Herbal Products and Their Active Constituents Used Alone and in Combination with Antifungal Drugs against Drug-Resistant Candida sp.
Abstract
1. Introduction
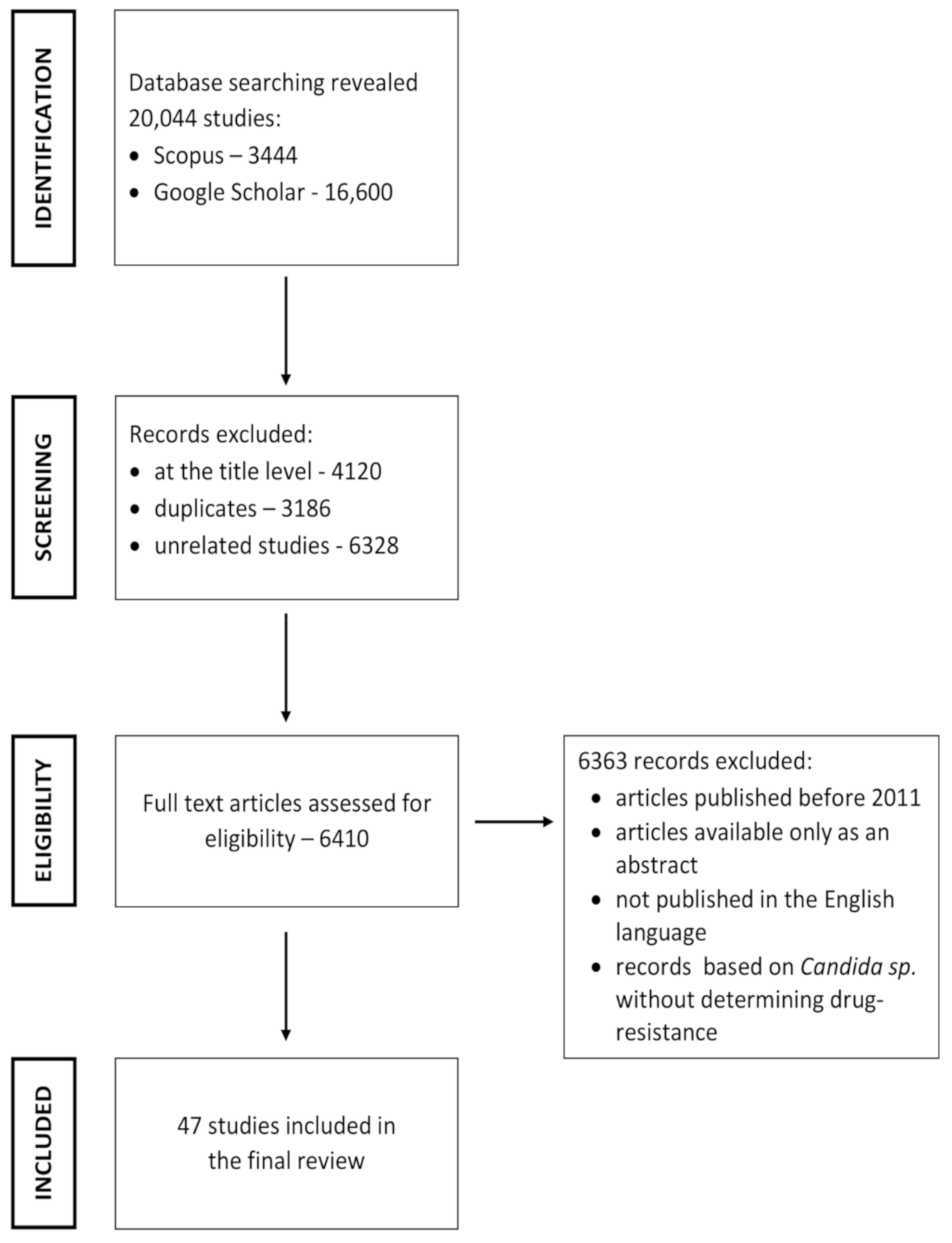
2. Literature Search Strategy
2.1. Inclusion and Exclusion Criteria
2.2. Study Selection
3. Herbs Used against Drug-Resistant Candida sp.
3.1. Herbal Products Used against Drug-Resistant Candida sp.
3.2. Herbal Products and their Combination with Antifungal Drugs Used against Drug-Resistant Candida sp.
4. Active Constituents Isolated from Herbs Used against Drug-Resistant Candida sp.
4.1. Herbal Active Constituents Used against Drug-Resistant Candida sp.
4.2. Herbal Active Constituents and Their Combination with Antifungal Drugs Used against Drug-Resistant Candida sp.
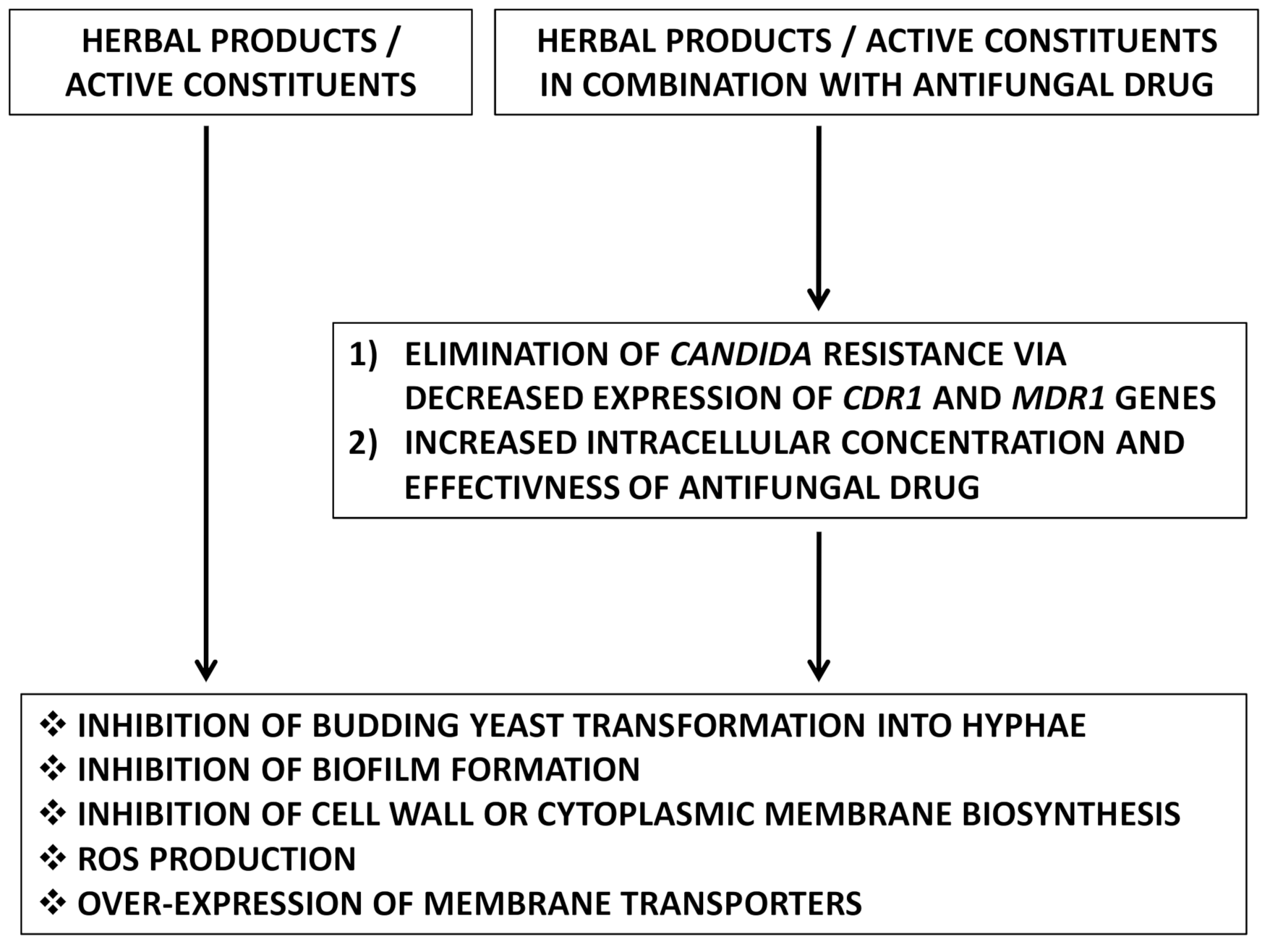
5. Mechanism of Action of Herbal Therapy against Drug-Resistant Candida sp.
5.1. Inhibition of Budding Yeast Transformation into Hyphae
5.2. Inhibition of Biofilm Formation
5.3. Inhibition of Cell Wall or Cytoplasmic Membrane Biosynthesis
5.4. Reactive Oxygen Species (ROS)Production
5.5. Over-Expression of Membrane Transporters
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Elsohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. Natural products inhibiting Candida albicans secreted aspartic proteases from Tovomitakrukovii. Planta Med. 2020, 68, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Sobel, J.D. Mucosal candidiasis. Infect. Dis. Clin. N. Am. 2002, 16, 793–820. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef]
- Bastert, J. Current and future approaches to antimycotic treatment in the era of resistant fungi and immunocompromised hosts. Int. J. Antimicrob. Agents 2001, 17, 81–91. [Google Scholar] [CrossRef]
- Hitchcock, C.A.; Pye, G.W.; Troke, P.F.; Johnson, E.M.; Warnock, D.W. Fluconazole resistance in Candida glabrata. Antimicrob. Agents Chemother. 1993, 37, 1962–1965. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Lockhartm, S.R.; Pujol, C.; Swails-Wenger, J.A.; Messer, S.A.; Edmond, M.B.; Jones, R.N.; Wenzel, R.P.; Soll, D.R. Hospital specificity, region specificity, and fluconazole resistance of Candida albicans bloodstream isolates. J. Clin. Microbiol. 1998, 36, 1518–1529. [Google Scholar] [CrossRef]
- Hsu, H.; Sheth, C.C.; Veses, V. Herbal extracts with antifungal activity against Candida albicans: A systematic review. Med. Chem. 2021, 21, 90–117. [Google Scholar]
- Kumar, D.; Ayesha, M.J.; Gautam, P.; Joshi, H.; Kumar, N. A recent report on ‘plants with anti-Candida properties’. Int. J. Cur. Res. Rev. 2020, 12, 25–34. [Google Scholar] [CrossRef]
