Back to Basics: Choosing the Appropriate Surface Disinfectant
Abstract
1. Introduction
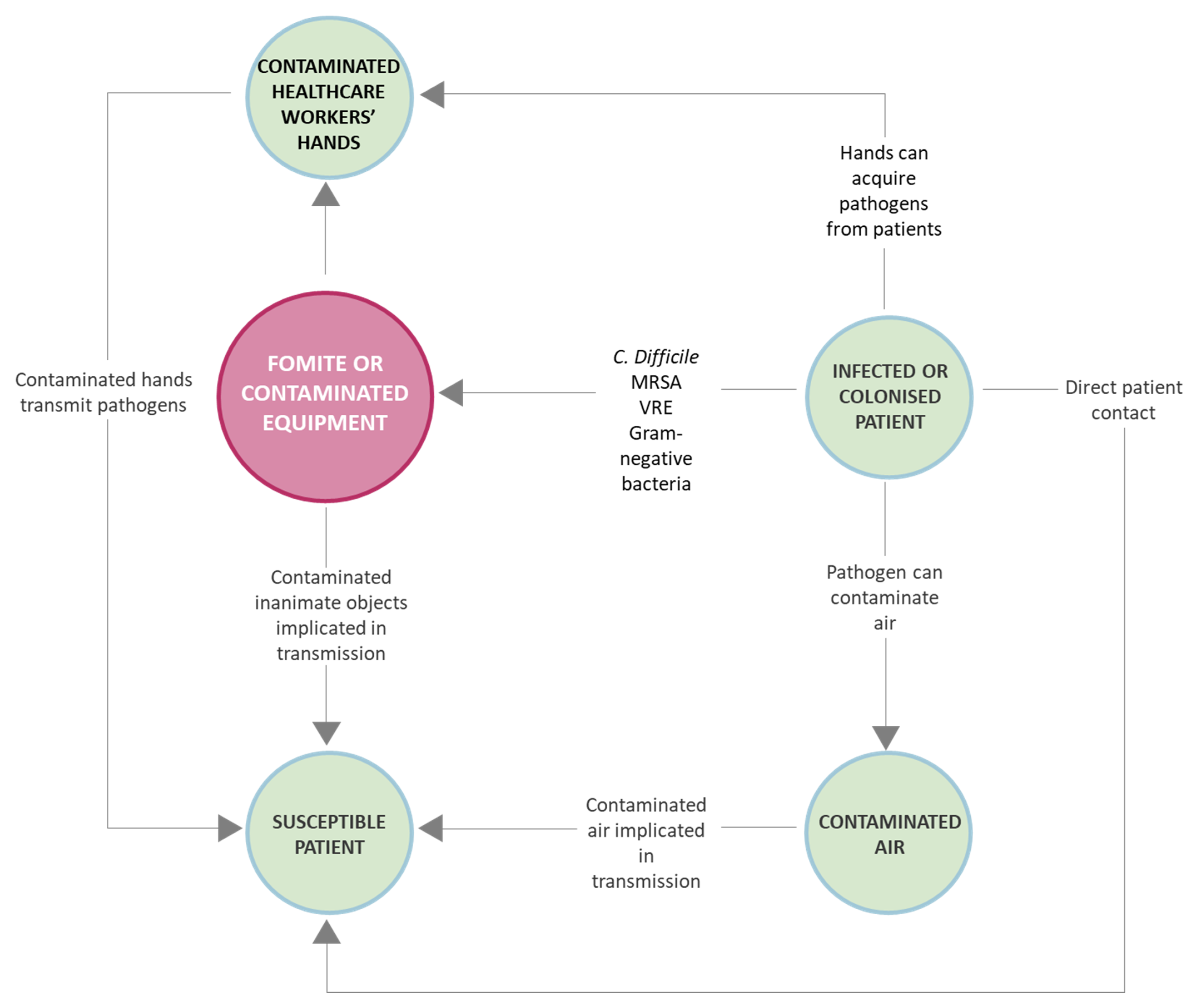
2. Most Common Microorganisms on Fomites and Associated Risks
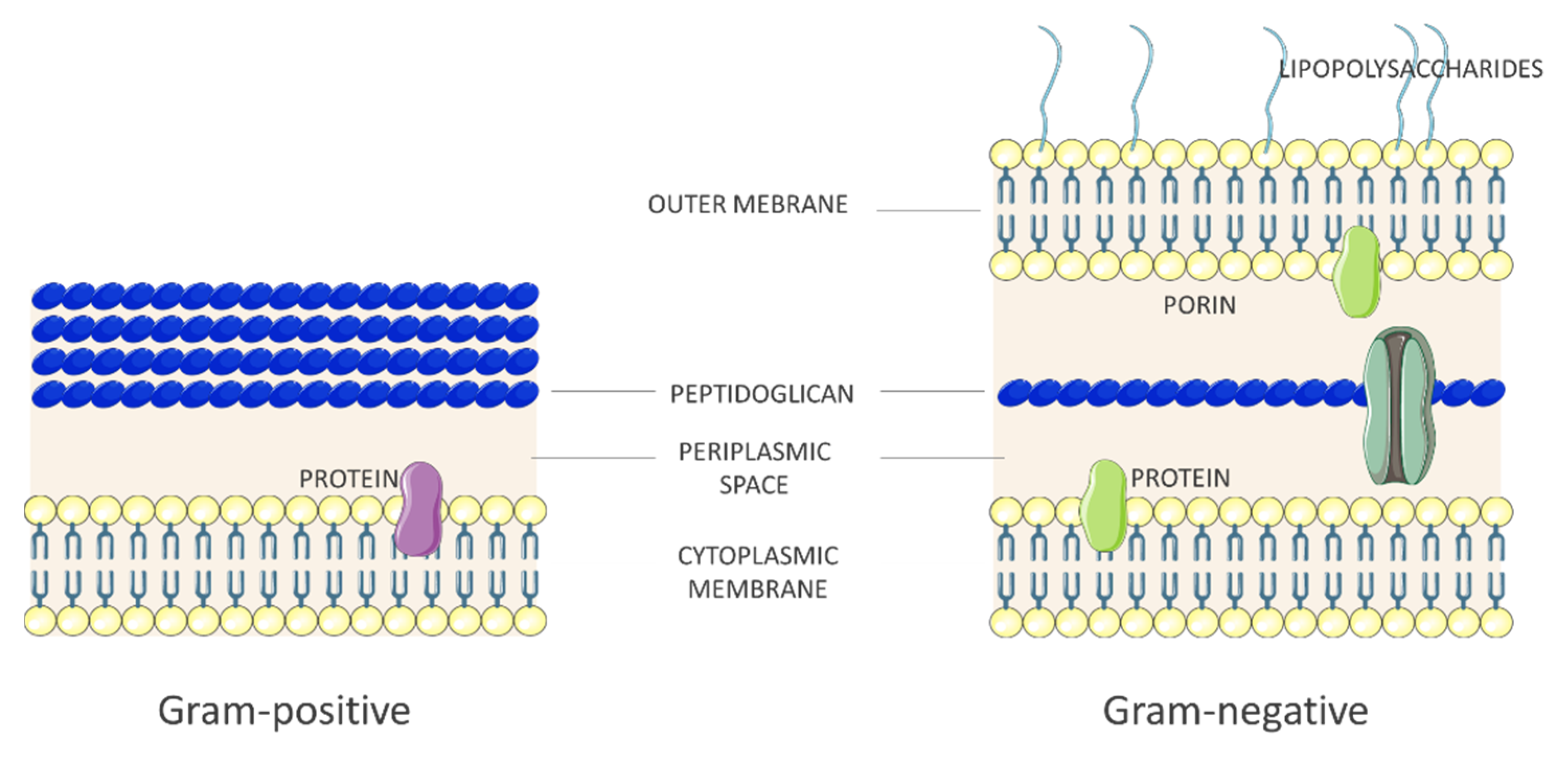
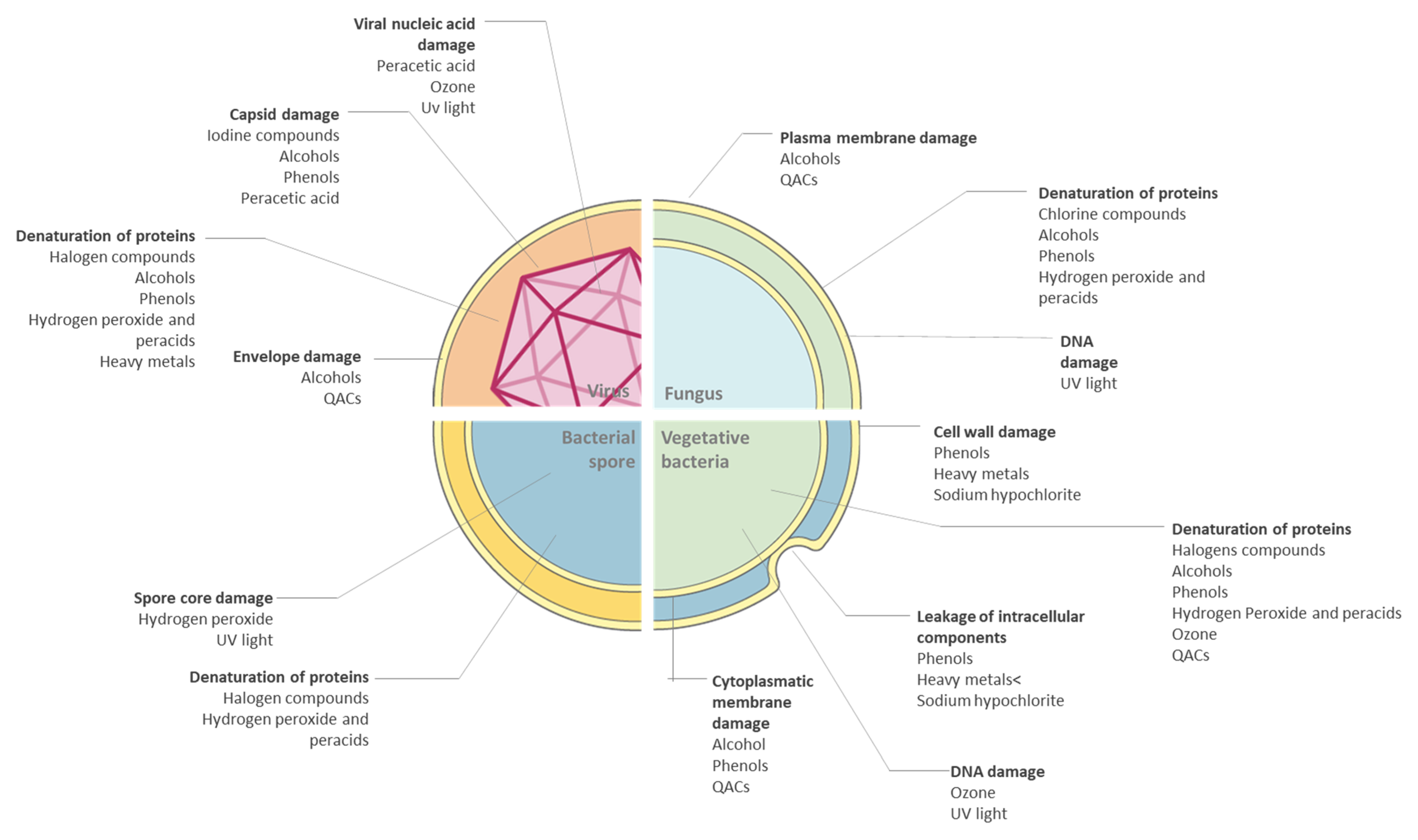
2.1. Bacteria
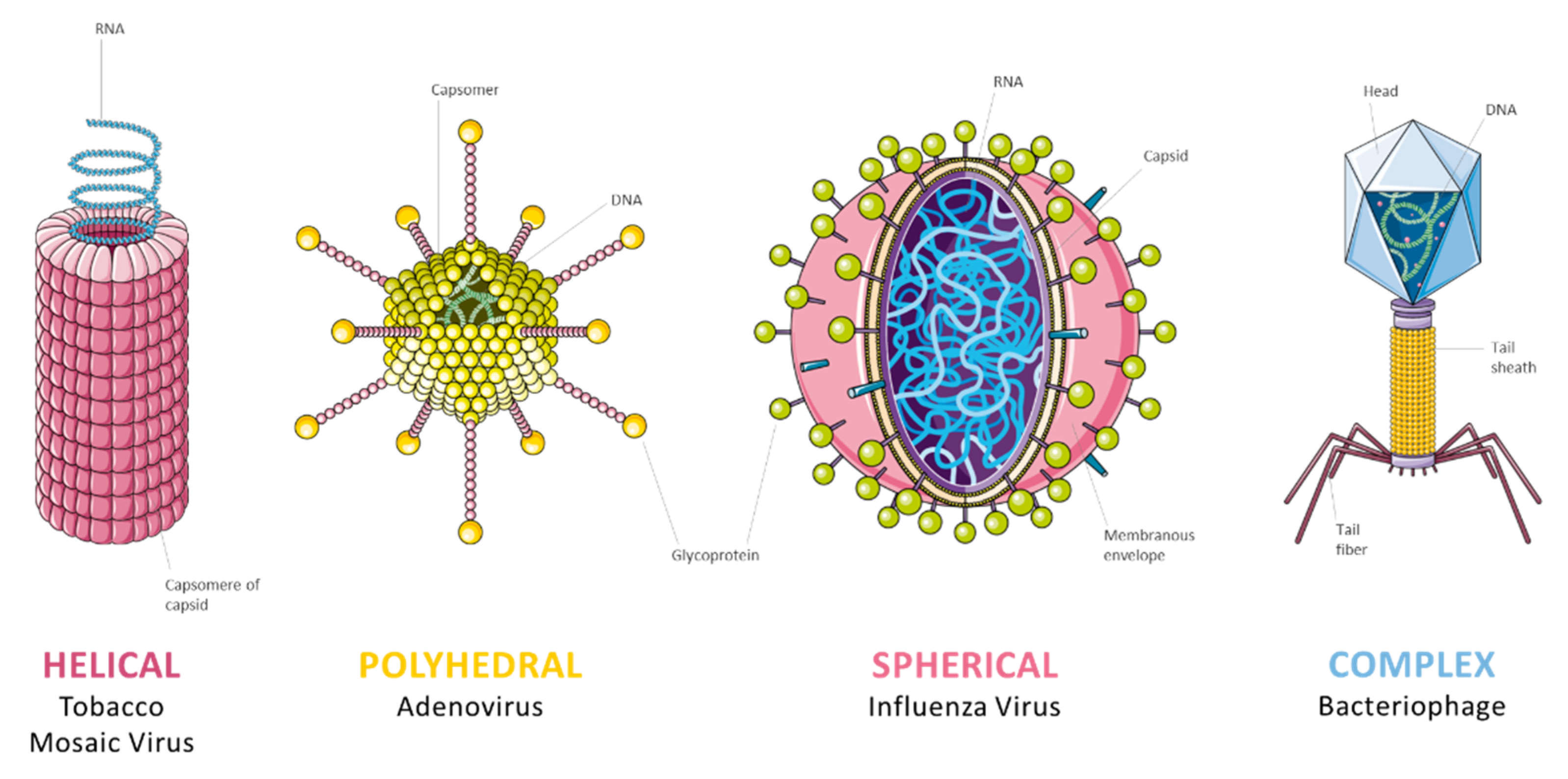
2.2. Virus
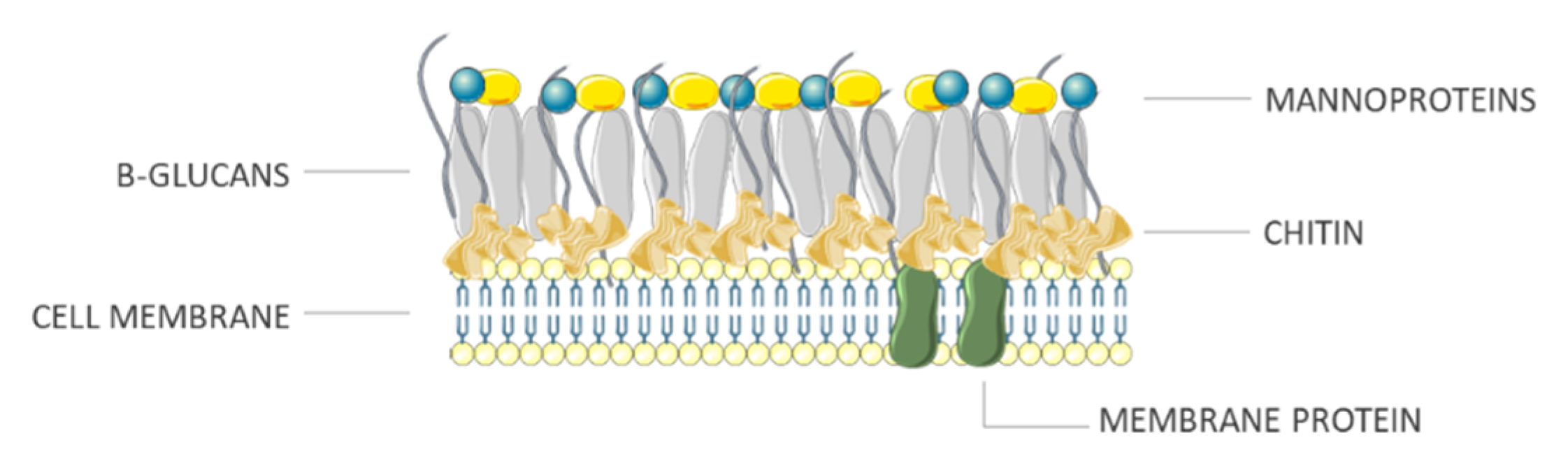
2.3. Fungi
2.4. Microbiological Risk Assessment
- Virulence—Ability of the microorganism to penetrate and multiplicate inside the host organism;
- Pathogenicity—Severity of the disease that may result;
- Transmissibility—Capability of the microorganism to be transmitted from one organism to another;
- Treatment—Availability, if any, of effective prophylaxis or therapy.
3. Factors That Affect the Activity of Antimicrobials
- Number and type of microorganismNo disinfectant can effectively act on all microorganism classes. So proper choice of chemical germicides is fundamental. Furthermore, some microbes can persist on surfaces showing resistance to these products: for example, the production of endospores or biofilm matrix protects the pathogens from environmental influences [13,30].
