New Quinone Antibiotics against Methicillin-Resistant S. aureus
Abstract
1. Introduction
2. Results
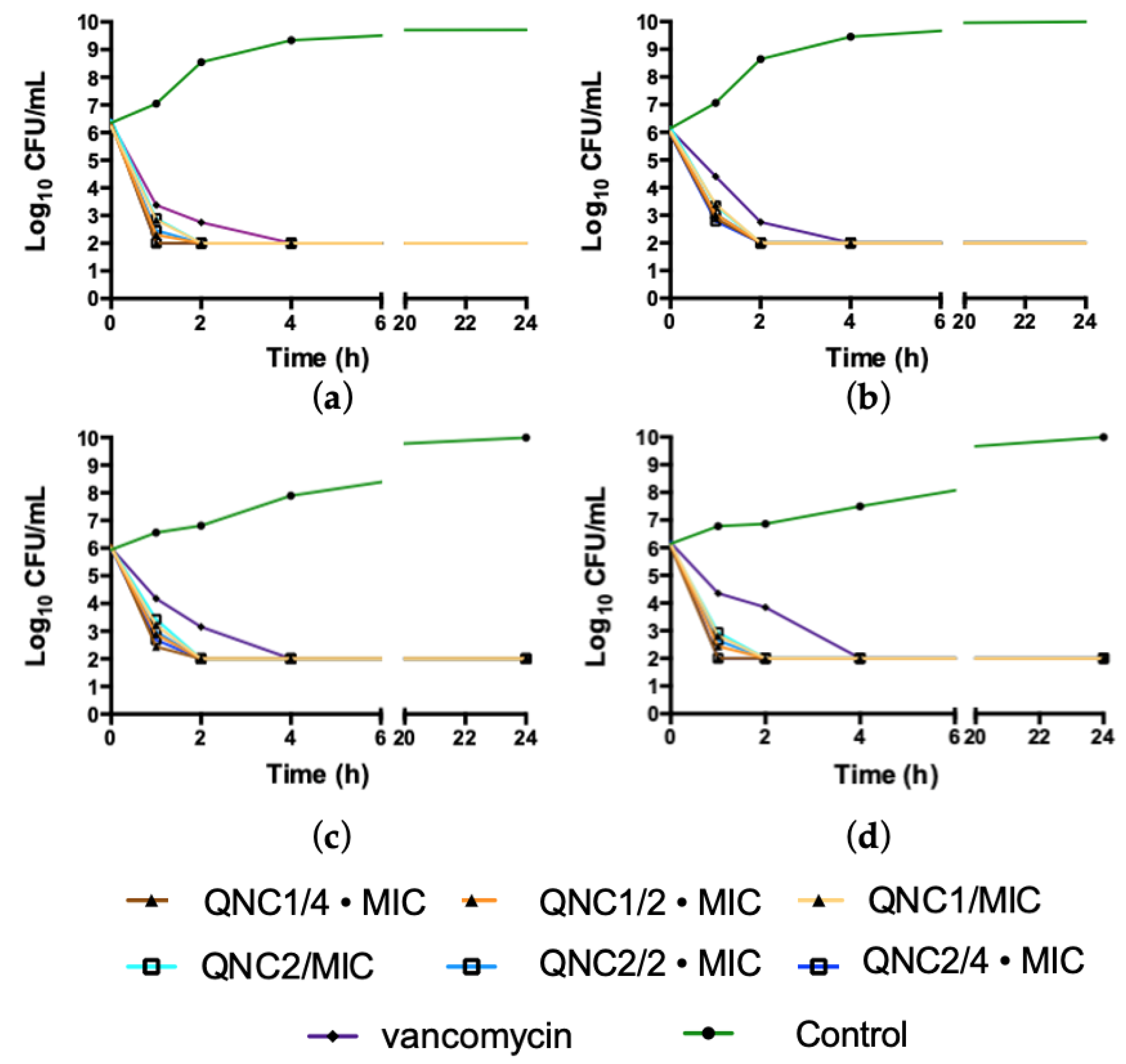
2.1. Kill Kinetics
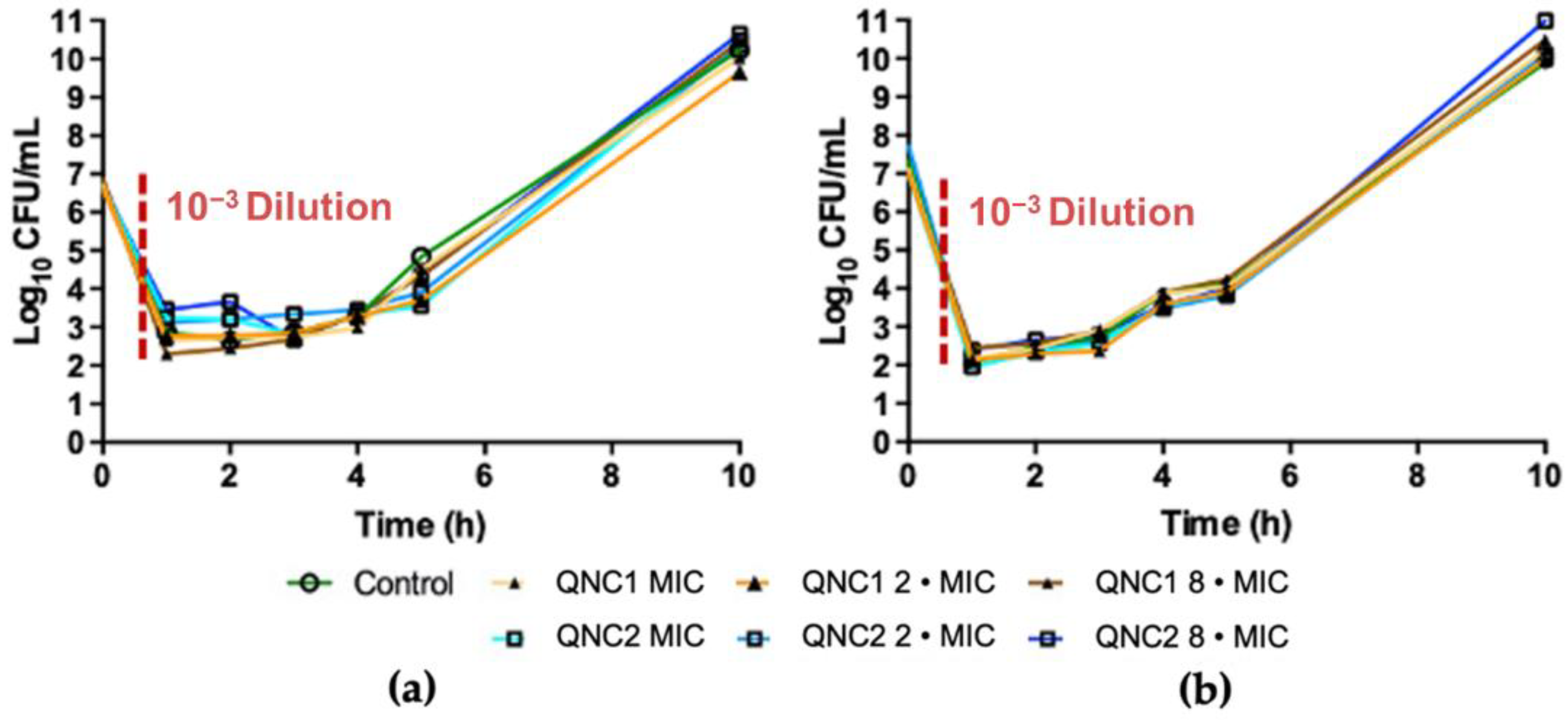
2.2. Post-Antibiotic Effect
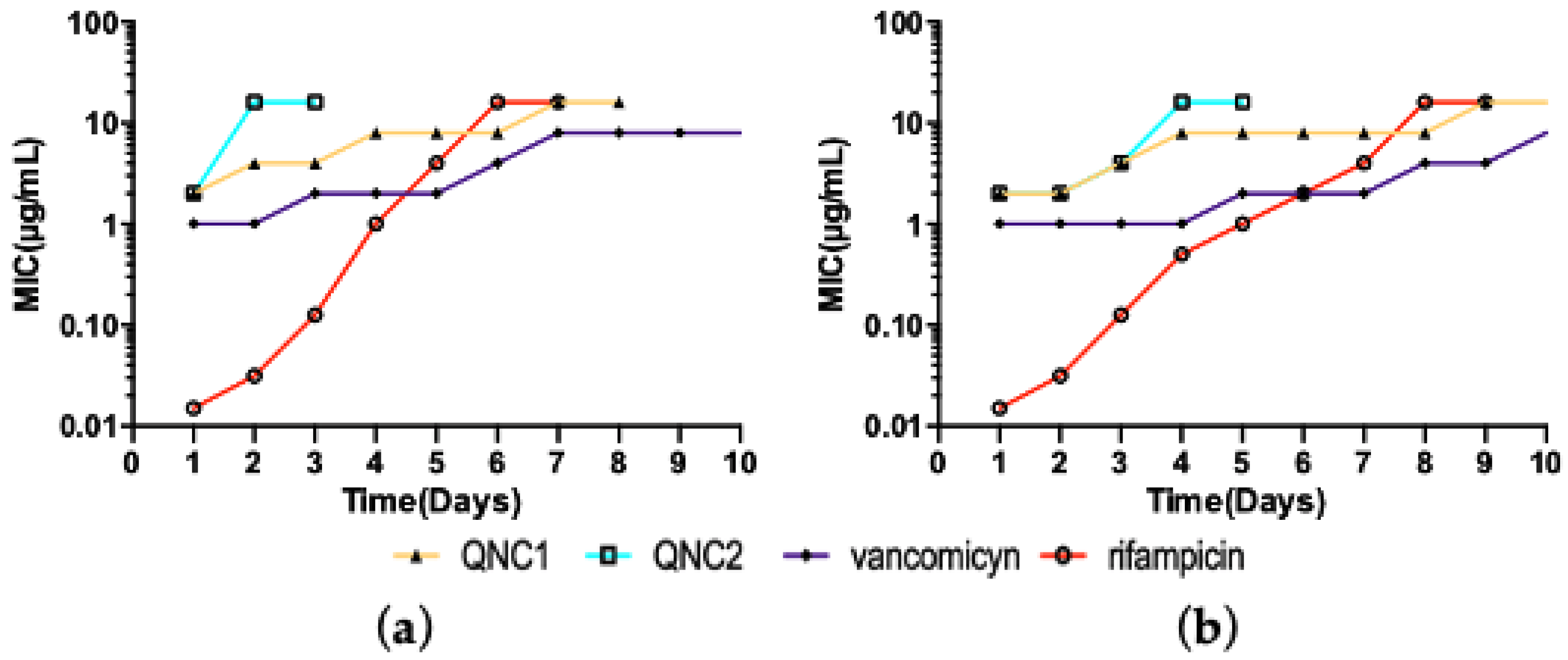
2.3. Resistance Studies
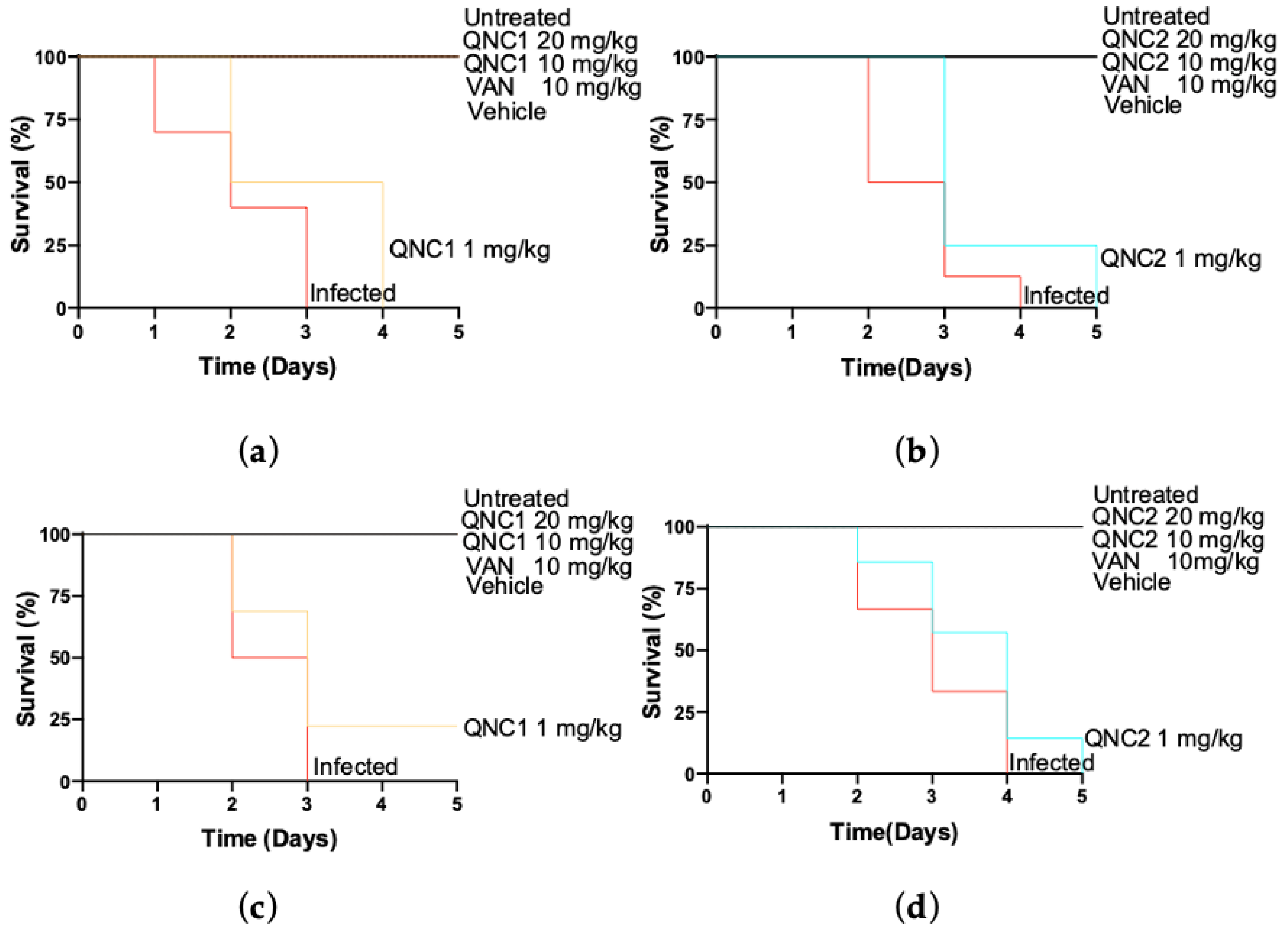
2.4. Effectiveness In Vivo
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Isolates
4.2. Media and Antibacterial Compounds
4.3. In Vitro Activity Characterization
4.3.1. Kill Kinetics
4.3.2. Post-Antibiotic Effect Determination
4.3.3. Serial Passage Assay
4.4. Galleria mellonella Infection Model
4.5. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, A.; Hughes, J.M. Critical importance of a one health approach to antimicrobial resistance. EcoHealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- O’neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review Paper; Wellcome Trust: London, UK, 2014. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F., III. Past, present, and future of antibacterial economics: Increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacotherapy 2017, 37, 71–84. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Campanini-Salinas, J.; Andrades-Lagos, J.; Mella-Raipan, J.; Vasquez-Velasquez, D. Novel classes of antibacterial drugs in clinical development, a hope in a post-antibiotic era. Clin. Microbiol. Rev. 2018, 18, 1188–1202. [Google Scholar] [CrossRef]
