Trans-Cinnamaldehyde Attenuates Enterococcus faecalis Virulence and Inhibits Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Growth Conditions, and Chemicals
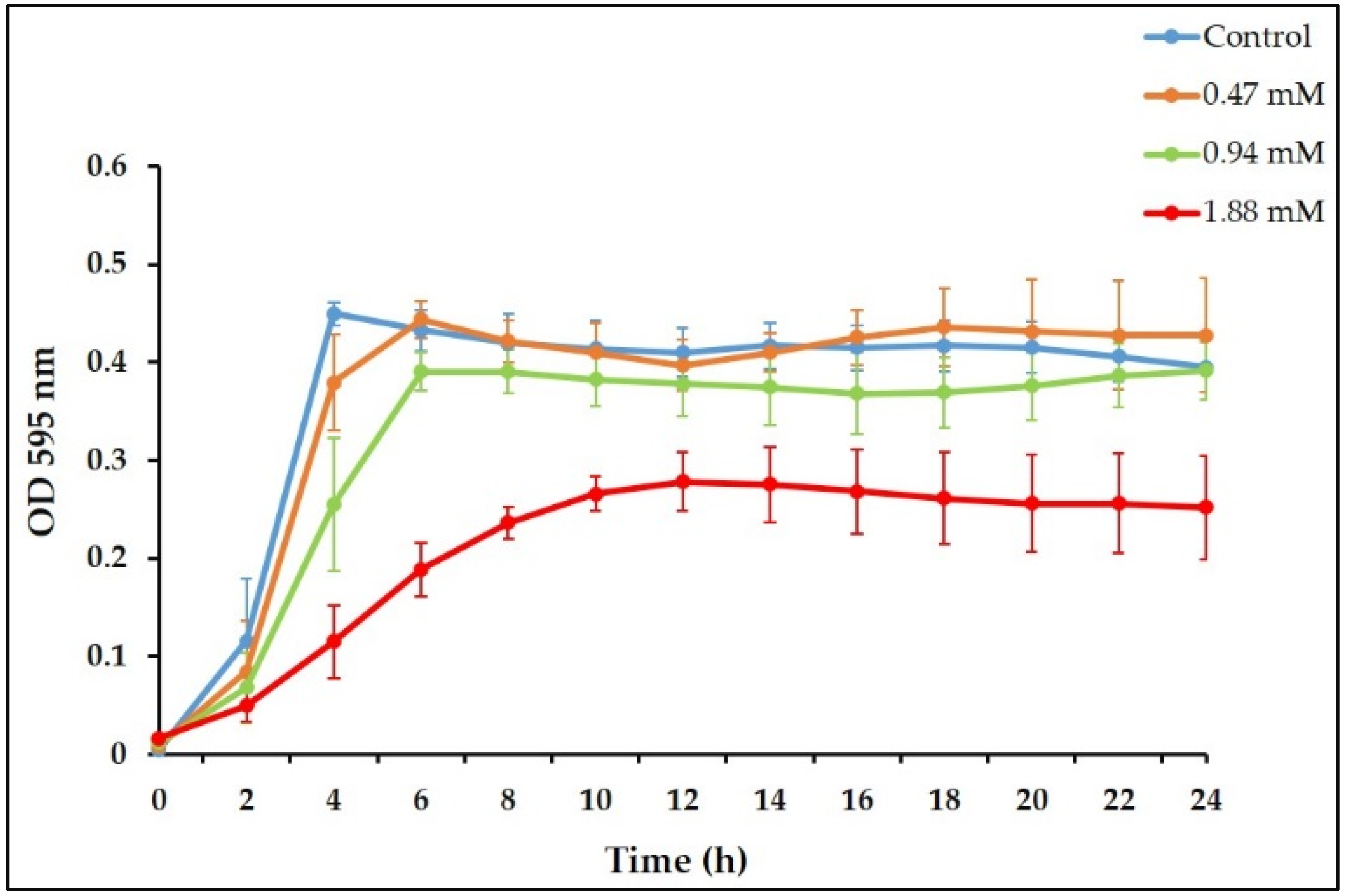
2.2. Planktonic Growth Assessment
2.3. Biofilm Formation Assays
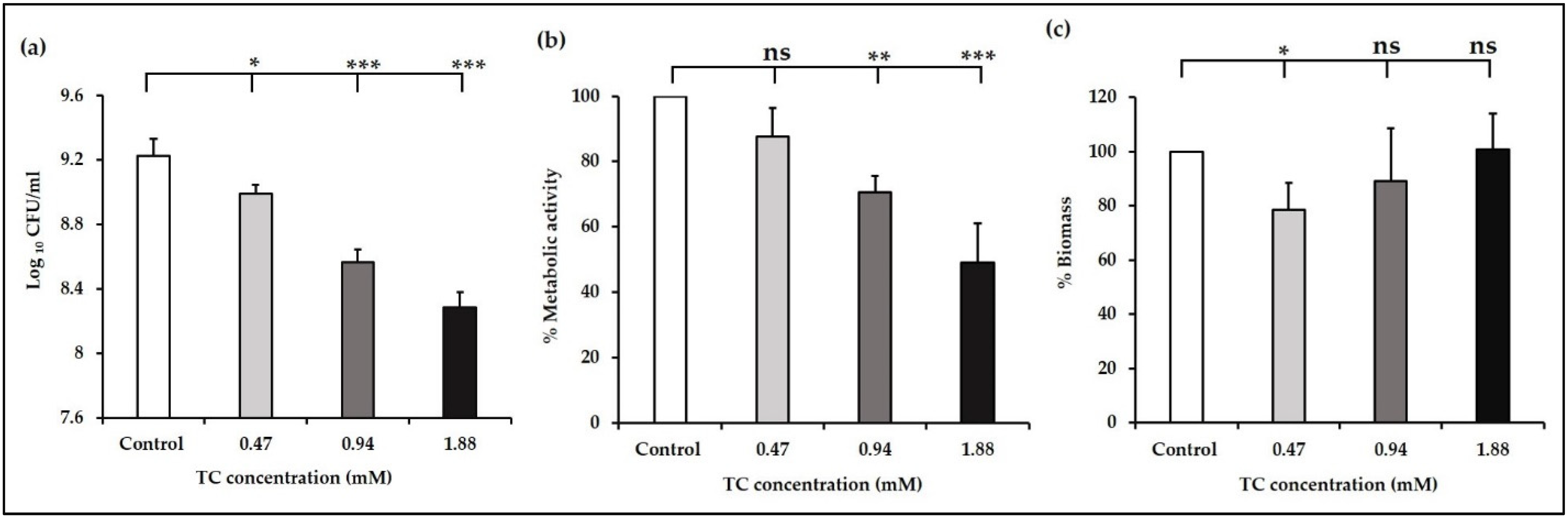
2.3.1. Quantification of Viable Cell Counts
2.3.2. Assessment of Biofilm Metabolic Activity
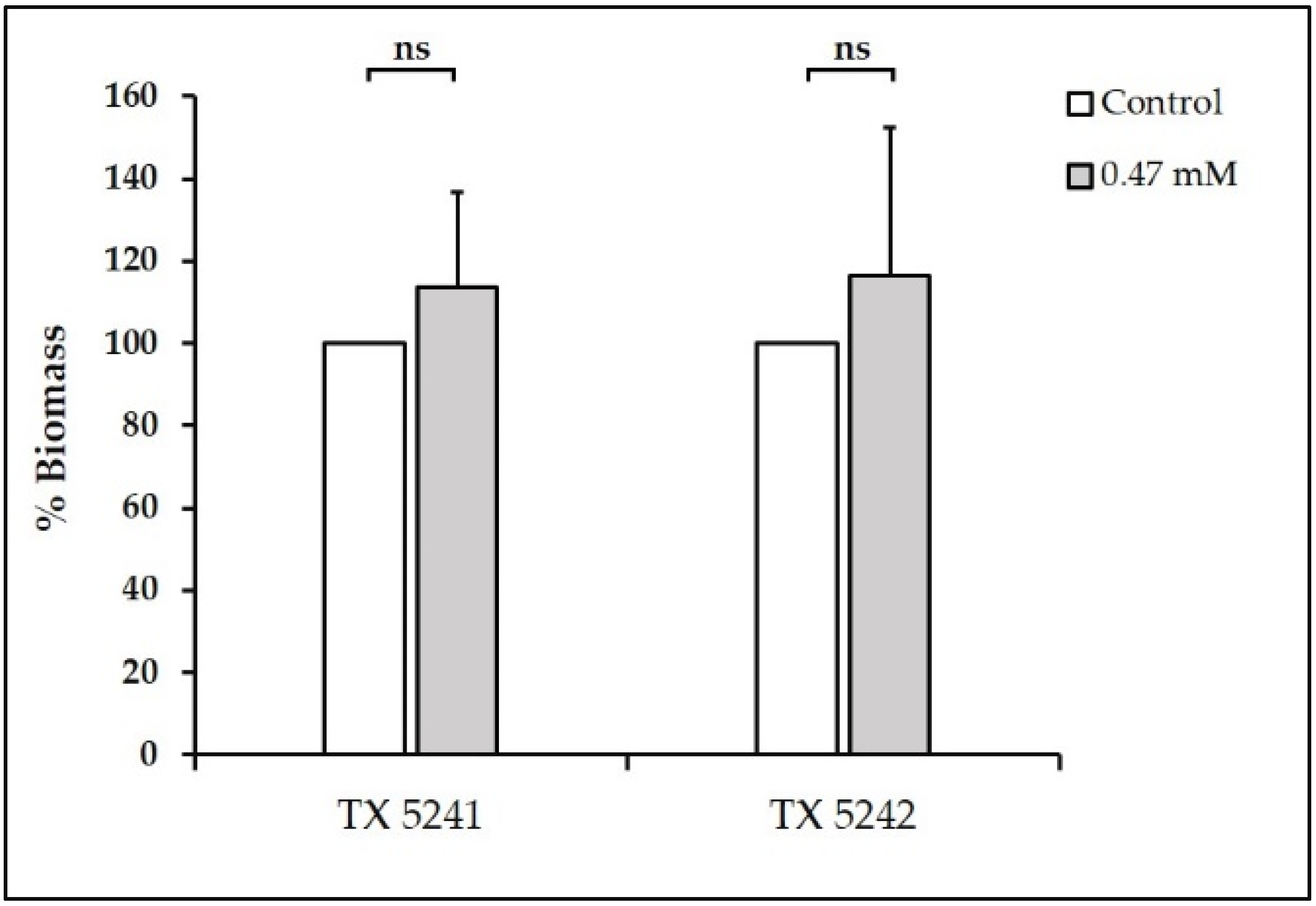
2.3.3. Biomass Quantification
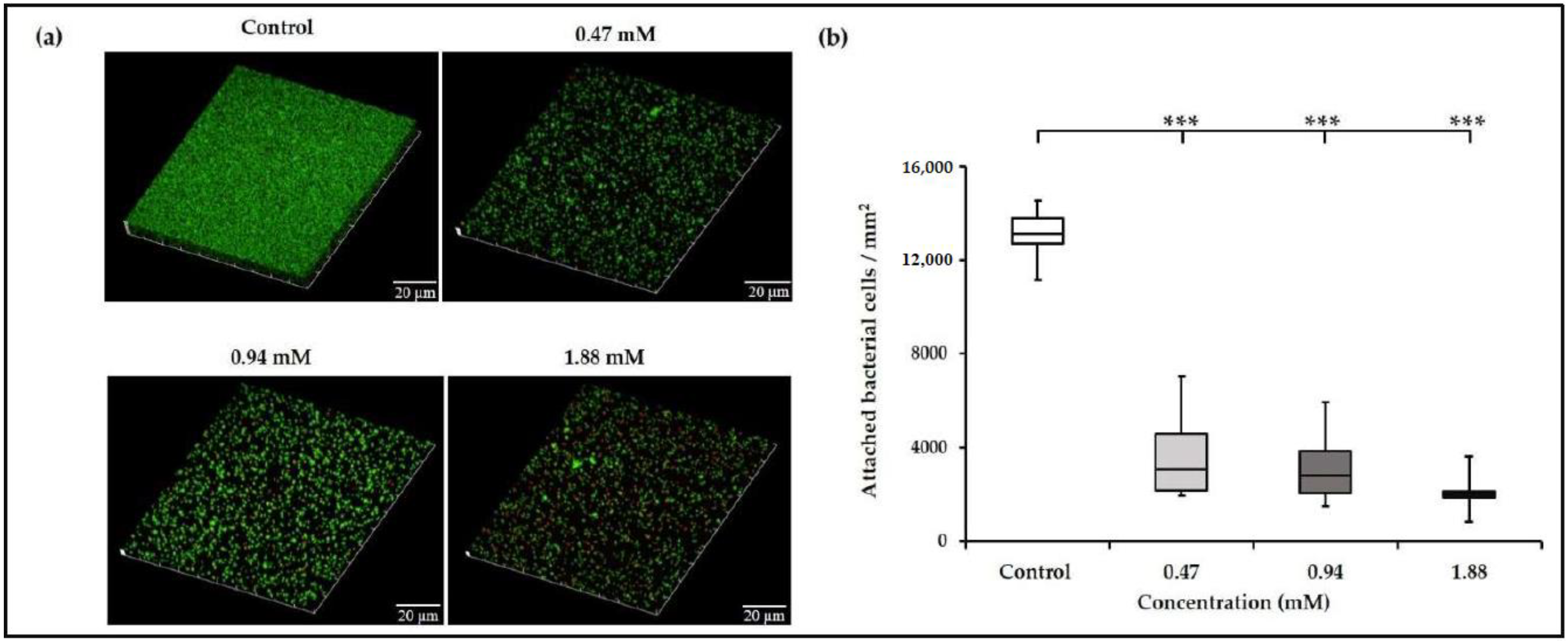
2.4. Confocal Microscopic Imaging of Biofilms
2.5. Quantification of Biofilm Exopolysaccharides
2.6. Extracellular Protease Production
2.7. Hemolytic Activity Analysis
2.8. Gene Expression Analysis of TC-Treated Biofilms
2.9. Statistical Analysis
3. Results and Discussion
3.1. Sub-Inhibitory Concentrations of TC inhibit E. faecalis Biofilm Formation
3.2. Sub-Inhibitory Concentration of TC Reduced E. faecalis Biofilm Exopolysaccharides
3.3. TC Reduced the Proteolytic and Hemolytic Activities of E. faecalis
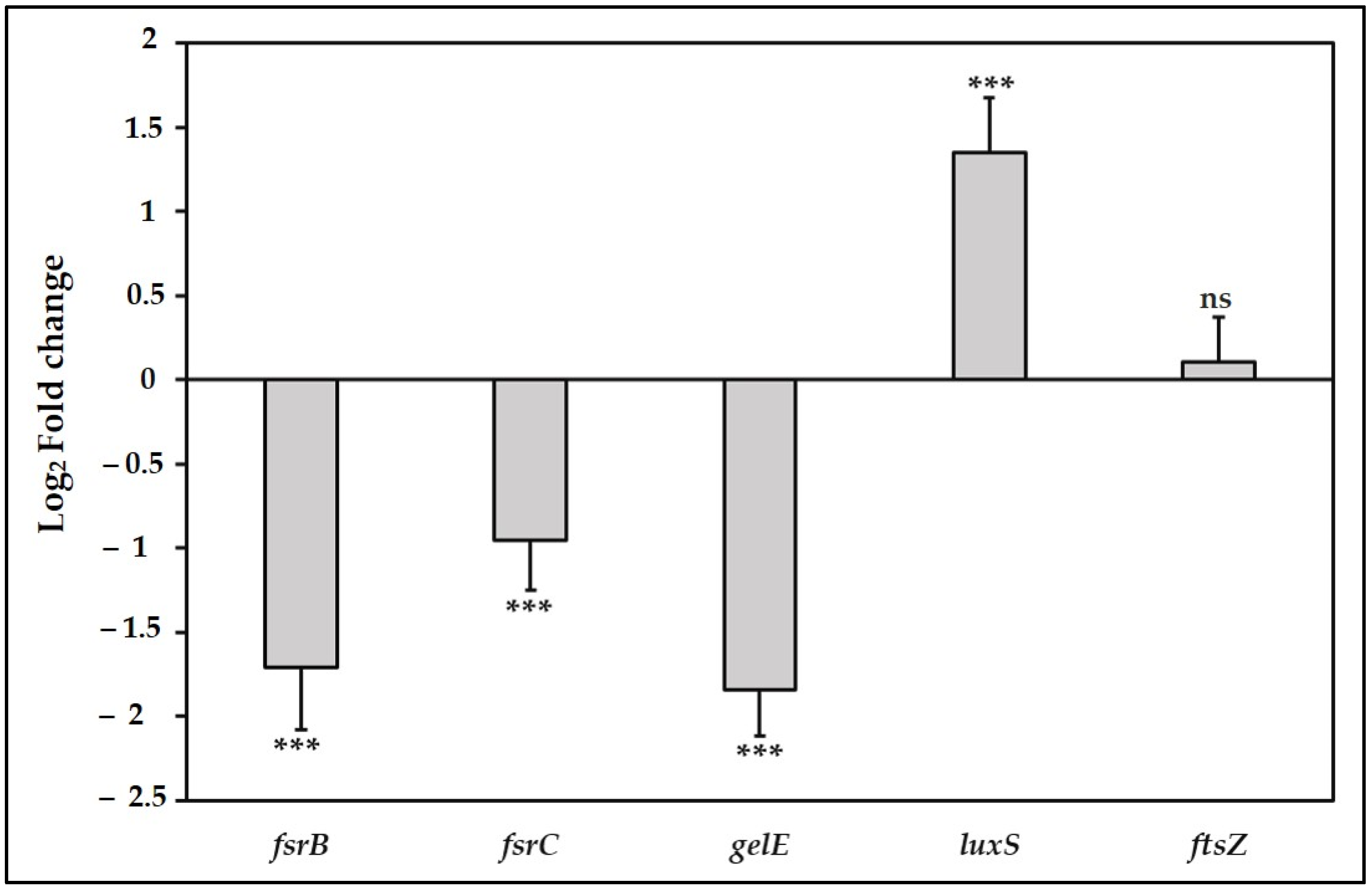
3.4. TC Suppress the Fsr Quorum Sensing Pathway and the Downstream gelE Gene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ch’ng, J.H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by Enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The Ecology, Epidemiology and Virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Willems, R.J.; Leavis, H.L. Optimizing Future Treatment of Enterococcal Infections: Attacking the Biofilm? Trends Microbiol. 2012, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- van Harten, R.M.; Willems, R.J.L.; Martin, N.I.; Hendrickx, A.P.A. Multidrug-Resistant Enterococcal Infections: New Compounds, Novel Antimicrobial Therapies? Trends Microbiol. 2017, 25, 467–479. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm Infections, Their Resilience to Therapy and Innovative Treatment Strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial Biofilm Development as a Multicellular Adaptation: Antibiotic Resistance and New Therapeutic Strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial Resistance Worldwide: Causes, Challenges and Responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Wilson, C.E.; Cathro, P.C.; Rogers, A.H.; Briggs, N.; Zilm, P.S. Clonal Diversity in Biofilm Formation by Enterococcus faecalis in Response to Environmental Stress Associated with Endodontic Irrigants and Medicaments. Int. Endod. J. 2015, 48, 210–219. [Google Scholar] [CrossRef]