- Type and concentration of the antimicrobialAfter choosing the proper disinfectant, the concentration of the active ingredient is a key factor: the influence of changing in the concentration of the active(s) can be measured experimentally, with the determination of the kinetics of inactivation. Moreover, the knowledge of the effect of dilution or concentration on the activity of a sanitizing agent provides some valuable information that could lead to a reduction in the exposure time.Furthermore, microbicidal concentration is also a central concept in the microbial resistance field and it is especially important nowadays with increasing knowledge and restrictions on the environmental discharges of potentially harmful chemicals [31].
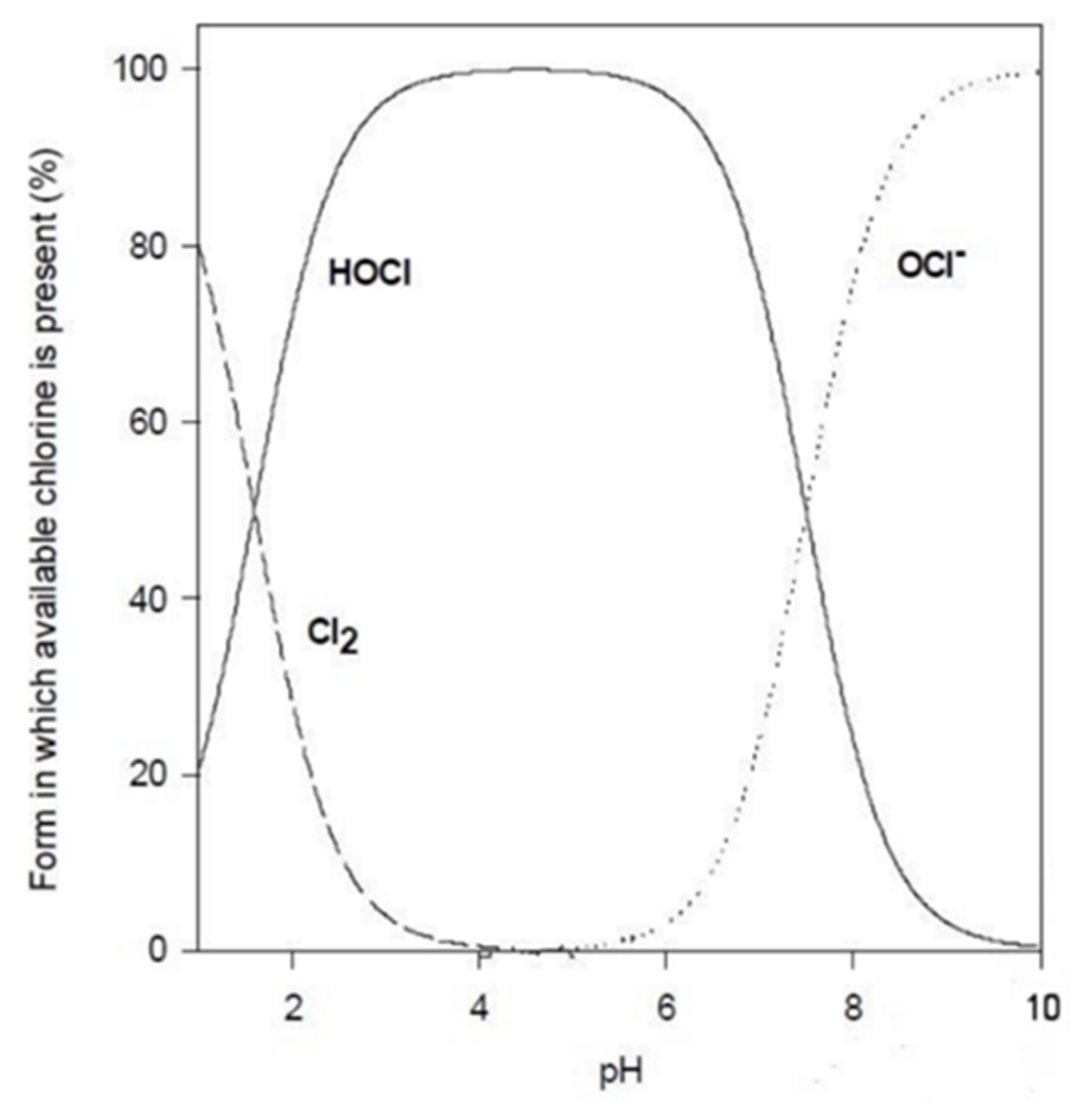
- pH of the solutionThe pH of the solution can affect the efficacy of the disinfection in two ways: a change in the agent itself and a change in the interactions between the microbicide and the microbial cell.For example, several microbicides are effective in their unionized form (Table 3). Thus, the pH level would affect their degree of dissociation and would decrease their overall activity. In contrast, other molecules are more effective in their ionized form. Besides these considerations, it should also be kept in mind that any alteration of the pH level could affect the compound’s stability.As a matter of fact, disinfectant products in the sanitary field are formulated to guarantee, at a certain level of pH, maximum germicidal efficacy.