- Simpkin, V.L.; Renwick, M.J.; Kelly, R.; Mossialos, E. Incentivising innovation in antibiotic drug discovery and development: Progress, challenges and next steps. J. Antibiot. 2017, 70, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76. [Google Scholar] [CrossRef]
- Ishidate, M.; Kobayashi, K.; Sakurai, Y.; Sato, H.; Yoshida, T. Experimental Studies on Chemotherapy of Malignant Growth Employing Yoshida Sarcoma Animals XI. Effect of quinone derivatives, antibiotics, alkaloids, organoarsen, and other miscellaneous compounds. Gan 1955, 46, 482–484. [Google Scholar]
- Resegotti, L.; Infelise, V.E. The effect of a combination of phenanthroline quinone plus iodochlorhydroxyquinoline in the prevention of dysvitaminosis caused by antibiotics. Minerva Med. 1966, 57, 947–949. [Google Scholar] [PubMed]
- Wanke, H.; Kersten, W.; Kersten, H. Polysomes in Bacillus subitilis: Influence of amino quinones and quinone antibiotics on the synthesis and stability of mRNA. Hoppe-Seyler’s Z. Physiol. Chem. 1969, 350, 1162–1163. [Google Scholar]
- Nagasawa, T.; Fukao, H.; Irie, H.; Yamada, H. Sakyomicins. A, B, C and D: New quinone-type antibiotics produced by a strain of nocardia taxonomy, production, isolation and biological properties. J. Antibiot. 1984, 37, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Campanini-Salinas, J.; Andrades-Lagos, J.; González-Rocha, G.; Choquesillo-Lazarte, D.; Dragnic, S.B.; Faúndez, M.; Alarcón, P.; Silva, F.; Vidal, R.; Salas-Huenuleo, E.; et al. A new kind of quinonic-antibiotic useful against multidrug-resistant S. aureus and E. faecium infections. Molecules 2018, 23, 1776. