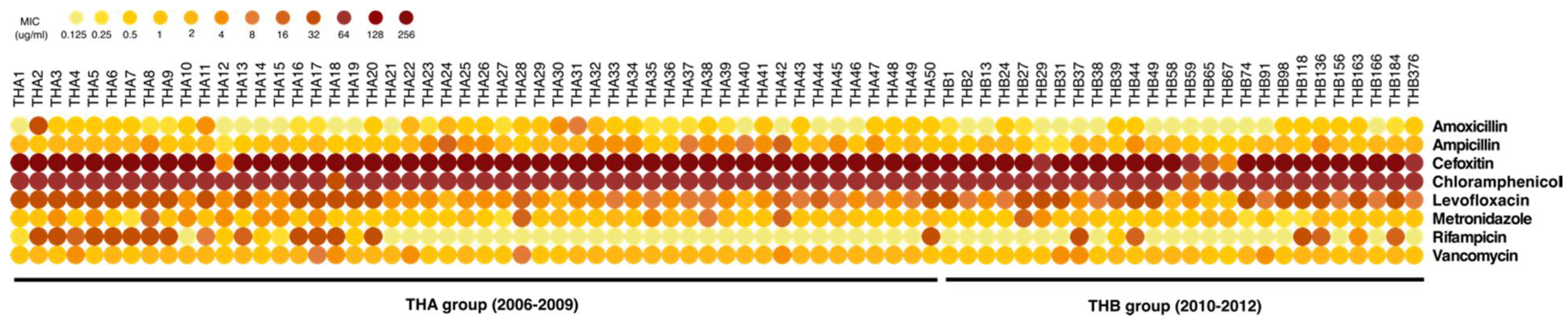
2.4. Antimicrobial Resistance Profiles of C. difficile Isolates
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Bacterial Culture
4.2. Toxin Genotyping
4.3. PCR Ribotyping
4.4. Quantification of Toxins A and B
4.5. Minimal Inhibitory Concentration (MIC) Testing
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, C.P.; Pothoulakis, C.; LaMont, J.T. Clostridium difficile colitis. N. Engl. J. Med. 1994, 330, 257–262. [Google Scholar] [CrossRef]
- Oren, A.; Rupnik, M. Clostridium difficile and Clostridioides difficile: Two validly published and correct names. Anaerobe 2018, 52, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Jawa, R.S.; Mercer, D.W. Clostridium difficile-associated infection: A disease of varying severity. Am. J. Surg. 2012, 204, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Sorg, J.A.; Sun, X. Clostridioides difficile Biology: Sporulation, Germination, and Corresponding Therapies for C. difficile Infection. Front. Cell. Infect. Microbiol. 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Burnham, C.A.; Carroll, K.C. Diagnosis of Clostridium difficile infection: An ongoing conundrum for clinicians and for clinical laboratories. Clin. Microbiol. Rev. 2013, 26, 604–630. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef]
- Cowardin, C.A.; Buonomo, E.L.; Saleh, M.M.; Wilson, M.G.; Burgess, S.L.; Kuehne, S.A.; Schwan, C.; Eichhoff, A.M.; Koch-Nolte, F.; Lyras, D.; et al. The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat. Microbiol. 2016, 1, 16108. [Google Scholar] [CrossRef]
- He, M.; Miyajima, F.; Roberts, P.; Ellison, L.; Pickard, D.J.; Martin, M.J.; Connor, T.R.; Harris, S.R.; Fairley, D.; Bamford, K.B.; et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 2013, 45, 109–113. [Google Scholar] [CrossRef]
- Borren, N.Z.; Ghadermarzi, S.; Hutfless, S.; Ananthakrishnan, A.N. The emergence of Clostridium difficile infection in Asia: A systematic review and meta-analysis of incidence and impact. PLoS ONE 2017, 12, e0176797. [Google Scholar] [CrossRef]
- Imwattana, K.; Wangroongsarb, P.; Riley, T.V. High prevalence and diversity of tcdA-negative and tcdB-positive, and non-toxigenic, Clostridium difficile in Thailand. Anaerobe 2019, 57, 4–10. [Google Scholar] [CrossRef]
- Putsathit, P.; Maneerattanaporn, M.; Piewngam, P.; Kiratisin, P.; Riley, T.V. Prevalence and molecular epidemiology of Clostridium difficile infection in Thailand. New Microbes New Infect. 2017, 15, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Imwattana, K.; Knight, D.R.; Kullin, B.; Collins, D.A.; Putsathit, P.; Kiratisin, P.; Riley, T.V. Clostridium difficile ribotype 017-characterization, evolution and epidemiology of the dominant strain in Asia. Emerg. Microbes Infect. 2019, 8, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Umeki, S.; Niki, Y.; Soejima, R. Angiotensin-converting enzyme activity and steroid therapy in sarcoidosis. Arch. Intern. Med. 1987, 147, 2056. [Google Scholar] [CrossRef]