- FormulationThe formulation of a disinfectant deeply affects its activity. Several excipients, such as solvents, surfactants, thickeners, chelating agents, colors, and fragrances, can be found in these products; they can interact with the microorganisms or with the active itself and ultimately affect the activity of the formulated product. Most of the information on the effect of different excipients on the activity of disinfectants are not available, since they are often trade secrets.
- Length of exposureThe microbicidal activity of chemicals usually increases with the rise of contact time. However, there is not a direct correlation between contact time and microbicidal activity, maybe due to other factors. Contact times for disinfectants are specific for each material and manufacturer. Therefore, all recommendations for use of disinfectants should follow manufacturers’ specifications that must be reported on the label.
- TemperatureTemperature can be an important parameter that influences the pathogen’s survival. High temperature can impact vital proteins and enzymes, as well as the genome. Moreover, high temperature can boost and speed up the germicidal activity of many chemicals resulting in reduced time and improved efficacy. As a drawback, high temperature can accelerate the evaporation of the chemicals and also degrade them. Particular care is needed in using and in stocking such chemicals in tropical regions, where their shelf-life may be reduced because of high room temperature;
- Type of surfaces and precleaning processThe location of microorganisms must be considered as well: to sanitize an instrument with multiple pieces or joints and channels is more difficult than a flat surface. Only surfaces that directly contact the germicide will be sanitized. Indeed, the presence of dirt is the principal reason for disinfection failure, since it could interact with the microbicide, reducing its availability or interact with the microorganisms, giving protection. Moreover, material characteristics of the surface may influence the survival of microorganisms as well: for example, porous surfaces are more difficult to clean and, consequently, to disinfect. Pretreatment of surfaces, especially when visibly soiled, is fundamental to ensure or improve the microbicidal efficacy of the disinfection procedure.