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Coote, P.J. Wax moth larva (Galleria mellonella): An in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Coote, P.J. Utility of greater wax moth larva (Galleria mellonella) for evaluating the toxicity and efficacy of new antimicrobial agents. Adv. Appl. Microbiol. 2012, 78, 25–53. [Google Scholar] [CrossRef]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Petronio, G.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef]
- Hornsey, M.; Wareham, D.W. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2011, 55, 3534–3537. [Google Scholar] [CrossRef]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Utilising Galleria mellonella larvae for studying in vivo activity of conventional and novel antimicrobial agents. Pathog. Dis. 2020, 78, ftaa059. [Google Scholar] [CrossRef]
- Craig, W.A. The postantibiotic effect. Clin. Microbiol. Newsl. 1991, 13, 121–124. [Google Scholar] [CrossRef]
- Srivastava, V.C. Glycerol as a Green Solvent in Organic Reactions. Mater. Res. Found. 2019, 54, 202–223. [Google Scholar] [CrossRef]
- Niazi, S.K. Handbook of Pharmaceutical Manufacturing Formulations: Volume Four, Semisolid Products; CRC Press: Boca Raton, FL, USA, 2019; ISBN 1351593331. [Google Scholar]
- Allegra, E.; Titball, R.W.; Carter, J.; Champion, O.L. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 2018, 198, 469–472. [Google Scholar] [CrossRef]