- Collins, D.A.; Putsathit, P.; Elliott, B.; Riley, T.V. Laboratory-based surveillance of Clostridium difficile strains circulating in the Australian healthcare setting in 2012. Pathology 2017, 49, 309–313. [Google Scholar] [CrossRef]
- Senoh, M.; Kato, H.; Fukuda, T.; Niikawa, A.; Hori, Y.; Hagiya, H.; Ito, Y.; Miki, H.; Abe, Y.; Furuta, K.; et al. Predominance of PCR-ribotypes, 018 (smz) and 369 (trf) of Clostridium difficile in Japan: A potential relationship with other global circulating strains? J. Med. Microbiol. 2015, 64, 1226–1236. [Google Scholar] [CrossRef]
- Jin, H.; Ni, K.; Wei, L.; Shen, L.; Xu, H.; Kong, Q.; Ni, X. Identification of Clostridium difficile RT078 From Patients and Environmental Surfaces in Zhejiang Province, China. Infect. Control. Hosp. Epidemiol. 2016, 37, 745–746. [Google Scholar] [CrossRef]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Loo, V.G.; Bourgault, A.M.; Poirier, L.; Lamothe, F.; Michaud, S.; Turgeon, N.; Toye, B.; Beaudoin, A.; Frost, E.H.; Gilca, R.; et al. Host and pathogen factors for Clostridium difficile infection and colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and treatment of Clostridium difficile in adults: A systematic review. JAMA 2015, 313, 398–408. [Google Scholar] [CrossRef]
- Ofosu, A. Clostridium difficile infection: A review of current and emerging therapies. Ann. Gastroenterol. 2016, 29, 147–154. [Google Scholar] [CrossRef]
- Goudarzi, M.; Goudarzi, H.; Alebouyeh, M.; Azimi Rad, M.; Shayegan Mehr, F.S.; Zali, M.R.; Aslani, M.M. Antimicrobial susceptibility of clostridium difficile clinical isolates in iran. Iran. Red Crescent Med. J. 2013, 15, 704–711. [Google Scholar] [CrossRef]
- Adler, A.; Miller-Roll, T.; Bradenstein, R.; Block, C.; Mendelson, B.; Parizade, M.; Paitan, Y.; Schwartz, D.; Peled, N.; Carmeli, Y.; et al. A national survey of the molecular epidemiology of Clostridium difficile in Israel: The dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn. Microbiol. Infect. Dis. 2015, 83, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Luo, Y.; Huang, C.; Cai, J.; Ye, J.; Zheng, Y.; Wang, L.; Zhao, P.; Liu, A.; Fang, W.; et al. Molecular Epidemiology of Clostridium difficile Infection in Hospitalized Patients in Eastern China. J. Clin. Microbiol. 2017, 55, 801–810. [Google Scholar] [CrossRef]
- Peng, Z.; Addisu, A.; Alrabaa, S.; Sun, X. Antibiotic Resistance and Toxin Production of Clostridium difficile Isolates from the Hospitalized Patients in a Large Hospital in Florida. Front. Microbiol. 2017, 8, 2584. [Google Scholar] [CrossRef] [PubMed]
- Putsathit, P.; Maneerattanaporn, M.; Piewngam, P.; Knight, D.R.; Kiratisin, P.; Riley, T.V. Antimicrobial susceptibility of Clostridium difficile isolated in Thailand. Antimicrob. Resist. Infect. Control. 2017, 6, 58. [Google Scholar] [CrossRef][Green Version]
- Imwattana, K.; Putsathit, P.; Leepattarakit, T.; Kiratisin, P.; Riley, T.V. Mild or Malign: Clinical Characteristics and Outcomes of Clostridium difficile Infection in Thailand. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Shen, A. Clostridium difficile toxins: Mediators of inflammation. J. Innate Immun. 2012, 4, 149–158. [Google Scholar] [CrossRef]