- Kafil, H.S.; Mobarez, A.M.; Moghadam, M.F.; Hashemi, Z.S.; Yousefi, M. Gentamicin Induces efaA Expression and Biofilm Formation in Enterococcus faecalis. Microb. Pathog. 2016, 92, 30–35. [Google Scholar] [CrossRef]
- Yu, W.; Hallinen, K.M.; Wood, K.B. Interplay between Antibiotic Efficacy and Drug-Induced Lysis Underlies Enhanced Biofilm Formation at Subinhibitory Drug Concentrations. Antimicrob. Agents Chemother. 2018, 62, e01603-17. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Suriyanarayanan, T.; Swarup, S.; Chia, K.H.B.; Nagarajan, N.; Zhang, C. Transcriptomics Analysis Reveals Putative Genes Involved in Biofilm Formation and Biofilm-associated Drug Resistance of Enterococcus faecalis. J. Endod. 2017, 43, 949–955. [Google Scholar] [CrossRef]
- Thieme, L.; Klinger-Strobel, M.; Hartung, A.; Stein, C.; Makarewicz, O.; Pletz, M.W. In vitro synergism and anti-biofilm activity of ampicillin, gentamicin, ceftaroline and ceftriaxone against Enterococcus faecalis. J. Antimicrob. Chemother. 2018, 73, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed. Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical Diversity and Defence Metabolism: How Plants Cope with Pathogens and Ozone Pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum Sensing and Phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting Quorum Sensing to Confuse Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial Properties and Major Bioactive Components of Cinnamon Stick (Cinnamomum burmannii): Activity against Foodborne Pathogenic Bacteria. J. Agric. Food. Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Kim, S.I.; Baek, K.H.; Lee, J. Cinnamon Bark Oil and Its Components Inhibit Biofilm Formation and Toxin Production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef]
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde Disrupts Biofilm Formation and Swarming Motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097. [Google Scholar] [CrossRef]
- Ferro, T.A.F.; Souza, E.B.; Suarez, M.A.M.; Rodrigues, J.F.S.; Pereira, D.M.S.; Mendes, S.J.F.; Gonzaga, L.F.; Machado, M.; Bomfim, M.R.Q.; Calixto, J.B.; et al. Topical Application of Cinnamaldehyde Promotes Faster Healing of Skin Wounds Infected with Pseudomonas aeruginosa. Molecules 2019, 24, 1627. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Narayanan, A.; Baskaran, S.A.; Venkitanarayanan, K. Antibiofilm Effect of Trans-cinnamaldehyde on Uropathogenic Escherichia coli. J. Urol. 2010, 184, 358–363. [Google Scholar] [CrossRef]
- Yuan, W.; Yuk, H.G. Effects of Sublethal Thymol, Carvacrol, and Trans-cinnamaldehyde Adaptation on Virulence Properties of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2019, 85, e00271-19. [Google Scholar] [CrossRef]