- Sharanappa, R.; Vidyasagar, G.M. Anti-Candida activity of medicinal plants. A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 9–16. [Google Scholar]
- Shin, J.; Prabhakaran, V.S.; Kim, K.S. The multi-faceted potential of plant-derived metabolites as antimicrobial agents against multidrug-resistant pathogens. Microb. Pathog. 2018, 116, 209–214. [Google Scholar] [CrossRef]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Plant natural products: Their pharmaceutical potential against disease and drug resistant microbial pathogens. J. Pharm. Res. 2011, 4, 1179–1185. [Google Scholar]
- Aiyegoro, O.A.; Okoh, A.I. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. J. Med. Plants Res. 2009, 3, 1147–1152. [Google Scholar]
- Chanda, S.; Rakholiya, K. Combination therapy: Synergism between natural plant extracts and antibiotics against infectious diseases. Microbiol. Book Ser. 2011, 1, 520–529. [Google Scholar]
- Inui, T.; Wang, Y.; Deng, S.; Smith, D.C.; Franzblau, S.G.; Pauli, G.F. Counter-current chromatography based analysis of synergy in an anti-tuberculosis ethnobotanical. J. Chromatogr. A 2007, 1151, 211–215. [Google Scholar] [CrossRef]
- Mundy, L.; Pendry, B.; Rahman, M. Antimicrobial resistance and synergy in herbal medicine. J. Herb. Med. 2016, 6, 53–58. [Google Scholar] [CrossRef]
- Obeidat, M. Antimicrobial activity of some medicinal plants against multidrug resistant skin pathogens. J. Med. Plants Res. 2011, 5, 3856–3860. [Google Scholar]
- Khajeh, E.; Hosseini Shokouh, S.J.; Rajabibazl, M.; Roudbary, M.; Rafiei, S.; Aslani, P.; Farahnejad, Z. Antifungal effect of Echinophoraplatyloba on expression of CDR1 and CDR2 genes in fluconazole-resistant Candida albicans. Br. J. Biomed. Sci. 2016, 73, 44–48. [Google Scholar] [CrossRef]
- Mathur, A.; Singh, R.; Yousuf, S.; Bhardwaj, A.; Verma, S.K.; Babu, P.; Gupta, V.; Prasad, G.B.K.S.; Dua, V.K. Antifungal activity of some plant extracts against clinical pathogens. Adv. Appl. Sci. Res. 2011, 2, 260–264. [Google Scholar]
- Dwivedi, M.; Muralidhar, S.; Saluja, D. Hibiscus sabdariffa extract inhibits adhesion, biofilm initiation and formation in Candida albicans. Ind. J. Microbiol. 2020, 60, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Jayan, L.; Priyadharsini, N.; Ramya, R.; Rajkumar, K. Evaluation of antifungal activity of mint, pomegranate and coriander on fluconazole-resistant Candida glabrata. J. Oral Maxillofac. Pathol. 2020, 24, 517–522. [Google Scholar] [PubMed]