- Livermore, D.M. The need for new antibiotics. Clin. Microbiol. Infect. 2004, 10, 1–9. [Google Scholar] [CrossRef]
- Kmietowicz, Z. Few novel antibiotics in the pipeline, WHO warns. BMJ 2017, 358, j4339. [Google Scholar] [CrossRef]
- Sakoulas, G.; Moise-Broder, P.A.; Schentag, J.; Forrest, A.; Moellering, R.C.; Eliopoulos, G.M. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 2004, 42, 2398–2402. [Google Scholar] [CrossRef]
- Kollef, M.H. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin. Infect. Dis. 2007, 45, S191–S195. [Google Scholar] [CrossRef] [PubMed]
- Mandell, G.L.; Douglas, R.G., Jr.; Bennett, J.E. Principles and Practice of Infectious Diseases; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 2, p. 233. ISBN 0471034894. [Google Scholar]
- Goodman, L.S. Goodman and Gilman’s the Pharmacological Basis of Therapeutics; McGraw-Hill: New York, NY, USA, 1996; Volume 1549, pp. 1361–1373. [Google Scholar]
- Craig, W. Pharmacodynamics of antimicrobial agents as a basis for determining dosage regimens. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, S6–S8. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L.; Baquero, F.; Andersson, D.I. Beyond serial passages: New methods for predicting the emergence of resistance to novel antibiotics. Curr. Opin. Pharmacol. 2011, 11, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Alder, J.; Thauvin-Eliopoulos, C.; Moellering, R.C.; Eliopoulos, G.M. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 2006, 50, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Kosowska-Shick, K.; McGhee, P.; Dewasse, B.; Beachel, L.; Appelbaum, P.C. Resistance selection studies comparing the activity of razupenem (PTZ601) to vancomycin and linezolid against eight methicillin-resistant and two methicillin-susceptible Staphylococcus aureus strains. Antimicrob. Agents Chemother. 2009, 53, 3118–3121. [Google Scholar] [CrossRef]
- Ramarao, N.; Nielsen-Leroux, C.; Lereclus, D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J. Vis. Exp. 2012, 70. [Google Scholar] [CrossRef]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.R.; Schroeder, G.N.; Collins, J.W.; Frankel, G. Use of Galleria mellonella as a model organism to study Legionella pneumophila infection. J. Vis. Exp. 2013, 81. [Google Scholar] [CrossRef]
- Mercier, R.-C.; Houlihan, H.H.; Rybak, M.J. Pharmacodynamic evaluation of a new glycopeptide, LY333328, and in vitro activity against Staphylococcus aureus and Enterococcus faecium. Antimicrob. Agents Chemother. 1997, 41, 1307–1312. [Google Scholar] [CrossRef]
- Silverman, J.A.; Oliver, N.; Andrew, T.; Li, T. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 2001, 45, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobi-Cally, Approved Standard, 8th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Luther, M.K.; Arvanitis, M.; Mylonakis, E.; LaPlante, K.L. Activity of daptomycin or linezolid in combination with rifampin or gentamicin against biofilm-forming Enterococcus faecalis or E. faecium in an in vitro pharmacodynamic model using simulated endocardial vegetations and an in vivo survival assay using Galleria mellonella larvae. Antimicrob. Agents Chemother. 2014, 58, 4612–4620. [Google Scholar] [CrossRef]