- Jia, P.; Xue, Y.J.; Duan, X.J.; Shao, S.H. Effect of Cinnamaldehyde on Biofilm Formation and sarA Expression by Methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Sytykiewicz, H.; Sprawka, I.; Witeska, M. Effect of Trans-cinnamaldehyde on Methicillin-resistant Staphylococcus aureus Biofilm Formation: Metabolic Activity Assessment and Analysis of the Biofilm-associated Genes Expression. Int. J. Mol. Sci. 2019, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.; Barbosa, L.N.; Furlanetto, A.; Lyra, L.; Rall, V.L.M.; Júnior, A.F. Antibacterial and Anti-biofilm Activities of Cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, Z.; Jiang, W.; Zhou, W. Antimicrobial Activity of Cinnamaldehyde on Streptococcus mutans Biofilms. Front. Microbiol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Chang, S.T.; Chen, P.F.; Chang, S.C. Antibacterial Activity of Leaf Essential Oils and Their Constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Ferro, T.A.; Araújo, J.M.; Dos Santos Pinto, B.L.; Dos Santos, J.S.; Souza, E.B.; da Silva, B.L.; Colares, V.L.; Novais, T.M.; Filho, C.M.; Struve, C.; et al. Cinnamaldehyde Inhibits Staphylococcus aureus Virulence Factors and Protects against Infection in a Galleria mellonella Model. Front. Microbiol. 2016, 7, 2052. [Google Scholar] [CrossRef]
- Ali, I.A.A.; Cheung, B.P.K.; Matinlinna, J.; Lévesque, C.M.; Neelakantan, P. Trans-cinnamaldehyde Potently Kills Enterococcus faecalis Biofilm Cells and Prevents Biofilm Recovery. Microb. Pathog. 2020, 149, 104482. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Q.; Zhang, C.; Cheung, G.S.; Shen, Y. Prevalence, Phenotype, and Genotype of Enterococcus faecalis Isolated from Saliva and Root Canals in Patients with Persistent Apical Periodontitis. J. Endod. 2010, 36, 1950–1955. [Google Scholar] [CrossRef]
- Kim, E.B.; Kopit, L.M.; Harris, L.J.; Marco, M.L. Draft Genome Sequence of the Quality Control Strain Enterococcus faecalis ATCC 29212. J. Bacteriol. 2012, 194, 6006–6007. [Google Scholar] [CrossRef]
- Qin, X.; Singh, K.V.; Weinstock, G.M.; Murray, B.E. Effects of Enterococcus faecalis fsr Genes on Production of Gelatinase and a Serine Protease and Virulence. Infect. Immun. 2000, 68, 2579–2586. [Google Scholar] [CrossRef]