4. Most Common Antimicrobial Classes
4.1. Halogens
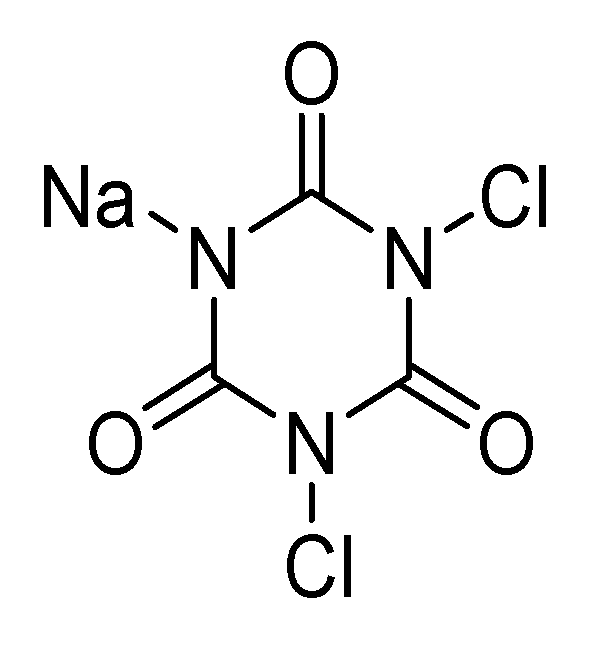
4.1.1. Chlorine Compounds
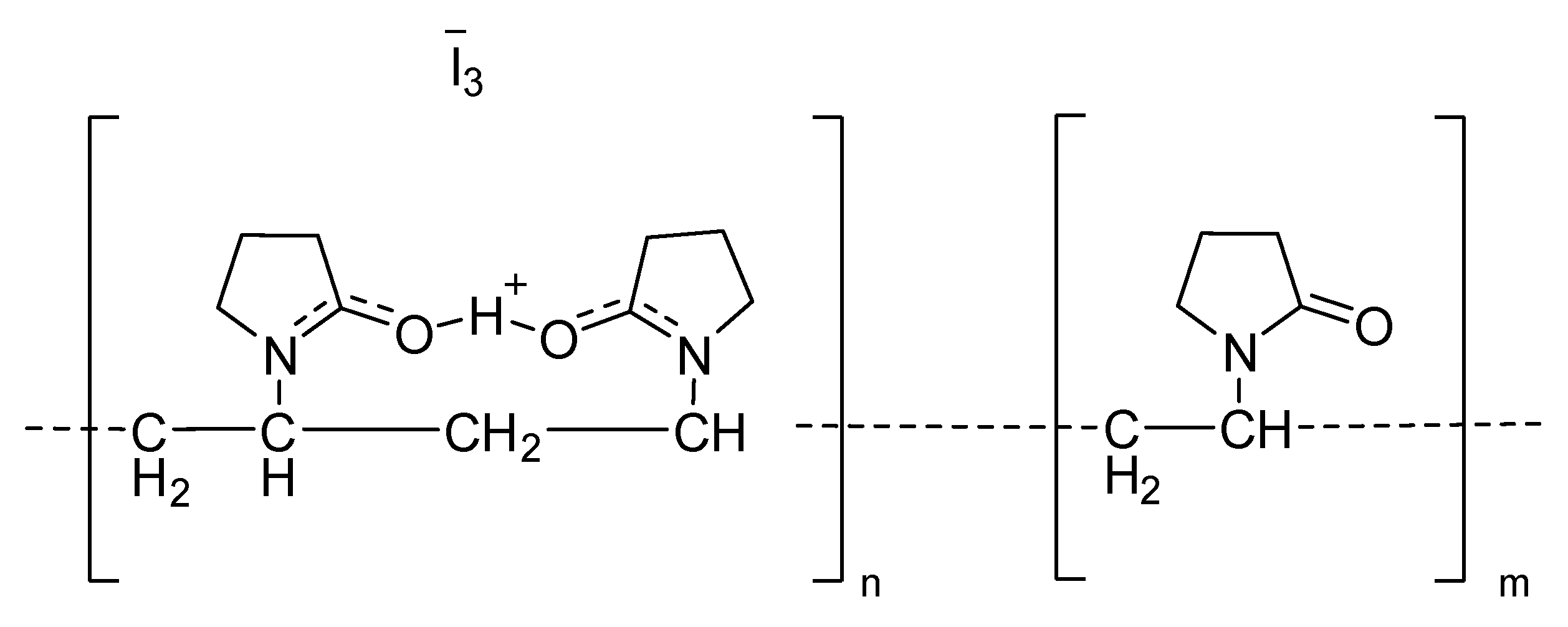
4.1.2. Iodine Compounds
4.2. Alcohols
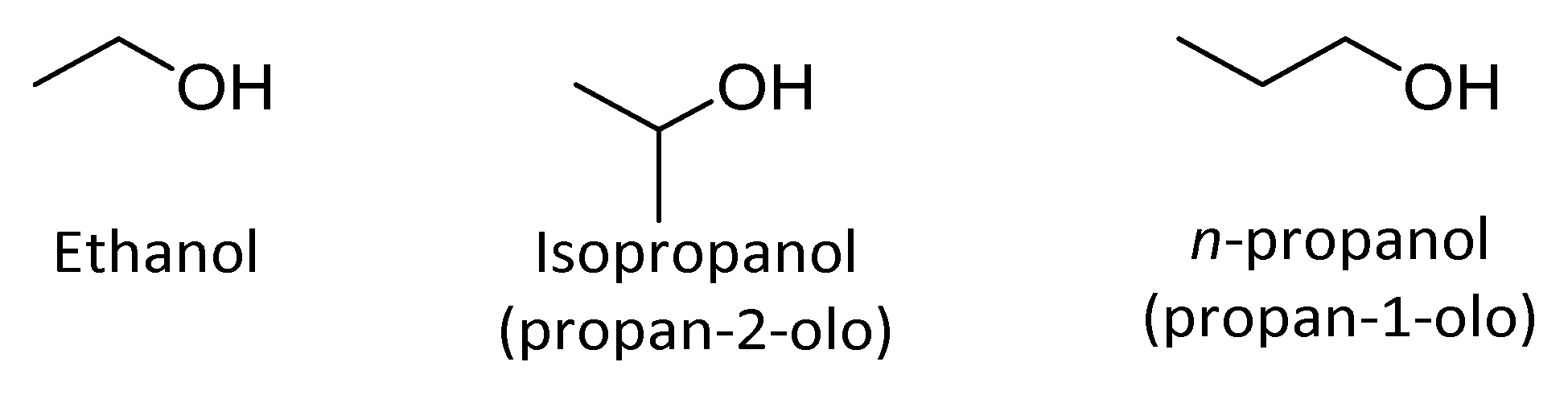
4.2.1. Alifatic Alcohols
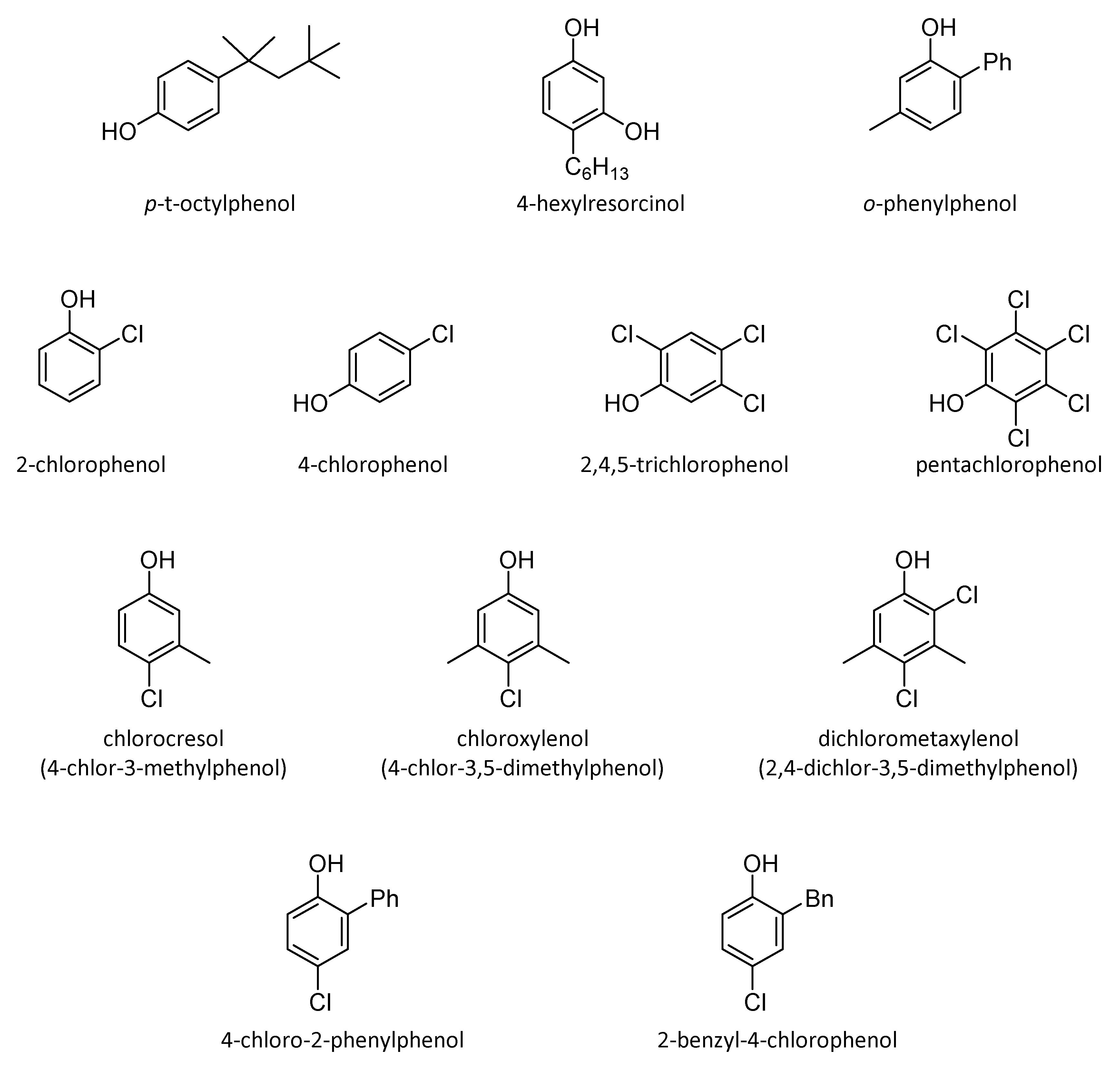
4.2.2. Aromatic Alcohols
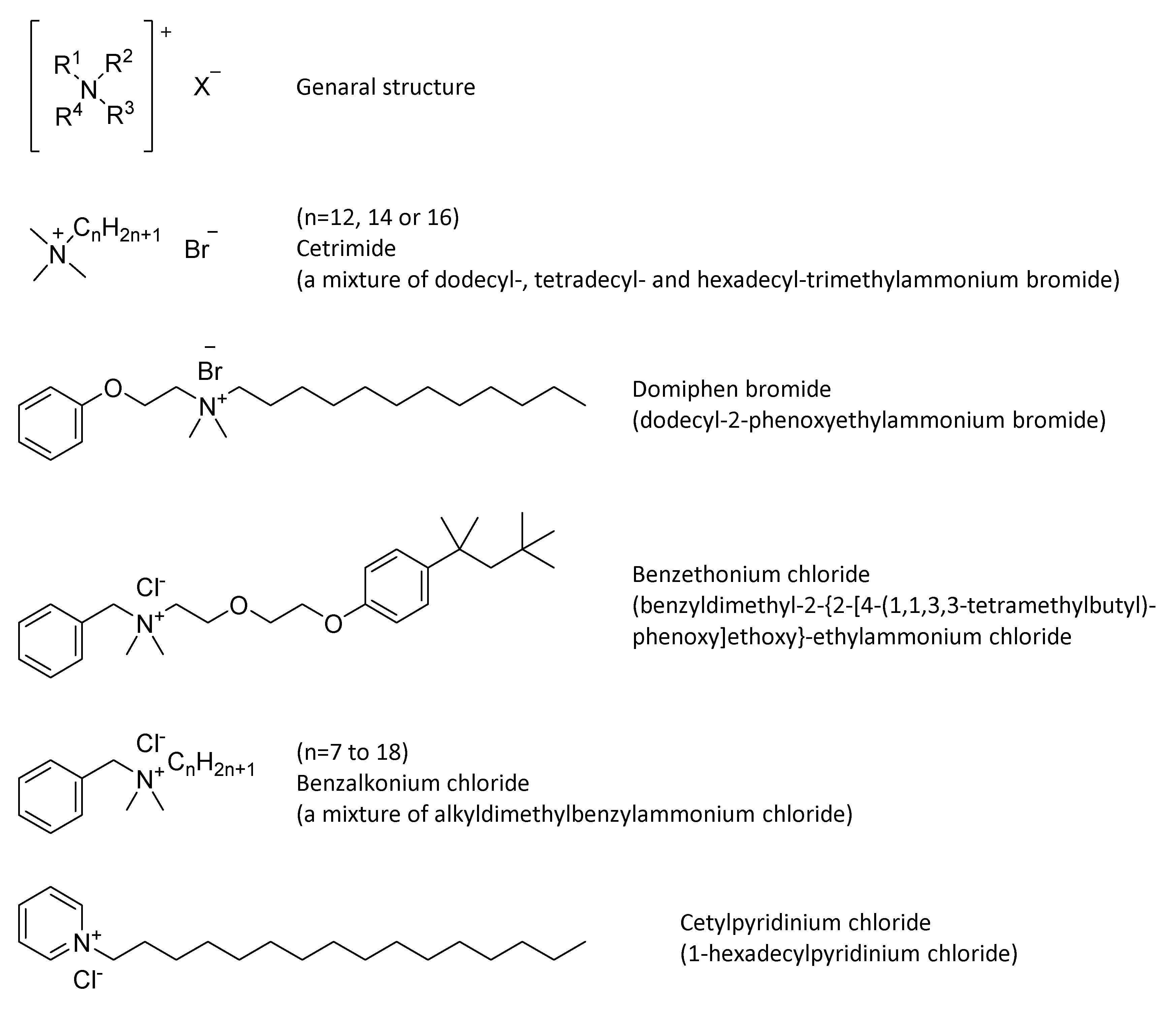
4.3. Quaternary Ammonium Compounds (QACs)
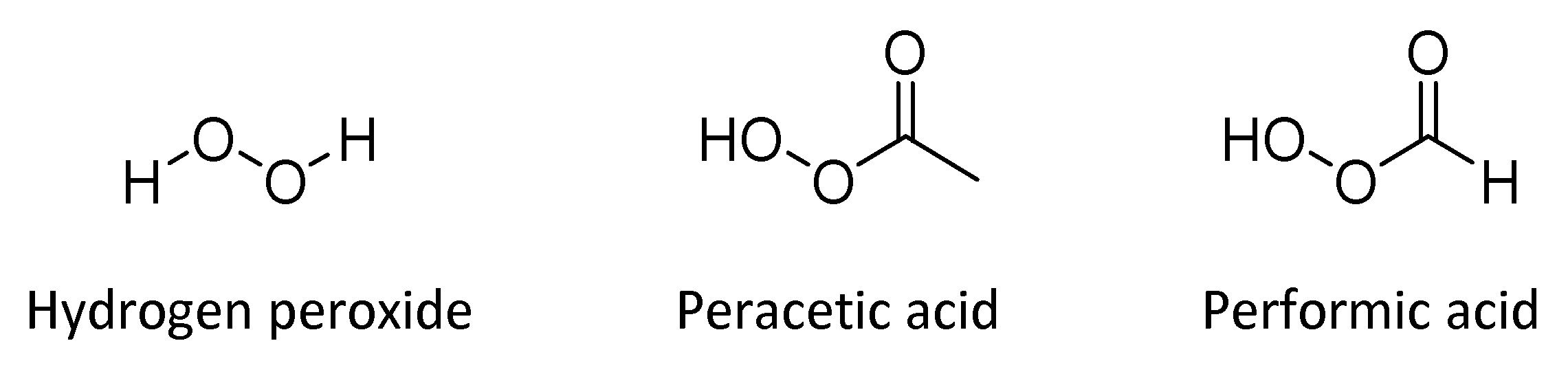
4.4. Hydrogen Peroxide and Peracids
4.5. Ozone
4.6. UV
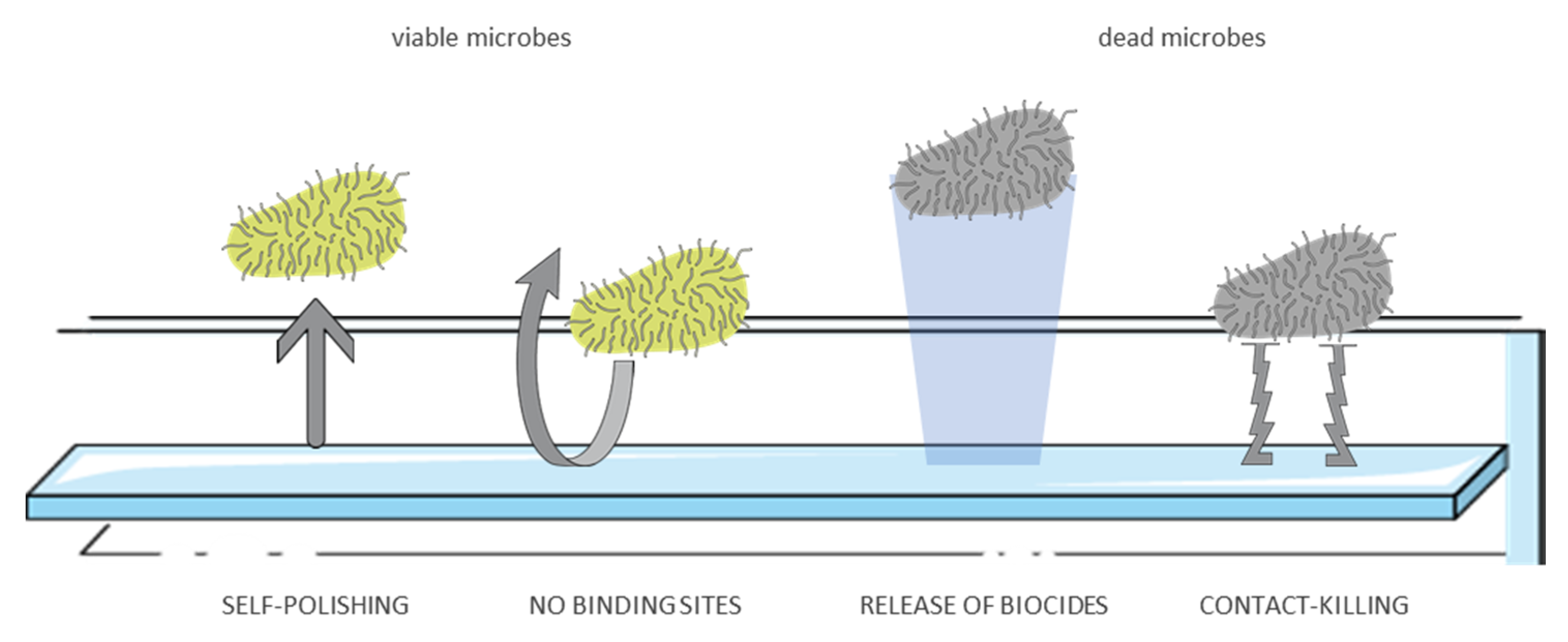
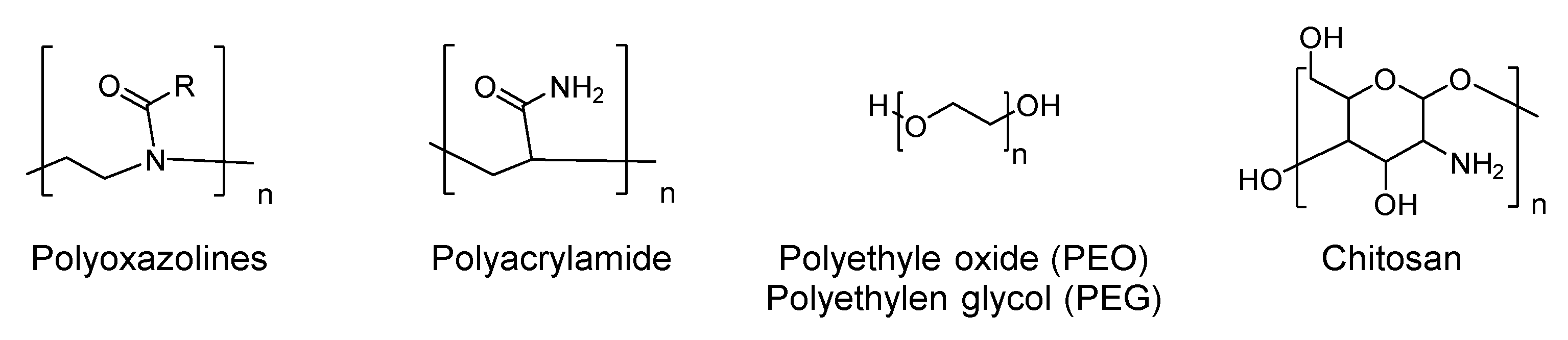
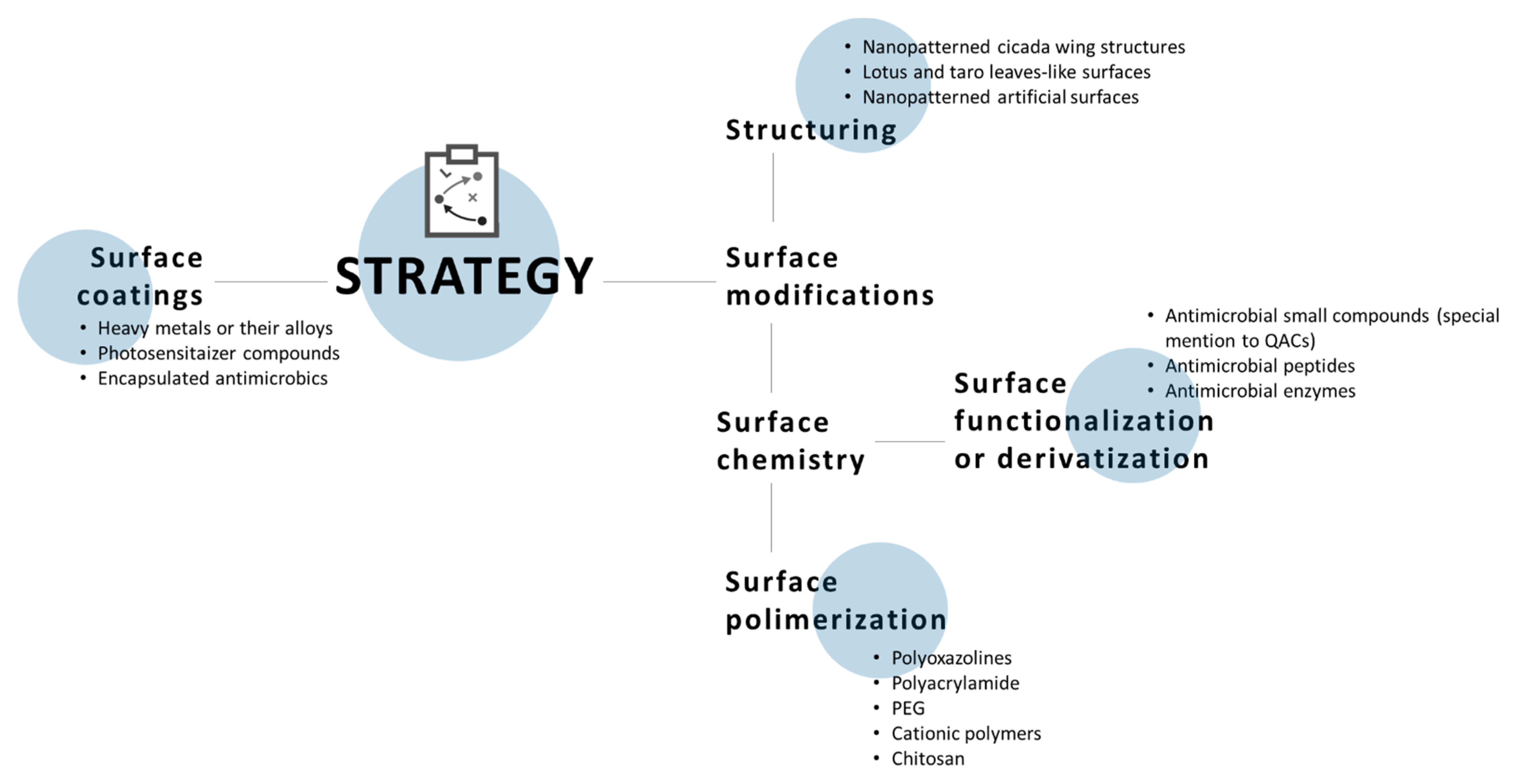
5. Antimicrobial Surfaces
6. Current and Future Issues
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Hayden, M.K.; Blom, D.W.; Lyle, E.A.; Moore, C.G.; Weinstein, R.A. Risk of Hand or Glove Contamination After Contact With Patients Colonized With Vancomycin-Resistant Enterococcus or the Colonized Patients’ Environment. Infect. Control Hosp. Epidemiol. 2008, 29, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Duckro, A.N.; Blom, D.W.; Lyle, E.A.; Weinstein, R.A.; Hayden, M.K. Transfer of vancomycin-resistant enterococci via health care worker hands. Arch. Intern. Med. 2005, 165, 302–307. [Google Scholar] [CrossRef]
- Guerrero, D.M.; Nerandzic, M.M.; Jury, L.A.; Jinno, S.; Chang, S.; Donskey, C.J. Acquisition of spores on gloved hands after contact with the skin of patients with Clostridium difficile infection and with environmental surfaces in their rooms. Am. J. Infect. Control 2012, 40, 556–558. [Google Scholar] [CrossRef]
- Stiefel, U.; Cadnum, J.L.; Eckstein, B.C.; Guerrero, D.M.; Tima, M.A.; Donskey, C.J. Contamination of Hands with Methicillin-Resistant Staphylococcus aureus after Contact with Environmental Surfaces and after Contact with the Skin of Colonized Patients. Infect. Control Hosp. Epidemiol. 2011, 32, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M. Infectious agents and how they cause disease. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001; p. 27114. [Google Scholar]
- Julian, T.R.; Tamayo, F.J.; Leckie, J.O.; Boehm, A.B. Comparison of surface sampling methods for virus recovery from fomites. Appl. Environ. Microbiol. 2011, 77, 6918–6925. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Auer, G.K.; Weibel, D.B. Bacterial Cell Mechanics. Biochemistry 2017, 56, 3710–3724. [Google Scholar] [CrossRef]