- Sultan, W.A.; Zghair, F.S. Susceptibility of clinical Candida albicans isolates to oak extract that resistance antifungal compounds. Eur. J. Mol. Clin. Med. 2020, 7, 5130–5137. [Google Scholar]
- Khan, S.; Imran, M.; Imran, M.; Pindari, N. Antimicrobial activity of various ethanolic plant extracts against pathogenic multi drug resistant Candida spp. Bioinformation 2017, 13, 67–72. [Google Scholar] [CrossRef]
- Anjos, M.N.V.; Araújo-Neto, L.N.D.; Silva Buonafina, M.D.; Pereira Neves, R.; Souza, E.R.D.; Bezerra, I.C.F.; Ferreira, M.R.A.; Soares, L.A.L.; Coutinho, H.D.M.; Martins, M.; et al. Ocotea glomerata (Nees) Mez extract and fractions: Chemical characterization, anti-Candida activity and related mechanism of action. Antibiotics 2020, 9, 394. [Google Scholar] [CrossRef]
- Saad, A.; Fadli, M.; Bouaziz, M.; Benharref, A.; Mezrioui, N.E.; Hassani, L. Anticandidal activity of the essential oils of Thymus maroccanus and Thymus broussonetii and their synergism with amphotericin B and fluconazol. Phytomedicine 2010, 17, 1057–1060. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnopharmacol. 2020, 262, 113135. [Google Scholar] [CrossRef]
- Göger, G.; Çomoğlu, B.A.; İşcan, G.; Demirci, F. Evaluation of anticandidal effects of essential oils of Lavender (Lavandula angustifolia Miller) in combination with ketoconazole against some Candida berkhout strains. Trak. Univ. J. Nat. Sci. 2020, 21, 13–19. [Google Scholar]
- Da Silva, A.R.; de Andrade Neto, J.B.; da Silva, C.R.; de Sousa Campos, R.; Silva, R.A.C.; Freitas, D.D.; do Nascimento, F.B.S.A.; de Andrade, L.N.D.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: Action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef]
- Sadeghi-Ghadi, Z.; Vaezi, A.; Ahangarkani, F.; Ilkit, M.; Ebrahimnejad, P.; Badali, H. Potent in vitro activity of curcumin and quercetin co-encapsulated in nanovesicles without hyaluronan against Aspergillus and Candida isolates. J. Mycol. Med. 2020, 30, 101014. [Google Scholar] [CrossRef]
- Iraji, A.; Yazdanpanah, S.; Alizadeh, F.; Mirzamohammadi, S.; Ghasemi, Y.; Pakshir, K.; Yang, Y.; Zomorodian, K. Screening the antifungal activities of monoterpenes and their isomers against Candida species. J. Appl. Microbiol. 2020, 129, 1541–1551. [Google Scholar] [CrossRef]