- Qin, X.; Singh, K.V.; Weinstock, G.M.; Murray, B.E. Characterization of Fsr, A Regulator Controlling Expression of Gelatinase and Serine Protease in Enterococcus faecalis OG1RF. J. Bacteriol. 2001, 183, 3372–3382. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Liu, F.; Jin, P.; Gong, H.; Sun, Z.; Du, L.; Wang, D. Antibacterial and Antibiofilm Activities of Thyme Oil against Foodborne Multiple Antibiotics-resistant Enterococcus faecalis. Poult. Sci. 2020, 99, 5127–5136. [Google Scholar] [CrossRef]
- Extremina, C.I.; Costa, L.; Aguiar, A.I.; Peixe, L.; Fonseca, A.P. Optimization of Processing Conditions for the Quantification of Enterococci Biofilms using Microtitre-plates. J. Microbiol. Methods 2011, 84, 167–173. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Cheung, B.P.K.; Yau, J.Y.Y.; Shanmugam, K.; Solomon, A.P.; Neelakantan, P. A Novel Small Molecule, 1,3-di-m-tolyl-urea, Inhibits and Disrupts Multispecies Oral Biofilms. Microorganisms 2020, 8, 1261. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Selinummi, J.; Seppälä, J.; Yli-Harja, O.; Puhakka, J.A. Software for Quantification of Labeled Bacteria from Digital Microscope Images by Automated Image Analysis. Biotechniques 2005, 39, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Fteita, D.; Könönen, E.; Söderling, E.; Gürsoy, U.K. Effect of Estradiol on Planktonic Growth, Coaggregation, and Biofilm Formation of the Prevotella intermedia Group Bacteria. Anaerobe 2014, 27, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Shrestha, A.; Kishen, A. Inflammatory Potential of Monospecies Biofilm Matrix Components. Int. Endod. J. 2019, 52, 1020–1027. [Google Scholar] [CrossRef]
- Pinkston, K.L.; Gao, P.; Diaz-Garcia, D.; Sillanpää, J.; Nallapareddy, S.R.; Murray, B.E.; Harvey, B.R. The Fsr Quorum-sensing System of Enterococcus faecalis Modulates Surface Display of the Collagen-binding MSCRAMM Ace through Regulation of gelE. J. Bacteriol. 2011, 193, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.L.; Nilson, J.L.; Barnes, A.M.T.; Dunny, G.M. Restructuring of Enterococcus faecalis Biofilm Architecture in Response to Antibiotic-induced Stress. NPJ Biofilms Microbiomes 2017, 3, 15. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shepard, B.D.; Gilmore, M.S. Differential Expression of Virulence-related Genes in Enterococcus faecalis in Response to Biological Cues in Serum and Urine. Infect. Immun. 2002, 70, 4344–4352. [Google Scholar] [CrossRef]