- Esko, J.D.; Doering, T.L.; Raetz, C.R. Eubacteria and Archaea. In Essentials of Glycobiology, 2nd ed.; Gerald, W.H., Marilynn, E.E., Eds.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2009; Chapter 20. [Google Scholar]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, Y.F.; Persat, A. Mechanomicrobiology: How bacteria sense and respond to forces. Nat. Rev. Microbiol. 2020, 18, 227–240. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Fajardo-Cavazos, P.; Rebeil, R.; Slieman, T.A.; Riesenman, P.J.; Law, J.F.; Xue, Y. Bacterial endospores and their significance in stress resistance. Antonie Van Leeuwenhoek 2002, 81, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Mallozzi, M.; Viswanathan, V.K.; Vedantam, G. Spore-forming Bacilli and Clostridia in human disease. Future Microbiol. 2010, 5, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Norde, W.; van der Mei, H.C.; Busscher, H.J. Bacterial cell surface deformation under external loading. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef]
- Gristina, A.G.; Costerton, J.W. Bacterial adherence and the glycocalyx and their role in musculoskeletal infection. Orthop. Clin. N. Am. 1984, 15, 517–535. [Google Scholar] [CrossRef]
- Gelderblom, H.R. Structure and Classification of Viruses. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 41. [Google Scholar]
- Summers, W.C. Virus Infection. In Encyclopedia of Microbiology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 546–552. [Google Scholar]
- Lucas, W. Viral Capsids and Envelopes: Structure and Function. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar]
- Louten, J. Virus Structure and Classification. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–29. [Google Scholar]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucans of fungi. A review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef]
- Taylor, T.N.; Osborn, J.M. The importance of fungi in shaping the paleoecosystem. Rev. Palaeobot. Palynol. 1996, 90, 249–262. [Google Scholar] [CrossRef]
- Classification of Biological Agents—Health and Safety Authority. Available online: https://www.hsa.ie/eng/publications_and_forms/publications/biological_agents/biological_agents_code_of_practice_2020.html (accessed on 20 April 2021).