- Åkerlund, T.; Persson, I.; Unemo, M.; Norén, T.; Svenungsson, B.; Wullt, M.; Burman, L.G. Increased Sporulation Rate of Epidemic Clostridium difficile Type 027/NAP1. J. Clin. Microbiol. 2008, 46, 1530. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.C.Y.; Kwong, T.N.Y.; So, E.W.M.; Ho, Y.I.I.; Wong, S.H.; Lai, R.W.M.; Chan, R.C.Y. Surveillance of antibiotic resistance among common Clostridium difficile ribotypes in Hong Kong. Sci. Rep. 2017, 7, 17218. [Google Scholar] [CrossRef]
- O’Connor, J.R.; Galang, M.A.; Sambol, S.P.; Hecht, D.W.; Vedantam, G.; Gerding, D.N.; Johnson, S. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob. Agents Chemother. 2008, 52, 2813–2817. [Google Scholar] [CrossRef]
- Robinson, C.D.; Auchtung, J.M.; Collins, J.; Britton, R.A. Epidemic Clostridium difficile strains demonstrate increased competitive fitness compared to nonepidemic isolates. Infect. Immun. 2014, 82, 2815–2825. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Bauer, M.P.; Baines, S.D.; Corver, J.; Fawley, W.N.; Goorhuis, B.; Kuijper, E.J.; Wilcox, M.H. The changing epidemiology of Clostridium difficile infections. Clin. Microbiol. Rev. 2010, 23, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Melendez, A.; Morfin-Otero, R.; Villarreal-Trevino, L.; Baines, S.D.; Camacho-Ortiz, A.; Garza-Gonzalez, E. Molecular epidemiology of predominant and emerging Clostridioides difficile ribotypes. J. Microbiol. Methods 2020, 175, 105974. [Google Scholar] [CrossRef] [PubMed]
- Schumann, P.; Pukall, R. The discriminatory power of ribotyping as automatable technique for differentiation of bacteria. Syst. Appl. Microbiol. 2013, 36, 369–375. [Google Scholar] [CrossRef]
- Wultanska, D.; Pituch, H.; Obuch-Woszczatynski, P.; Meisel-Mikolajczyk, F.; Luczak, M. Profile of toxigenicity of Clostridium difficile strains isolated from paediatric patients with clinical diagnosis of antibiotic associated diarrhea (AAD). Med. Dosw. Mikrobiol. 2005, 57, 377–382. [Google Scholar] [PubMed]
- Deniz, U.; Ulger, N.; Aksu, B.; Karavus, M.; Soyletir, G. Investigation of toxin genes of Clostridium difficile strains isolated from hospitalized patients with diarrhoea at Marmara University Hospital. Mikrobiyol. Bul. 2011, 45, 1–10. [Google Scholar]
- Aliramezani, A.; Talebi, M.; Baghani, A.; Hajabdolbaghi, M.; Salehi, M.; Abdollahi, A.; Afhami, S.; Marjani, M.; Golbabaei, F.; Boroumand, M.A.; et al. Pathogenicity locus determinants and toxinotyping of Clostridioides difficile isolates recovered from Iranian patients. New Microbes New Infect. 2018, 25, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Torpdahl, M.; Olsen, K.E. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin. Microbiol. Infect. 2008, 14, 1057–1064. [Google Scholar] [CrossRef]
- Chankhamhaengdecha, S.; Hadpanus, P.; Aroonnual, A.; Ngamwongsatit, P.; Chotiprasitsakul, D.; Chongtrakool, P.; Janvilisri, T. Evaluation of multiplex PCR with enhanced spore germination for detection of Clostridium difficile from stool samples of the hospitalized patients. BioMed Res. Int. 2013, 2013, 875437. [Google Scholar] [CrossRef]