- He, Z.; Liang, J.; Zhou, W.; Xie, Q.; Tang, Z.; Ma, R.; Huang, Z. Effect of the Quorum-Sensing luxS Gene on Biofilm Formation by Enterococcus faecalis. Eur. J. Oral Sci. 2016, 124, 234–240. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, R.; Mishra, S. Pharmacological Properties and their Medicinal Uses of Cinnamomum: A Review. J. Pharm. Pharmacol. 2019, 71, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; de Carvalho, M.G.; Catunda, F.E.A., Jr. Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pang, D.; Liao, S.; Zou, Y.; Zhou, P.; Li, E.; Wang, W. Synergistic Effects of Cinnamaldehyde and Cinnamic Acid in Cinnamon Essential Oil against S. pullorum. Ind. Crop. Prod. 2021, 162, 113296. [Google Scholar] [CrossRef]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of Cinnamon Bark Oil and Cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the Synergistic Effects in Combination with Other Antimicrobial Agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef]
- Honraet, K.; Goetghebeur, E.; Nelis, H.J. Comparison of Three Assays for the Quantification of Candida Biomass in Suspension and CDC Reactor Grown Biofilms. J. Microbiol. Methods 2005, 63, 287–295. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Balkis, M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Uses and Limitations of the XTT Assay in Studies of Candida Growth and Metabolism. J. Clin. Microbiol. 2003, 41, 506–508. [Google Scholar] [CrossRef]
- Kitagawa, H.; Izutani, N.; Kitagawa, R.; Maezono, H.; Yamaguchi, M.; Imazato, S. Evolution of Resistance to Cationic Biocides in Streptococcus mutans and Enterococcus faecalis. J. Dent. 2016, 47, 18–22. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Ren, B.; Li, H.; Weir, M.D.; Zhou, X.; Oates, T.W.; Cheng, L.; Xu, H.H.K. Do Quaternary Ammonium Monomers Induce Drug Resistance in Cariogenic, Endodontic and Periodontal Bacterial Species? Dent. Mater. 2017, 33, 1127–1138. [Google Scholar] [CrossRef]
- Schwarz, S.R.; Hirsch, S.; Hiergeist, A.; Kirschneck, C.; Muehler, D.; Hiller, K.A.; Maisch, T.; Al-Ahmad, A.; Gessner, A.; Buchalla, W.; et al. Limited antimicrobial Efficacy of Oral Care Antiseptics in Microcosm Biofilms and Phenotypic Adaptation of Bacteria upon Repeated Exposure. Clin. Oral Investig. 2021, 25, 2939–2950. [Google Scholar] [CrossRef]
- Beema Shafreen, R.M.; Selvaraj, C.; Singh, S.K.; Karutha Pandian, S. In Silico and In Vitro Studies of Cinnamaldehyde and Their Derivatives against LuxS in Streptococcus pyogenes: Effects on Biofilm and Virulence Genes. J. Mol. Recognit. 2014, 27, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rudden, M.; Smyth, T.J.; Dooley, J.S.G.; Marchant, R.; Banat, I.M. Natural Quorum Sensing Inhibitors Effectively Downregulate Gene Expression of Pseudomonas aeruginosa Virulence Factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef]