- Li, Z.; Yin, H.; Chen, W.; Jiang, C.; Hu, J.; Xue, Y.; Yao, D.; Peng, Y.; Hu, X. Synergistic effect of pseudolaric acid B with fluconazole against resistant isolates and biofilm of Candida tropicalis. Infect. Drug Resist. 2020, 13, 2733–2743. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, H.; Wang, D.; Zhang, M.; Sun, S.; Zhao, Y. The synergistic antifungal effects of gypenosides combined with fluconazole against resistant Candida albicans via inhibiting the drug efflux and biofilm formation. Biomed. Pharmacother. 2020, 130, 110580. [Google Scholar] [CrossRef]
- Xu, J.; Liu, R.; Sun, F.; An, L.; Shang, Z.; Kong, L.; Yang, M. Eucalyptal D enhances the antifungal effect of fluconazole on fluconazole-resistant Candida albicans by competitively inhibiting efflux pump. Front. Cell. Infect. Microbiol. 2019, 9, 211. [Google Scholar] [CrossRef]
- Singh, S.; Fatima, Z.; Ahmad, K.; Hameed, S. Fungicidal action of geraniol against Candida albicans is potentiated by abrogated CaCdr1p drug efflux and fluconazole synergism. PLoS ONE 2018, 13, e0203079. [Google Scholar]
- Sun, L.M.; Liao, K.; Liang, S.; Yu, P.H.; Wang, D.Y. Synergistic activity of magnolol with azoles and its possible antifungal mechanism against Candida albicans. J. Appl. Microbiol. 2015, 118, 826–838. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Gao, L.; Wang, L.; Song, F.; Zhang, L.; Wan, Y. The synergistic antifungal activity of resveratrol with azoles against Candida albicans. Lett. Appl. Microbiol. 2021, 72, 688–697. [Google Scholar] [CrossRef]
- Jafri, H.; Banerjee, G.; Khan, M.S.A.; Ahmad, I.; Abulreesh, H.H.; Althubiani, A.S. Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Nagy, F.; Vitális, E.; Jakab, Á.; Borman, A.M.; Forgács, L.; Tóth, Z.; Majoros, L.; Kovács, R. In vitro and in vivo effect of exogenous farnesol exposure against Candida auris. Front. Microbiol. 2020, 11, 975. [Google Scholar] [CrossRef]
- Silva, D.; Diniz-Neto, H.; Cordeiro, L.; Silva-Neta, M.; Silva, S.; Andrade-Júnior, F.; Leite, M.; Nóbrega, J.; Morais, M.; Souza, J.; et al. (R)-(+)-β-Citronellol and (S)-(−)-β-Citronellol in combination with amphotericin B against Candida spp. Int. J. Mol. Sci. 2020, 21, 1785. [Google Scholar] [CrossRef]
- Monalis, H.; Sujith, R.; Leela, K.V.; Balamurali, V. Antibiotics in combination with antifungals to combat drug resistant Candida–a concept on drug repurposing. J. Adv. Microbiol. 2020, 20, 42–48. [Google Scholar] [CrossRef]
- Salazar, S.B.; Simões, R.S.; Pedro, N.A.; Pinheiro, M.J.; Carvalho, M.F.N.; Mira, N.P. An overview on conventional and non-conventional therapeutic approaches for the treatment of candidiasis and underlying resistance mechanisms in clinical strains. J. Fungi 2020, 6, 23. [Google Scholar] [CrossRef]