- Chotiprasitsakul, D.; Janvilisri, T.; Kiertiburanakul, S.; Watcharananun, S.; Chankhamhaengdecha, S.; Hadpanus, P.; Malathum, K. A superior test for diagnosis of Clostridium difficile-associated diarrhea in resource-limited settings. Jpn. J. Infect. Dis. 2012, 65, 326–329. [Google Scholar] [CrossRef][Green Version]
- Eckert, C.; Emirian, A.; Le Monnier, A.; Cathala, L.; De Montclos, H.; Goret, J.; Berger, P.; Petit, A.; De Chevigny, A.; Jean-Pierre, H.; et al. Prevalence and pathogenicity of binary toxin-positive Clostridium difficile strains that do not produce toxins A and B. New Microbes New Infect. 2015, 3, 12–17. [Google Scholar] [CrossRef]
- McGovern, A.M.; Androga, G.O.; Knight, D.R.; Watson, M.W.; Elliott, B.; Foster, N.F.; Chang, B.J.; Riley, T.V. Prevalence of binary toxin positive Clostridium difficile in diarrhoeal humans in the absence of epidemic ribotype 027. PLoS ONE 2017, 12, e0187658. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Godoy-Ruiz, R.; Adipietro, K.A.; Peralta, C.; Ben-Hail, D.; Varney, K.M.; Cook, M.E.; Roth, B.M.; Wilder, P.T.; Cleveland, T.; et al. Structure of the cell-binding component of the Clostridium difficile binary toxin reveals a di-heptamer macromolecular assembly. Proc. Natl. Acad. Sci. USA 2020, 117, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Papatheodorou, P.; Schwan, C. Binary Clostridium difficile toxin (CDT)–A virulence factor disturbing the cytoskeleton. Anaerobe 2018, 53, 21–29. [Google Scholar] [CrossRef]
- Stubbs, S.L.; Brazier, J.S.; O’Neill, G.L.; Duerden, B.I. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 1999, 37, 461–463. [Google Scholar] [CrossRef]
- Gurtler, V. Typing of Clostridium difficile strains by PCR-amplification of variable length 16S-23S rDNA spacer regions. J. Gen. Microbiol. 1993, 139, 3089–3097. [Google Scholar] [CrossRef]
- Indra, A.; Schmid, D.; Huhulescu, S.; Hell, M.; Gattringer, R.; Hasenberger, P.; Fiedler, A.; Wewalka, G.; Allerberger, F. Characterization of clinical Clostridium difficile isolates by PCR ribotyping and detection of toxin genes in Austria, 2006-2007. J. Med. Microbiol. 2008, 57, 702–708. [Google Scholar] [CrossRef]
- Hung, Y.P.; Huang, I.H.; Lin, H.J.; Tsai, B.Y.; Liu, H.C.; Liu, H.C.; Lee, J.C.; Wu, Y.H.; Tsai, P.J.; Ko, W.C. Predominance of Clostridium difficile Ribotypes 017 and 078 among Toxigenic Clinical Isolates in Southern Taiwan. PLoS ONE 2016, 11, e0166159. [Google Scholar] [CrossRef]
- Killgore, G.; Thompson, A.; Johnson, S.; Brazier, J.; Kuijper, E.; Pepin, J.; Frost, E.H.; Savelkoul, P.; Nicholson, B.; van den Berg, R.J.; et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: Restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J. Clin. Microbiol. 2008, 46, 431–437. [Google Scholar] [CrossRef]