- Ali, L.; Goraya, M.U.; Arafat, Y.; Ajmal, M.; Chen, J.L.; Yu, D. Molecular Mechanism of Quorum-Sensing in Enterococcus faecalis: Its Role in Virulence and Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 960. [Google Scholar] [CrossRef]
- Hancock, L.E.; Perego, M. The Enterococcus faecalis Fsr Two-component System Controls Biofilm Development through Production of Gelatinase. J. Bacteriol. 2004, 186, 5629–5639. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Van Calenbergh, S.; Nelis, H.; Coenye, T. Cinnamaldehyde and Cinnamaldehyde Derivatives Reduce Virulence in Vibrio spp. by Decreasing the DNA-Binding Activity of the Quorum Sensing Response Regulator LuxR. BMC Microbiol. 2008, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Antibiofilm Effect of Plant Derived Antimicrobials on Listeria monocytogenes. Food Microbiol. 2013, 36, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Jett, B.D.; Huycke, M.M.; Gilmore, M.S. Virulence of Enterococci. Clin. Microbiol. Rev. 1994, 7, 462–478. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.M.; Lee, J.H.; Seo, S.J.; Lee, I.H. Extracellular Gelatinase of Enterococcus faecalis Destroys a Defense System in Insect Hemolymph and Human Serum. Infect. Immun. 2007, 75, 1861–1869. [Google Scholar] [CrossRef]
- Steck, N.; Hoffmann, M.; Sava, I.G.; Kim, S.C.; Hahne, H.; Tonkonogy, S.L.; Mair, K.; Krueger, D.; Pruteanu, M.; Shanahan, F.; et al. Enterococcus faecalis Metalloprotease Compromises Epithelial Barrier and Contributes to Intestinal Inflammation. Gastroenterology 2011, 141, 959–971. [Google Scholar] [CrossRef]
- Cox, C.R.; Coburn, P.S.; Gilmore, M.S. Enterococcal Cytolysin: A Novel Two Component Peptide System that Serves as a Bacterial Defense against Eukaryotic and Prokaryotic Cells. Curr. Protein Pept. Sci. 2005, 6, 77–84. [Google Scholar] [CrossRef]
- Jett, B.D.; Jensen, H.G.; Nordquist, R.E.; Gilmore, M.S. Contribution of the pAD1-encoded Cytolysin to the Severity of Experimental Enterococcus faecalis Endophthalmitis. Infect. Immun. 1992, 60, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Huycke, M.M.; Gilmore, M.S. Frequency of Aggregation Substance and Cytolysin Genes among Enterococcal Endocarditis Isolates. Plasmid 1995, 34, 152–156. [Google Scholar] [CrossRef]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wölber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from Food, Clinical Specimens, and Oral Sites: Prevalence of Virulence Factors in Association with Biofilm Formation. Front. Microbiol. 2015, 6, 1534. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.A.; Huang, W.; Nallapareddy, S.R.; Teng, F.; Murray, B.E. Influence of Origin of Isolates, especially Endocarditis Isolates, and Various Genes on Biofilm Formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 3658–3663. [Google Scholar] [CrossRef] [PubMed]