- Ruiz-Baca, E.; Arredondo-Sánchez, R.I.; Corral-Pérez, K.; López-Rodríguez, A.; Meneses-Morales, I.; Ayala-García, V.M.; Martínez-Rocha, A.L. Molecular mechanisms of resistance to antifungals in Candida albicans. In Candida albicans; IntechOpen: London, UK, 2021. [Google Scholar]
- Hamdy, R.; Soliman, S.S.; Alsaadi, A.I.; Fayed, B.; Hamoda, A.M.; Elseginy, S.A.; Husseiny, M.I.; Ibrahim, A.S. Design and synthesis of new drugs inhibitors of Candida albicans hyphae and biofilm formation by upregulating the expression of TUP1 transcription repressor gene. Eur. J. Pharm. Sci. 2020, 148, 105327. [Google Scholar] [CrossRef]
- Angiolella, L. Synergistic activity of Pelargonium capitatum and Cymbopogon martini essential oils against C. albicans. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef]
- Lee, D.G.; Jung, H.J.; Woo, E.R. Antimicrobial property of (+)-lyoniresinol-3α-O-β-D-Glucopyranoside isolated from the root bark of Lyciumchinense Miller against human pathogenic microorganisms. Arch. Pharm. Res. 2005, 28, 1031–1036. [Google Scholar] [CrossRef]
- da Nóbrega Alves, D.; Monteiro, A.F.M.; Andrade, P.N.; Lazarini, J.G.; Abílio, G.M.F.; Guerra, F.Q.S.; Scotti, M.T.; Scotti, L.; Rosalen, P.L.; Castro, R.D.D. Docking prediction, antifungal activity, anti-biofilm effects on Candida spp., and toxicity against human cells of cinnamaldehyde. Molecules 2020, 25, 5969. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.; Sanglard, D.; Soković, M. Flavones, flavonols, and glycosylated derivatives impact on Candida albicans growth and virulence, expression of CDR1 and ERG11, cytotoxicity. Pharmaceuticals 2021, 14, 27. [Google Scholar]
- Yong, J.; Zu, R.; Huang, X.; Ge, Y.; Li, Y. Synergistic effect of berberine hydrochloride and fluconazole against Candida albicans resistant isolates. Front. Microbiol. 2020, 11, 1498. [Google Scholar] [CrossRef] [PubMed]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans antifungal resistance and tolerance in bloodstream infections: The triad yeast-host-antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Andes, D.R. Contributions of the biofilm matrix to Candida pathogenesis. J. Fungi 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef]
- Stojković, D.; Dias, M.I.; Drakulić, D.; Barros, L.; Stevanović, M.; Ferreira, I.C.F.R.; Soković, M.D. Methanolic extract of the herb Ononis spinosa L. is an antifungal agent with no cytotoxicity to primary human cells. Pharmaceuticals 2020, 13, 78. [Google Scholar] [CrossRef]
- Girardot, M.; Barbot, V.; Costa, D.; Chaftar, N.; Imbert, C. Anti-Candida potential of red fruits: Antifungal and anti-biofilm effects. Planta Med. 2012, 78, 167. [Google Scholar] [CrossRef]