- Hayden, M.K.; Bonten, M.J.M.; Blom, D.W.; Lyle, E.A.; van de Vijver, D.A.M.C.; Weinstein, R.A. Reduction in Acquisition of Vancomycin-Resistant Enterococcus after Enforcement of Routine Environmental Cleaning Measures. Clin. Infect. Dis. 2006, 42, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Dettenkofer, M.; Block, C. Hospital disinfection: Efficacy and safety issues. Curr. Opin. Infect. Dis. 2005, 18, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Johnson, A.C.; Jin, X.; Nakada, N.; Sumpter, J.P. Learning from the past and considering the future of chemicals in the environment. Science 2020, 367, 384–387. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Biosafety Manual, 3rd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Albrich, J.M.; McCarthy, C.A.; Hurst, J.K. Biological reactivity of hypochlorous acid: Implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA 1981, 78, 210–214. [Google Scholar] [CrossRef]
- Brazis, A.R.; Leslie, J.E.; Kabler, P.W.; Woodward, R.L. The inactivation of spores of Bacillus globigii and Bacillus anthracis by free available chlorine. Appl. Microbiol. 1958, 6, 338–342. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 2003, 25, 259–274. [Google Scholar] [CrossRef]
- Winter, J.; Ilbert, M.; Graf, P.C.F.; Özcelik, D.; Jakob, U. Bleach Activates a Redox-Regulated Chaperone by Oxidative Protein Unfolding. Cell 2008, 135, 691–701. [Google Scholar] [CrossRef]
- Adam, L.C.; Suzuki, K.; Gordon, G.; Fábián, I. Hypochlorous Acid Decomposition in the pH 5–8 Region. Inorg. Chem. 1992, 31, 3534–3541. [Google Scholar] [CrossRef]
- White, G.C. Handbook of Chlorination and Alternative Disinfectants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1999; Volume 77. [Google Scholar]
- Heseltine, P. Disinfection, Sterilization, and Preservation, 5th ed. SS Block, ed.; Philadelphia: Lippincott Williams & Wilkins, 2001; 1,504 pages. Infect. Control Hosp. Epidemiol. 2002, 23, 109. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Kuroiwa, K.; Nakayama, H.; Kuwahara, T.; Tamagawa, K.; Hattori, K.; Murakami, K.; Korai, H.; Ohnishi, Y. Augmenting effect of acetic acid for acidification on bactericidal activity of hypochlorite solution. Lett. Appl. Microbiol. 2003, 36, 46–49. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Evaluation of active substances Assessment Report Cholecalciferol PT 14 (Rodenticides). In Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products; ECHA: Helsinki, Finland, 2018. [Google Scholar]
- Iqbal, Q.; Lubeck-Schricker, M.; Wells, E.; Wolfe, M.K.; Lantagne, D. Shelf-Life of Chlorine Solutions Recommended in Ebola Virus Disease Response. PLoS ONE 2016, 11, e0156136. [Google Scholar] [CrossRef]
- Coates, D. A comparison of sodium hypochlorite and sodium dichloroisocyanurate products. J. Hosp. Infect. 1985, 6, 31–40. [Google Scholar] [CrossRef]
- Bloomfield, S.F.; Uso, E.E. The antibacterial properties of sodium hypochlorite and sodium dichloroisocyanurate as hospital disinfectants. J. Hosp. Infect. 1985, 6, 20–30. [Google Scholar] [CrossRef]
- Coates, D. Comparison of sodium hypochlorite and sodium dichloroisocyanurate disinfectants: Neutralization by serum. J. Hosp. Infect. 1988, 11, 60–67. [Google Scholar] [CrossRef]
- Bloomfield, S.F.; Arthur, M.; Begun, K.; Patel, H. Comparative testing of disinfectants using proposed European surface test methods. Lett. Appl. Microbiol. 1993, 17, 119–125. [Google Scholar] [CrossRef]
- Gallandat, K.; Wolfe, M.K.; Lantagne, D. Surface Cleaning and Disinfection: Efficacy Assessment of Four Chlorine Types Using Escherichia coli and the Ebola Surrogate Phi6. Environ. Sci. Technol. 2017, 51, 4624–4631. [Google Scholar] [CrossRef]
- Aarnisalo, K.; Salo, S.; Miettinen, H.; Suihko, M.L.; Wirtanen, G.; Autio, T.; Lundén, J.; Korkeala, H.; Sjöberg, A.M. Bactericidal efficiencies of commercial disinfectants against listeria monocytogenes on surfaces. J. Food Saf. 2000, 20, 237–250. [Google Scholar] [CrossRef]
- Mbithi, J.N.; Springthorpe, V.S.; Sattar, S.A. Chemical Disinfection of Hepatitis A Virus on Environmental Surfaces. Appl. Environ. Microbiol. 1990, 56, 3601–3604. [Google Scholar] [CrossRef]
- Bichsel, Y. Behavior of Iodine Species in Oxidative Processing during Drinking Water Treatment. Ph.D. Thesis, Swiss Federal Institute of Technology Zurich, Zürich, Switzerland, 2000. [Google Scholar]
- Al-Adham, I.; Haddadin, R.; Collier, P. Types of Microbicidal and Microbistatic Agents. In Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 5–70. [Google Scholar]
- Block, C.; Robenshtok, E.; Simhon, A.; Shapiro, M. Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. J. Hosp. Infect. 2000, 46, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Morton, H.E. The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann. N. Y. Acad. Sci. 1950, 53, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, C.E.; Sykes, G. The germicidal effect of alcohol with special reference to its action on bacterial spores. Pharm. J. 1936, 137, 79–81. [Google Scholar]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities; CDC: Atlanta, GA, USA, 2008.
- Doerrbecker, J.; Friesland, M.; Ciesek, S.; Erichsen, T.J.; Mateu-Gelabert, P.; Steinmann, J.; Pietschmann, T.; Steinmann, E. Inactivation and survival of hepatitis C virus on inanimate surfaces. J. Infect. Dis. 2011, 204, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Chemical Disinfectants. Disinfection & Sterilization Guidelines. Guidelines Library. Infection Control. CDC. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html# (accessed on 8 January 2021).
- Gilbert, P.; McBain, A.J. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin. Microbiol. Rev. 2003, 16, 189–208. [Google Scholar] [CrossRef]
- Fraise, A.P. Choosing disinfectants. J. Hosp. Infect. 1999, 43, 255–264. [Google Scholar] [CrossRef]
- Harrington, C.; Walker, H. The Germicidal Action of Alcohol. Boston Med. Surg. J. 1903, 148, 548–552. [Google Scholar] [CrossRef]
- Salvage, R.; Hull, C.M.; Kelly, D.E.; Kelly, S.L. Use of 70% alcohol for the routine removal of microbial hard surface bioburden in life science cleanrooms. Future Microbiol. 2014, 9, 1123–1130. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Monitoring and improving the effectiveness of surface cleaning and disinfection. Am. J. Infect. Control 2016, 44, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Rotter, M.L. Arguments for alcoholic hand disinfection. J. Hosp. Infect. 2001, 48, S4–S8. [Google Scholar] [CrossRef]
- Withell, E.R. The evaluation of bactericides. J. Hyg. (Lond.) 1942, 42, 339–353. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Lambert, P.A.; Hammond, S.M. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem. Biophys. Res. Commun. 1973, 54, 796–799. [Google Scholar] [CrossRef]
- Judis, J. Mechanism of action of phenolic disinfectants IV. Effects on induction of and accessibility of substrate to β-galactosidase in Escherichia coli. J. Pharm. Sci. 1965, 54, 417–420. [Google Scholar] [CrossRef]
- Suter, C.M. Relationships between the structure and the bactericidal properties of phenols. Chem. Rev. 1941, 28, 269–299. [Google Scholar] [CrossRef]
- Terleckyj, B.; Axler, D.A. Quantitative neutralization assay of fungicidal activity of disinfectants. Antimicrob. Agents Chemother. 1987, 31, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.S.; McDougal, J.S.; Loskoski, S.L. Disinfection and Inactivation of the Human T Lymphotropic Virus Type III/Lymphadenopathy-Associated Virus. J. Infect. Dis. 1985, 152, 400–403. [Google Scholar] [CrossRef]
- Sattar, S.A.; Springthorpe, V.S. Survival and disinfectant inactivation of the human immunodeficiency virus: A critical review. Rev. Infect. Dis. 1991, 13, 430–447. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Eklund, C.A.; Trost, P.A.; Jinneman, K.C.; Abeyta, C.; Kaysner, C.A.; Hill, W.E. Comparative sensitivity of 13 species of pathogenic bacteria to seven chemical germicides. Am. J. Infect. Control 1997, 25, 335–339. [Google Scholar] [CrossRef]
- Narang, H.K.; Codd, A.A. Action of commonly used disinfectants against enteroviruses. J. Hosp. Infect. 1983, 4, 209–212. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: An Overview and Current Issues. Infect. Dis. Clin. 2016, 30, 609–637. [Google Scholar] [CrossRef]
- Doan, H.M.; Keith, L.; Shennan, A.T. Phenol and Neonatal Jaundice. Pediatrics 1979, 64, 324–325. [Google Scholar] [PubMed]
- Calafat, A.M.; Weuve, J.; Ye, X.; Jia, L.T.; Hu, H.; Ringer, S.; Huttner, K.; Hauser, R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009, 117, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Role of Quaternary Ammonium Compounds on Antimicrobial Resistance in the Environment. In Antimicrobial Resistance in the Environment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 349–387. [Google Scholar]
- Fumagalli, L.; Regazzoni, L.G.; Straniero, V.; Valoti, E.; Aldini, G.; Vistoli, G.; Carini, M.; Picozzi, C. Stressed degradation studies of domiphen bromide by LC-ESI-MS/MS identify a novel promising antimicrobial agent. J. Pharm. Biomed. Anal. 2018, 159, 224–228. [Google Scholar] [CrossRef]