- Tanner, H.E.; Hardy, K.J.; Hawkey, P.M. Coexistence of multiple multilocus variable-number tandem-repeat analysis subtypes of Clostridium difficile PCR ribotype 027 strains within fecal specimens. J. Clin. Microbiol. 2010, 48, 985–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzales-Luna, A.J.; Carlson, T.J.; Dotson, K.M.; Poblete, K.; Costa, G.; Miranda, J.; Lancaster, C.; Walk, S.T.; Tupy, S.; Begum, K.; et al. PCR ribotypes of Clostridioides difficile across Texas from 2011 to 2018 including emergence of ribotype 255. Emerg. Microbes Infect. 2020, 9, 341–347. [Google Scholar] [CrossRef]
- Tenover, F.C.; Akerlund, T.; Gerding, D.N.; Goering, R.V.; Bostrom, T.; Jonsson, A.M.; Wong, E.; Wortman, A.T.; Persing, D.H. Comparison of strain typing results for Clostridium difficile isolates from North America. J. Clin. Microbiol. 2011, 49, 1831–1837. [Google Scholar] [CrossRef]
- Elliott, B.; Androga, G.O.; Knight, D.R.; Riley, T.V. Clostridium difficile infection: Evolution, phylogeny and molecular epidemiology. Infect. Genet. Evol. 2017, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Drudy, D.; Fanning, S.; Kyne, L. Toxin A-negative, toxin B-positive Clostridium difficile. Int. J. Infect. Dis. 2007, 11, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.M.; Kuak, E.Y.; Yoo, S.J.; Shin, W.C.; Yoo, H.M. Emerging toxin A-B+ variant strain of Clostridium difficile responsible for pseudomembranous colitis at a tertiary care hospital in Korea. Diagn. Microbiol. Infect. Dis. 2008, 60, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, X.; Jia, X.; Cheng, Y.; Luo, Y.; Yuan, L.; Wang, Y.; Zhao, C.; Guo, S.; Li, L.; et al. Impacts of infection with different toxigenic Clostridium difficile strains on faecal microbiota in children. Sci. Rep. 2014, 4, 7485. [Google Scholar] [CrossRef]
- Fatima, R.; Aziz, M. The Hypervirulent Strain of Clostridium Difficile: NAP1/B1/027–A Brief Overview. Cureus 2019, 11, e3977. [Google Scholar] [CrossRef]
- Merrigan, M.; Venugopal, A.; Mallozzi, M.; Roxas, B.; Viswanathan, V.K.; Johnson, S.; Gerding, D.N.; Vedantam, G. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J. Bacteriol. 2010, 192, 4904–4911. [Google Scholar] [CrossRef]
- Warny, M.; Pepin, J.; Fang, A.; Killgore, G.; Thompson, A.; Brazier, J.; Frost, E.; McDonald, L.C. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005, 366, 1079–1084. [Google Scholar] [CrossRef]
- Lyon, S.A.; Hutton, M.L.; Rood, J.I.; Cheung, J.K.; Lyras, D. CdtR Regulates TcdA and TcdB Production in Clostridium difficile. PLoS Pathog. 2016, 12, e1005758. [Google Scholar] [CrossRef]
- Buchler, A.C.; Rampini, S.K.; Stelling, S.; Ledergerber, B.; Peter, S.; Schweiger, A.; Ruef, C.; Zbinden, R.; Speck, R.F. Antibiotic susceptibility of Clostridium difficile is similar worldwide over two decades despite widespread use of broad-spectrum antibiotics: An analysis done at the University Hospital of Zurich. BMC Infect. Dis. 2014, 14, 607. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.R.; Giglio, S.; Huntington, P.G.; Korman, T.M.; Kotsanas, D.; Moore, C.V.; Paterson, D.L.; Prendergast, L.; Huber, C.A.; Robson, J.; et al. Surveillance for antimicrobial resistance in Australian isolates of Clostridium difficile, 2013–2014. J. Antimicrob. Chemother. 2015, 70, 2992–2999. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.J.; Park, K.H.; Park, D.A.; Park, J.; Bang, B.W.; Lee, S.S.; Lee, E.J.; Lee, H.J.; Hong, S.K.; Kim, Y.R. Guideline for the Antibiotic Use in Acute Gastroenteritis. Infect. Chemother. 2019, 51, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S.; DuPont, H.L.; Connor, B.A. ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults. Am. J. Gastroenterol. 2016, 111, 602–622. [Google Scholar] [CrossRef]
- Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther. Adv. Infect. Dis. 2016, 3, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Slimings, C.; Riley, T.V. Antibiotics and hospital-acquired Clostridium difficile infection: Update of systematic review and meta-analysis. J. Antimicrob. Chemother. 2014, 69, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Johanesen, P.A.; Mackin, K.E.; Hutton, M.L.; Awad, M.M.; Larcombe, S.; Amy, J.M.; Lyras, D. Disruption of the Gut Microbiome: Clostridium difficile Infection and the Threat of Antibiotic Resistance. Genes 2015, 6, 1347–1360. [Google Scholar] [CrossRef]
- Freeman, J.; Vernon, J.; Morris, K.; Nicholson, S.; Todhunter, S.; Longshaw, C.; Wilcox, M.H.; Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes’ Study, G. Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin. Microbiol. Infect. 2015, 21, 248.e9–248.e16. [Google Scholar] [CrossRef]
- Kim, J.; Kang, J.O.; Pai, H.; Choi, T.Y. Association between PCR ribotypes and antimicrobial susceptibility among Clostridium difficile isolates from healthcare-associated infections in South Korea. Int. J. Antimicrob. Agents 2012, 40, 24–29. [Google Scholar] [CrossRef]
- Obuch-Woszczatynski, P.; Dubiel, G.; Harmanus, C.; Kuijper, E.; Duda, U.; Wultanska, D.; van Belkum, A.; Pituch, H. Emergence of Clostridium difficile infection in tuberculosis patients due to a highly rifampicin-resistant PCR ribotype 046 clone in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kato, N.; Katow, S.; Maegawa, T.; Nakamura, S.; Lyerly, D.M. Deletions in the repeating sequences of the toxin A gene of toxin A-negative, toxin B-positive Clostridium difficile strains. FEMS Microbiol. Lett. 1999, 175, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bidet, P.; Barbut, F.; Lalande, V.; Burghoffer, B.; Petit, J.C. Development of a new PCR-ribotyping method for Clostridium difficile based on ribosomal RNA gene sequencing. FEMS Microbiol. Lett. 1999, 175, 261–266. [Google Scholar] [CrossRef] [PubMed]


| Toxigenic Profile | No. of Isolates | No. of Isolates of | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THA (n = 50) | THB (n = 26) | RT017 | RT020 | NN01 | NN02 | NN03 | NN04 | NN05 | NN06 | NN07 | NT01 | NT02 | NT03 | NT04 | NT05 | NT06 | NT07 | NT08 | NT09 | NT10 | NT11 | |
| A+B+CDT+ | 6 | 1 | 1 | 2 | 1 | 1 | ||||||||||||||||
| A+B+CDT- | 2 | 2 | ||||||||||||||||||||
| A-B+CDT+ | 5 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| A-B+CDT- | 27 | 7 | 20 | 9 | 1 | 1 | 3 | |||||||||||||||
| A-B-CDT- | 23 | 6 | 3 | 1 | 1 | 1 | 6 | 3 | 14 | |||||||||||||
| Antibiotics | MIC Range (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | Breakpoints (µg/mL) | Susceptible (%) | Intermediate (%) | Resistant (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THA (n = 50) | THB (n = 26) | THA | THB | THA | THB | S/I/R | THA | THB | THA | THB | THA | THB | |