- Muslim, S.N.; Hussin, Z.S. Chemical compounds and synergistic antifungal properties of Thymus kotschanus essential oil plus ketoconazole against Candida spp. Gene Rep. 2020, 21, 100916. [Google Scholar] [CrossRef]
- Kanchanapiboon, J.; Kongsa, U.; Pattamadilok, D.; Kamponchaidet, S.; Wachisunthon, D.; Poonsatha, S.; Tuntoaw, S. Boesenbergia rotunda extract inhibits Candida albicans biofilm formation by pinostrobin and pinocembrin. J. Ethnopharmacol. 2020, 261, 113193. [Google Scholar] [CrossRef]
- Kim, H.R.; Eom, Y.B. Antifungal and anti-biofilm effects of 6-shogaol against Candida auris. J. Appl. Microbiol. 2020, 130, 1142–1153. [Google Scholar] [CrossRef]
- Das, S.; Czuni, L.; Báló, V.; Papp, G.; Gazdag, Z.; Papp, N.; Kőszegi, T. Cytotoxic action of artemisinin and scopoletin on planktonic forms and on biofilms of Candida species. Molecules 2020, 25, 476. [Google Scholar] [CrossRef]
- Park, K.S.; Kang, K.C.; Kim, J.H.; Adams, D.J.; Johng, T.N.; Paik, Y.K. Differential inhibitory effects of protoberberines on sterol and chitin biosyntheses in Candida albicans. J. Antimicrob. Chemother. 1999, 43, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, S.; Zhang, S. Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J. Appl. Microbiol. 2020, 130, 1154–1172. [Google Scholar] [CrossRef] [PubMed]
- Aijaz Ahmad, A.; Khan, A.; Kumar, P.; Bhatt, R.P.; Manzoor, N. Antifungal activity of Coriarianepalensis essential oil by disrupting ergosterol biosynthesis and membrane integrity against Candida. Yeast 2011, 28, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shan, M.; Zhu, Y.; Yao, H.; Li, H.; Gu, B.; Zhu, Z. Kalopanaxsaponin A induces reactive oxygen species mediated mitochondrial dysfunction and cell membrane destruction in Candida albicans. PLoS ONE 2020, 15, e0243066. [Google Scholar]
- Sharma, Y.; Rastogi, S.K.; Ahmedi, S.; Manzoor, N. Antifungal activity of β-citronellol against two non-albicans Candida species. J. Essent. Oil Res. 2020, 32, 198–208. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Duartea, A.; Mendonc, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef]