- Bourgogne, A.; Garsin, D.A.; Qin, X.; Singh, K.V.; Sillanpaa, J.; Yerrapragada, S.; Ding, Y.; Dugan-Rocha, S.; Buhay, C.; Shen, H.; et al. Large-Scale Variation in Enterococcus faecalis Illustrated by the Genome Analysis of Strain OG1RF. Genome Biol. 2008, 9, R110. [Google Scholar] [CrossRef]
- Pillai, S.K.; Sakoulas, G.; Gold, H.S.; Wennersten, C.; Eliopoulos, G.M.; Moellering, R.C., Jr.; Inouye, R.T. Prevalence of the fsr Locus in Enterococcus faecalis Infections. J. Clin. Microbiol. 2002, 40, 2651–2652. [Google Scholar] [CrossRef]
- Nakayama, J.; Chen, S.; Oyama, N.; Nishiguchi, K.; Azab, E.A.; Tanaka, E.; Kariyama, R.; Sonomoto, K. Revised Model for Enterococcus faecalis Fsr Quorum-Sensing System: The Small Open Reading Frame fsrD Encodes the Gelatinase Biosynthesis-Activating Pheromone Propeptide Corresponding to Staphylococcal Agrd. J. Bacteriol. 2006, 188, 8321–8326. [Google Scholar] [CrossRef]
- Sifri, C.D.; Mylonakis, E.; Singh, K.V.; Qin, X.; Garsin, D.A.; Murray, B.E.; Ausubel, F.M.; Calderwood, S.B. Virulence Effect of Enterococcus faecalis Protease Genes and the Quorum-Sensing Locus fsr in Caenorhabditis elegans and Mice. Infect. Immun. 2002, 70, 5647–5650. [Google Scholar] [CrossRef]
- Balasubramanian, A.R.; Vasudevan, S.; Shanmugam, K.; Lévesque, C.M.; Solomon, A.P.; Neelakantan, P. Combinatorial Effects of Trans-cinnamaldehyde with Fluoride and Chlorhexidine on Streptococcus mutans. J. Appl. Microbiol. 2021, 130, 382–393. [Google Scholar] [CrossRef]
- Thomas, V.C.; Hiromasa, Y.; Harms, N.; Thurlow, L.; Tomich, J.; Hancock, L.E. A Fratricidal Mechanism is Responsible for eDNA Release and Contributes to Biofilm Development of Enterococcus faecalis. Mol. Microbiol. 2009, 72, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Strateva, T.; Atanasova, D.; Savov, E.; Petrova, G.; Mitov, I. Incidence of Virulence Determinants in Clinical Enterococcus faecalis and Enterococcus faecium Isolates Collected in Bulgaria. Braz. J. Infect. Dis. 2016, 20, 127–133. [Google Scholar] [CrossRef]