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharmacol. 2013, 67, 456–467. [Google Scholar] [CrossRef]
- Zinchenko, A.A.; Sergeyev, V.G.; Yamabe, K.; Murata, S.; Yoshikawa, K. DNA compaction by divalent cations: Structural specificity revealed by the potentiality of designed quaternary diammonium salts. ChemBioChem 2004, 5, 360–368. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Rutala, W.A.; White, M.S.; Gergen, M.F.; Weber, D.J. Bacterial Contamination of Keyboards: Efficacy and Functional Impact of Disinfectants. Infect. Control Hosp. Epidemiol. 2006, 27, 372–377. [Google Scholar] [CrossRef]
- Brown, E.; Dhanireddy, K.; Teska, P.; Eifert, J.; Williams, R.C.; Boyer, R. Influence of drying time on prewetted disinfectant towelettes to disinfect glass surfaces. Am. J. Infect. Control 2020, 48, 846–848. [Google Scholar] [CrossRef]
- Cousins, C.M.; Clegg, L.F.L. The effect of water hardness and temperature on water sterilization by mixtures of detergents and quaternary ammonium compounds. J. Appl. Bacteriol. 1956, 19, 250–255. [Google Scholar] [CrossRef]
- Song, X.; Vossebein, L.; Zille, A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: A review. Antimicrob. Resist. Infect. Control 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Kopferschmitt-Kubler, M.C.; Moreau, C.; Popin, E.; Blaumeiser, M.; Pauli, G. Quaternary ammonium compounds and occupational asthma. Int. Arch. Occup. Environ. Health 2000, 73, 423–427. [Google Scholar] [CrossRef]
- Schyllert, C.; Rönmark, E.; Andersson, M.; Hedlund, U.; Lundbäck, B.; Hedman, L.; Lindberg, A. Occupational exposure to chemicals drives the increased risk of asthma and rhinitis observed for exposure to vapours, gas, dust and fumes: A cross-sectional population-based study. Occup. Environ. Med. 2016, 73, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zock, J.P.; Plana, E.; Jarvis, D.; Antó, J.M.; Kromhout, H.; Kennedy, S.M.; Künzli, N.; Villani, S.; Olivieri, M.; Torén, K.; et al. The use of household cleaning sprays and adult asthma: An international longitudinal study. Am. J. Respir. Crit. Care Med. 2007, 176, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Degenhardt, S.; Lackner, S.; Jesse, K.; von Baum, H.; Ostermeyer, C. Poorly processed reusable surface disinfection tissue dispensers may be a source of infection. BMC Infect. Dis. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Baldry, M.G.C. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 1983, 54, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Absalan, A.; Ehrampoush, M.; Davoudi, M.; Vakili, T.; Ebrahimi, A. Antibacterial effects of hydrogen peroxide and silver composition on selected pathogenic enterobacteriaceae. Int. J. Environ. Health Eng. 2012, 1, 23. [Google Scholar] [CrossRef]
- Steinberg, D.; Heling, I.; Daniel, I.; Ginsburg, I. Antibacterial synergistic effect of chlorhexidine and hydrogen peroxide against Streptococcus sobrinus, Streptococcus faecalis and Staphylococcus aureus. J. Oral Rehabil. 1999, 26, 151–156. [Google Scholar] [CrossRef]
- Martin, N.L.; Bass, P.; Liss, S.N. Antibacterial properties and mechanism of activity of a novel silver-stabilized hydrogen peroxide. PLoS ONE 2015, 10, e0131345. [Google Scholar] [CrossRef]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In Patai’s Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–34. [Google Scholar]
- Fraise, A.P.; Lambert, P.A.; Maillard, J.Y. Russell, Hugo and Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Totaro, M.; Casini, B.; Profeti, S.; Tuvo, B.; Privitera, G.; Baggiani, A. Role of hydrogen peroxide vapor (HPV) for the disinfection of hospital surfaces contaminated by multiresistant bacteria. Pathogens 2020, 9, 408. [Google Scholar] [CrossRef]
- Weber, D.J.; Kanamori, H.; Rutala, W.A. “No touch” technologies for environmental decontamination: Focus on ultraviolet devices and hydrogen peroxide systems. Curr. Opin. Infect. Dis. 2016, 29, 424–431. [Google Scholar] [CrossRef]
- Tuladhar, E.; Terpstra, P.; Koopmans, M.; Duizer, E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J. Hosp. Infect. 2012, 80, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Orlando, P.; Cristina, M.L.; Dallera, M.; Ottria, G.; Vitale, A.; Badolati, G. Surface disinfection: Evaluation of the efficacy of a nebulization system spraying hydrogen peroxide. J. Prev. Med. Hyg. 2008, 49, 116–119. [Google Scholar] [CrossRef]
- Domínguez Henao, L.; Turolla, A.; Antonelli, M. Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: A review. Chemosphere 2018, 213, 25–40. [Google Scholar] [CrossRef]
- Cutts, T.; Kasloff, S.; Safronetz, D.; Krishnan, J. Decontamination of common healthcare facility surfaces contaminated with SARS-CoV-2 using peracetic acid dry fogging. J. Hosp. Infect. 2021, 109, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Mcdonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. New disinfection and sterilization methods. Emerg. Infect. Dis. 2001, 7, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Gehr, R.; Chen, D.; Moreau, M. Performic acid (PFA): Tests on an advanced primary effluent show promising disinfection performance. Water Sci. Technol. 2009, 59, 89–96. [Google Scholar] [CrossRef]
- Rickloff, J.R. An evaluation of the sporicidal activity of ozone. Appl. Environ. Microbiol. 1987, 53, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Giovannangeli, F.; Rotunno, S.; Trombetta, C.M.; Montomoli, E. Water and air ozone treatment as an alternative sanitizing technology. J. Prev. Med. Hyg. 2017, 58, E48–E52. [Google Scholar] [CrossRef]
- Breidablik, H.J.; Lysebo, D.E.; Johannessen, L.; Skare, Å.; Andersen, J.R.; Kleiven, O.T. Ozonized water as an alternative to alcohol-based hand disinfection. J. Hosp. Infect. 2019, 102, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Megahed, A.; Aldridge, B.; Lowe, J. The microbial killing capacity of aqueous and gaseous ozone on different surfaces contaminated with dairy cattle manure. PLoS ONE 2018, 13, e0196555. [Google Scholar] [CrossRef]
- Burleson, G.R.; Murray, T.M.; Pollard, M. Inactivation of Viruses and Bacteria by Ozone, With and Without Sonication. Appl. Microbiol. 1975, 29, 340–344. [Google Scholar] [CrossRef]
- Albert, S.; Amarilla, A.A.; Trollope, B.; Sng, J.D.J.; Setoh, Y.X.; Deering, N.; Modhiran, N.; Weng, S.H.; Melo, M.C.; Hutley, N.; et al. Assessing the potential of unmanned aerial vehicle spraying of aqueous ozone as an outdoor disinfectant for SARS-CoV-2. Environ. Res. 2021, 196, 110944. [Google Scholar] [CrossRef]
- Megahed, A.; Aldridge, B.; Lowe, J. Comparative study on the efficacy of sodium hypochlorite, aqueous ozone, and peracetic acid in the elimination of Salmonella from cattle manure contaminated various surfaces supported by Bayesian analysis. PLoS ONE 2019, 14, e0217428. [Google Scholar] [CrossRef] [PubMed]
- Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19. Gases 2020, 1, 19–32. [Google Scholar] [CrossRef]
- Selma, M.V.; Allende, A.; López-Gálvez, F.; Conesa, M.A.; Gil, M.I. Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. 2008, 25, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Zoutman, D.; Shannon, M.; Mandel, A. Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces. Am. J. Infect. Control 2011, 39, 873–879. [Google Scholar] [CrossRef]
- Fang, J.; Liu, H.; Shang, C.; Zeng, M.; Ni, M.; Liu, W.E. coli and bacteriophage MS2 disinfection by UV, ozone and the combined UV and ozone processes. Front. Environ. Sci. Eng. 2014, 8, 547–552. [Google Scholar] [CrossRef]
- Fan, L.; Song, J.; Hildebrand, P.D.; Forney, C.F. Interaction of ozone and negative air ions to control micro-organisms. J. Appl. Microbiol. 2002, 93, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.F. Ultraviolet Disinfection. In Microbiology of Waterborne Diseases, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 617–630. [Google Scholar]
- Bolton, J.R.; Dussert, B.; Bukhari, Z.; Hargy, T.; Clancy, J.L. Inactivation of Cryptosporidium parvum by medium-pressure ultraviolet light in finished drinking water. In Proceedings of the AWWA 1998 Annual Conference, Dallas, TX, USA, 21–25 June 1998; Volume A, pp. 389–403. [Google Scholar]
- Rutala, W.A.; Gergen, M.F.; Weber, D.J. Room Decontamination with UV Radiation. Infect. Control Hosp. Epidemiol. 2010, 31, 1025–1029. [Google Scholar] [CrossRef]
- Lindblad, M.; Tano, E.; Lindahl, C.; Huss, F. Ultraviolet-C decontamination of a hospital room: Amount of UV light needed. Burns 2020, 46, 842–849. [Google Scholar] [CrossRef]
- Oguma, K.; Katayama, H.; Ohgaki, S. Photoreactivation of Escherichia coli after low- or medium-pressure UV disinfection determined by an endonuclease sensitive site assay. Appl. Environ. Microbiol. 2002, 68, 6029–6035. [Google Scholar] [CrossRef] [PubMed]
- Casini, B.; Tuvo, B.; Cristina, M.L.; Spagnolo, A.M.; Totaro, M.; Baggiani, A.; Privitera, G.P. Evaluation of an ultraviolet C (UVC) light-emitting device for disinfection of high touch surfaces in hospital critical areas. Int. J. Environ. Res. Public Health 2019, 16, 3572. [Google Scholar] [CrossRef]