- Seyedjavadi, S.S.; Khani, S.; Eslamifar, A.; Ajdary, S.; Goudarzi, M.; Halabian, R.; Akbari, R.; Zare-Zardini, H.; Fooladi, A.A.I.; Amani, J.; et al. The antifungal peptide MCh-AMP1 derived from Matricaria chamomilla inhibits Candida albicans growth via inducing ROS generation and altering fungal cell membrane permeability. Front. Microbiol. 2020, 10, 3150. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Wang, C.; Shao, J.; Wang, T.; Wu, D.; Ma, K.; Yan, G.; Yin, D. Antifungal evaluation of traditional herbal monomers and their potential for inducing cell wall remodeling in Candida albicans and Candida auris. Biofouling 2020, 36, 319–331. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol induces Candida albicans apoptosis associated with Ca2+/Calcineurin pathway. Front. Cell. Inf. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Fu, Z.; Lu, H.; Zhu, Z.; Yan, L.; Jiang, Y.; Cao, Y. Combination of baicalein and amphotericin B accelerates Candida albicans apoptosis. Biol. Pharm. Bull. 2011, 34, 214–218. [Google Scholar] [CrossRef]
- Poopedi, E.; Marimani, M.; Alomar, S.Y.; Aldahmash, B.; Ahmad, A. Modulation of antioxidant defence system in response to berberine in Candida albicans. Yeast 2021, 38, 157–169. [Google Scholar] [CrossRef]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef]
- Keereedach, P.; Hrimpeng, K.; Boonbumrung, K. Antifungal activity of Thai cajuput oil and its effect on efflux-pump gene expression in fluconazole-resistant Candida albicans clinical isolates. Int. J. Microbiol. 2020, 2020, 5989206. [Google Scholar] [CrossRef]
- Moraes, D.C.; de Sá, L.F.R.; Domingos, L.T.S.; Pinto, M.D.C.F.R.; de Araújo Soares, R.M.; Ferreira-Pereira, A. Synergistic interactions between β-lapachone and fluconazole in the inhibition of CaCdr2p and CaMdr1p in Candida albicans. Rev. Micol. 2020, 37, 104–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, A.; Herman, A.P. Herbal Products and Their Active Constituents Used Alone and in Combination with Antifungal Drugs against Drug-Resistant Candida sp. Antibiotics 2021, 10, 655. https://doi.org/10.3390/antibiotics10060655
Herman A, Herman AP. Herbal Products and Their Active Constituents Used Alone and in Combination with Antifungal Drugs against Drug-Resistant Candida sp. Antibiotics. 2021; 10(6):655. https://doi.org/10.3390/antibiotics10060655
Chicago/Turabian StyleHerman, Anna, and Andrzej Przemysław Herman. 2021. "Herbal Products and Their Active Constituents Used Alone and in Combination with Antifungal Drugs against Drug-Resistant Candida sp." Antibiotics 10, no. 6: 655. https://doi.org/10.3390/antibiotics10060655
APA StyleHerman, A., & Herman, A. P. (2021). Herbal Products and Their Active Constituents Used Alone and in Combination with Antifungal Drugs against Drug-Resistant Candida sp. Antibiotics, 10(6), 655. https://doi.org/10.3390/antibiotics10060655