| Amoxicillin | ≤0.125-32 | ≤0.125-0.5 | 0.5 | ≤0.125 | 2 | 0.5 | ≤4/8/≥ 16 ≤2/4/≥ 8 | 96 | 100 | 2 | 0 | 2 | 0 |
| Ampicillin | 0.25-16 | 0.25-4 | 2 | 2 | 4 | 2 | ≤0.5/1/≥ 2 | 92 | 100 | 4 | 0 | 4 | 0 |
| Cefoxitin | 4-256 | 4-256 | 256 | 256 | 256 | 256 | ≤16/32/≥ 64 | 2 | 7.69 | 0 | 0 | 98 | 92.31 |
| Chloramphenicol | 32- ≥ 64 | 16- ≥ 64 | ≥64 | ≥64 | ≥64 | ≥64 | ≤8/16/≥ 32 | 0 | 0 | 0 | 3.85 | 100 | 96.15 |
| Levofloxacin | 2- ≥ 32 | 1- ≥ 32 | 8 | 8 | ≥32 | ≥32 | -/-/≥8 a | - | - | - | - | 56 | 11.54 |
| Metronidazole | 0.25-16 | 0.25- ≥ 16 | 1 | 1 | 4 | 2 | ≤8/16/≥ 32 | 96 | 96.15 | 4 | 3.85 | 0 | 0 |
| Rifampicin | ≤0.125- ≥ 32 | ≤0.125- ≥ 32 | ≤0.125 | ≤0.125 | ≥32 | ≥32 | ≤0.06/0.012-16/≥ 32 b | 0 | 0 | 74 | 92.31 | 26 | 7.69 |
| Vancomycin | 1-8 | 0.5-4 | 2 | 1 | 4 | 4 | ≤2/-/> 2 c | 88 | 88.46 | - | - | 12 | 11.54 |
| Analysis | Target Gene | Primer Name | Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|---|
| Multiplex PCR | tcdA | tcdA-F | GTATGGATAGGTGGAGAAGTCAGTG | 632 |
| tcdA-R | CGGTCTAGTCCAATAGAGCTAGGTC | |||
| tcdB | tcdB-F | GAAGATTTAGGAAATGAAGAAGGTGA | 441 | |
| tcdB-R | AACCACTATATTCAACTGCTTGTCC | |||
| cdtA | cdtA-F | ATGCACAAGACTTACAAAGCTATAGTG | 260 | |
| cdtA-R | CGAGAATTTGCTTCTATTTGATAATC | |||
| cdtB | cdtB-F | ATTGGCAATAATCTATCTCCTGGA | 179 | |
| cdtB-R | CTTGTTCTGGTACCAAATAATCCG | |||
| 16S rRNA | UFU-L | GCCTAACACATGCAAGTCGA | 800 | |
| 802-R | TACCAGGGTATCTAATCC | |||
| tcdA 3′-end deletion | tcdA | NK9 | CCACCAGCTGCAGCCATA | 2535 |
| NKV011 | TTTTGATCCTATAGAATYTAACTTAGTAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongkuna, S.; Janvilisri, T.; Phanchana, M.; Harnvoravongchai, P.; Aroonnual, A.; Aimjongjun, S.; Malaisri, N.; Chankhamhaengdecha, S. Temporal Variations in Patterns of Clostridioides difficile Strain Diversity and Antibiotic Resistance in Thailand. Antibiotics 2021, 10, 714. https://doi.org/10.3390/antibiotics10060714
Wongkuna S, Janvilisri T, Phanchana M, Harnvoravongchai P, Aroonnual A, Aimjongjun S, Malaisri N, Chankhamhaengdecha S. Temporal Variations in Patterns of Clostridioides difficile Strain Diversity and Antibiotic Resistance in Thailand. Antibiotics. 2021; 10(6):714. https://doi.org/10.3390/antibiotics10060714
Chicago/Turabian StyleWongkuna, Supapit, Tavan Janvilisri, Matthew Phanchana, Phurt Harnvoravongchai, Amornrat Aroonnual, Sathid Aimjongjun, Natamon Malaisri, and Surang Chankhamhaengdecha. 2021. "Temporal Variations in Patterns of Clostridioides difficile Strain Diversity and Antibiotic Resistance in Thailand" Antibiotics 10, no. 6: 714. https://doi.org/10.3390/antibiotics10060714
APA StyleWongkuna, S., Janvilisri, T., Phanchana, M., Harnvoravongchai, P., Aroonnual, A., Aimjongjun, S., Malaisri, N., & Chankhamhaengdecha, S. (2021). Temporal Variations in Patterns of Clostridioides difficile Strain Diversity and Antibiotic Resistance in Thailand. Antibiotics, 10(6), 714. https://doi.org/10.3390/antibiotics10060714