- Gibreel, T.M.; Upton, M. Synthetic epidermicin NI01 can protect Galleria mellonella larvae from infection with Staphylococcus aureus. J. Antimicrob. Chemother. 2013, 68, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanini-Salinas, J.; Andrades-Lagos, J.; Hinojosa, N.; Moreno, F.; Alarcón, P.; González-Rocha, G.; Burbulis, I.E.; Vásquez-Velásquez, D. New Quinone Antibiotics against Methicillin-Resistant S. aureus. Antibiotics 2021, 10, 614. https://doi.org/10.3390/antibiotics10060614
Campanini-Salinas J, Andrades-Lagos J, Hinojosa N, Moreno F, Alarcón P, González-Rocha G, Burbulis IE, Vásquez-Velásquez D. New Quinone Antibiotics against Methicillin-Resistant S. aureus. Antibiotics. 2021; 10(6):614. https://doi.org/10.3390/antibiotics10060614
Chicago/Turabian StyleCampanini-Salinas, Javier, Juan Andrades-Lagos, Nicolás Hinojosa, Fabián Moreno, Pedro Alarcón, Gerardo González-Rocha, Ian E. Burbulis, and David Vásquez-Velásquez. 2021. "New Quinone Antibiotics against Methicillin-Resistant S. aureus" Antibiotics 10, no. 6: 614. https://doi.org/10.3390/antibiotics10060614
APA StyleCampanini-Salinas, J., Andrades-Lagos, J., Hinojosa, N., Moreno, F., Alarcón, P., González-Rocha, G., Burbulis, I. E., & Vásquez-Velásquez, D. (2021). New Quinone Antibiotics against Methicillin-Resistant S. aureus. Antibiotics, 10(6), 614. https://doi.org/10.3390/antibiotics10060614