- Lins, R.X.; de Oliveira Andrade, A.; Hirata Junior, R.; Wilson, M.J.; Lewis, M.A.; Williams, D.W.; Fidel, R.A. Antimicrobial Resistance and Virulence Traits of Enterococcus faecalis from Primary Endodontic Infections. J. Dent. 2013, 41, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ribeiro, M.; De-Jesus-Soares, A.; Zaia, A.A.; Ferraz, C.C.; Almeida, J.F.; Gomes, B.P. Antimicrobial Susceptibility and Characterization of Virulence Genes of Enterococcus faecalis Isolates from Teeth with Failure of the Endodontic Treatment. J. Endod. 2016, 42, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Federle, M.J.; Bassler, B.L. Interspecies Communication in Bacteria. J. Clin. Investig. 2003, 112, 1291–1299. [Google Scholar] [CrossRef]
- Schauder, S.; Shokat, K.; Surette, M.G.; Bassler, B.L. The LuxS Family of Bacterial Autoinducers: Biosynthesis of a Novel Quorum-Sensing Signal Molecule. Mol. Microbiol. 2001, 41, 463–476. [Google Scholar] [CrossRef]
- Brackman, G.; Celen, S.; Hillaert, U.; Van Calenbergh, S.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-activity Relationship of Cinnamaldehyde Analogs as Inhibitors of AI-2 based Quorum Sensing and their Effect on Virulence of Vibrio spp. PLoS ONE 2011, 6, e16084. [Google Scholar] [CrossRef]
- Geisinger, E.; Chen, J.; Novick, R.P. Allele-Dependent Differences in Quorum-Sensing Dynamics Result in Variant Expression of Virulence Genes in Staphylococcus aureus. J. Bacteriol. 2012, 194, 2854–2864. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Hou, B.; Zhang, C. Quorum sensing LuxS/autoinducer-2 Inhibits Enterococcus faecalis Biofilm Formation Ability. J. Appl. Oral Sci. 2018, 26, e20170566. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.A.; Stephens, J.C. A review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of Bacterial Cell Division Protein FtsZ by Cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.A.A.; Matinlinna, J.P.; Lévesque, C.M.; Neelakantan, P. Trans-Cinnamaldehyde Attenuates Enterococcus faecalis Virulence and Inhibits Biofilm Formation. Antibiotics 2021, 10, 702. https://doi.org/10.3390/antibiotics10060702
Ali IAA, Matinlinna JP, Lévesque CM, Neelakantan P. Trans-Cinnamaldehyde Attenuates Enterococcus faecalis Virulence and Inhibits Biofilm Formation. Antibiotics. 2021; 10(6):702. https://doi.org/10.3390/antibiotics10060702
Chicago/Turabian StyleAli, Islam A. A., Jukka P. Matinlinna, Celine M. Lévesque, and Prasanna Neelakantan. 2021. "Trans-Cinnamaldehyde Attenuates Enterococcus faecalis Virulence and Inhibits Biofilm Formation" Antibiotics 10, no. 6: 702. https://doi.org/10.3390/antibiotics10060702
APA StyleAli, I. A. A., Matinlinna, J. P., Lévesque, C. M., & Neelakantan, P. (2021). Trans-Cinnamaldehyde Attenuates Enterococcus faecalis Virulence and Inhibits Biofilm Formation. Antibiotics, 10(6), 702. https://doi.org/10.3390/antibiotics10060702