- Kumar, A.; Sagdeo, A.; Sagdeo, P.R. Possibility of using ultraviolet radiation for disinfecting the novel COVID-19. Photodiagn. Photodyn. Ther. 2021, 34, 102234. [Google Scholar] [CrossRef]
- Kitagawa, H.; Nomura, T.; Nazmul, T.; Omori, K.; Shigemoto, N.; Sakaguchi, T.; Ohge, H. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control 2021, 49, 299–301. [Google Scholar] [CrossRef]
- Jelden, K.C.; Gibbs, S.G.; Smith, P.W.; Hewlett, A.L.; Iwen, P.C.; Schmid, K.K.; Lowe, J.J. Comparison of hospital room surface disinfection using a novel ultraviolet germicidal irradiation (UVGI) generator. J. Occup. Environ. Hyg. 2016, 13, 690–698. [Google Scholar] [CrossRef]
- Umezawa, K.; Asai, S.; Inokuchi, S.; Miyachi, H. A comparative study of the bactericidal activity and daily disinfection housekeeping surfaces by a new portable pulsed UV radiation device. Curr. Microbiol. 2012, 64, 581–587. [Google Scholar] [CrossRef]
- Kovach, C.R.; Taneli, Y.; Neiman, T.; Dyer, E.M.; Arzaga, A.J.A.; Kelber, S.T. Evaluation of an ultraviolet room disinfection protocol to decrease nursing home microbial burden, infection and hospitalization rates. BMC Infect. Dis. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Gergen, M.F.; Tande, B.M.; Weber, D.J. Rapid Hospital Room Decontamination Using Ultraviolet (UV) Light with a Nanostructured UV-Reflective Wall Coating. Infect. Control Hosp. Epidemiol. 2013, 34, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C. Antimicrobial surfaces. Adv. Polym. Sci. 2010, 240, 193–217. [Google Scholar]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Webb, H.K.; Hasan, J.; Truong, V.K.; Crawford, R.J.; Ivanova, E.P. Nature Inspired Structured Surfaces for Biomedical Applications. Curr. Med. Chem. 2011, 18, 3367–3375. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.H.T.; Webb, H.K.; Hasan, J.; Tobin, M.J.; Crawford, R.J.; Ivanova, E.P. Dual role of outer epicuticular lipids in determining the wettability of dragonfly wings. Colloids Surf. B Biointerfaces 2013, 106, 126–134. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Webb, H.K.; Hasan, J.; Truong, V.K.; Lamb, R.N.; Duan, X.; Tobin, M.J.; Mahon, P.J.; Crawford, R.J. Molecular Organization of the Nanoscale Surface Structures of the Dragonfly Hemianax papuensis Wing Epicuticle. PLoS ONE 2013, 8, e67893. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef]
- Weaver, L.; Noyce, J.O.; Michels, H.T.; Keevil, C.W. Potential action of copper surfaces on meticillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2010, 109, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Jin, H.E. Antimicrobial activity of zinc oxide nano/microparticles and their combinations against pathogenic microorganisms for biomedical applications: From physicochemical characteristics to pharmacological aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Valeria Prado, J.; Roberto Vidal, A.; Claudia Durán, T. Aplicación de la capacidad bactericida del cobre en la práctica médica. Rev. Med. Chil. 2012, 140, 1325–1332. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef]
- Villapún, V.M.; Dover, L.G.; Cross, A.; González, S. Antibacterial metallic touch surfaces. Materials 2016, 9, 736. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Mendonça, P.V.; Branco, R.; Sousa, R.; Dias, C.; Serra, A.C.; Fernandes, J.R.; Magalhães, F.D.; Morais, P.V.; Coelho, J.F.J. Light-Activated Antimicrobial Surfaces Using Industrial Varnish Formulations to Mitigate the Incidence of Nosocomial Infections. ACS Appl. Mater. Interfaces 2021, 13, 7567–7579. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Y.Y.; Kushida, Y.; Bhayana, B.; Hamblin, M.R. Broad-spectrum antimicrobial photocatalysis mediated by titanium dioxide and UVA is potentiated by addition of bromide ion via formation of hypobromite. Free Radic. Biol. Med. 2016, 95, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Visnapuu, M.; Rosenberg, M.; Truska, E.; Nõmmiste, E.; Šutka, A.; Kahru, A.; Rähn, M.; Vija, H.; Orupõld, K.; Kisand, V.; et al. UVA-induced antimicrobial activity of ZnO/Ag nanocomposite covered surfaces. Colloids Surf. B Biointerfaces 2018, 169, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical Instruments. ACS Appl. Mater. Interfaces 2009, 1, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Rudra, J.S.; Dave, K.; Haynie, D.T. Antimicrobial polypeptide multilayer nanocoatings. J. Biomater. Sci. Polym. Ed. 2006, 17, 1301–1315. [Google Scholar] [CrossRef]
- Waschinski, C.J.; Herdes, V.; Schueler, F.; Tiller, J.C. Influence of satellite groups on telechelic antimicrobial functions of polyoxazolines. Macromol. Biosci. 2005, 5, 149–156. [Google Scholar] [CrossRef]
- Fundeanu, I.; van der Mei, H.C.; Schouten, A.J.; Busscher, H.J. Polyacrylamide brush coatings preventing microbial adhesion to silicone rubber. Colloids Surf. B 2008, 64, 297–301. [Google Scholar] [CrossRef]
- Chapman, R.G.; Ostuni, E.; Liang, M.N.; Meluleni, G.; Kim, E.; Yan, L.; Pier, G.; Warren, H.S.; Whitesides, G.M. Polymeric thin films that resist the adsorption of proteins and the adhesion of bacteria. Langmuir 2001, 17, 1225–1233. [Google Scholar] [CrossRef]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef]
- Alves, D.; Olívia Pereira, M. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef]
- Zubris, D.; Minbiole, K.; Wuest, W. Polymeric Quaternary Ammonium Compounds: Versatile Antimicrobial Materials. Curr. Top. Med. Chem. 2016, 17, 305–318. [Google Scholar] [CrossRef]
- Grigoras, A.G. Natural and synthetic polymeric antimicrobials with quaternary ammonium moieties: A review. Environ. Chem. Lett. 2021, 1, 3. [Google Scholar]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad. 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Amsalu, A.; Sapula, S.A.; Lopes, M.D.B.; Hart, B.J.; Nguyen, A.H.; Drigo, B.; Turnidge, J.; Leong, L.E.; Venter, H. Efflux pump-driven antibiotic and biocide cross-resistance in pseudomonas Aeruginosa isolated from different ecological Niches: A case study in the development of multidrug resistance in environmental hotspots. Microorganisms 2020, 8, 1647. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Chakraborty, R.; Mandal, S.M. Biocides and health-care agents are more than just antibiotics: Inducing cross to co-resistance in microbes. Ecotoxicol. Environ. Saf. 2019, 174, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Li, J.; Mao, L.; Nguyen, S.H.; Duarte, T.; Coin, L.; Bond, P.; Yuan, Z.; Guo, J. Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera. Environ. Int. 2018, 121, 1217–1226. [Google Scholar] [CrossRef]
- Jennings, M.C.; Forman, M.E.; Duggan, S.M.; Minbiole, K.P.C.; Wuest, W.M. Efflux Pumps Might Not Be the Major Drivers of QAC Resistance in Methicillin-Resistant Staphylococcus aureus. ChemBioChem 2017, 18, 1573–1577. [Google Scholar] [CrossRef]
- Morrison, K.R.; Allen, R.A.; Minbiole, K.P.C.; Wuest, W.M. More QACs, more questions: Recent advances in structure activity relationships and hurdles in understanding resistance mechanisms. Tetrahedron Lett. 2019, 60, 150935. [Google Scholar] [CrossRef]
- Dukan, S.; Touati, D. Hypochlorous acid stress in Escherichia coli: Resistance, DNA damage, and comparison with hydrogen peroxide stress. J. Bacteriol. 1996, 178, 6145–6150. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Simões, L.C.; Simões, M. Biocides. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 478–490. [Google Scholar]
- Siedlecka, A. Antibiotic and Disinfectant Resistance in Tap Water Strains—Insight into the Resistance of Environmental Bacteria. Pol. J. Microbiol. 2021, 70, 57–67. [Google Scholar] [CrossRef] [PubMed]
- McBain, A.J.; Bartolo, R.G.; Catrenich, C.E.; Charbonneau, D.; Ledder, R.G.; Price, B.B.; Gilbert, P. Exposure of sink drain microcosms to triclosan: Population dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 2003, 69, 5433–5442. [Google Scholar] [CrossRef]
- Kümmerer, K. Resistance in the environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
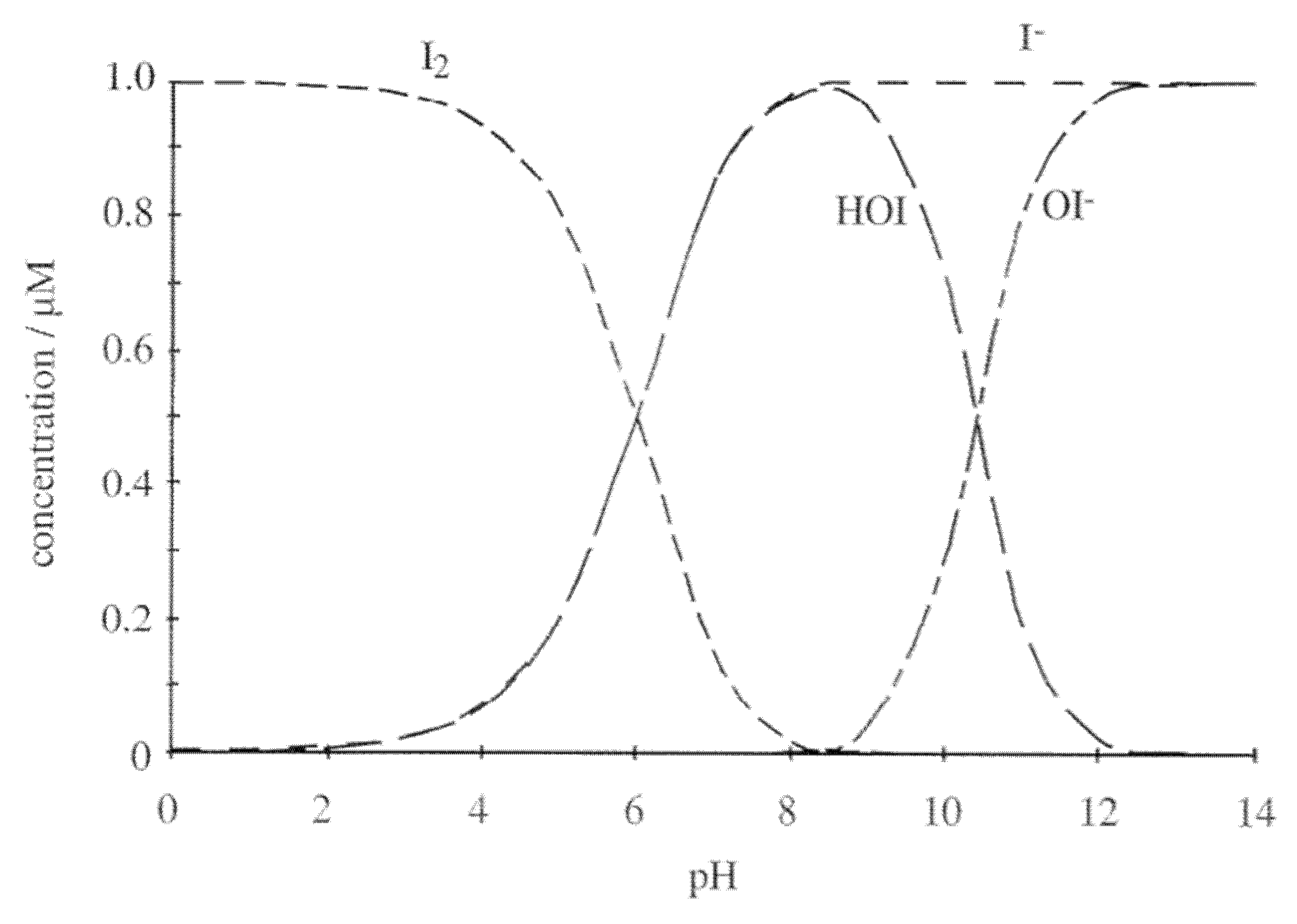
| Bacterial Species | Clinical Manifestation |
|---|---|
| B. anthracis | anthrax |
| B. cereus | foodborne illness |
| B. subtilis | not pathogen |
| C. botulinum | botulism |
| C. perfringens | gas gangrene |
| C. tetani | tetanus |
| Risk Classification | Description | Examples | Heading |
|---|---|---|---|
| Category 1 | Pathogen with a low probability of developing diseases in the human organism | Nonpathogenic strains of Escherichia |  |
| Category 2 | Pathogen that may cause pathology in humans and be a potential hazard for workers; it’s unlikely that can be spread in the community; usually, there are effective treatments | Measles virus, Salmonella, Legionella | |
| Category 3 | Pathogen that may cause severe illness in humans and be a serious hazard for workers; the biological agent may spread in the community, but usually effective treatments are available | HIV, Bacillus anthracis, HBV, HCV, Mycobacterium tuberculosis SARS-CoV-2 | |
| Category 4 | Pathogen that may cause severe illness in humans and may be a serious hazard for workers; the biological agent can spread in the community, and usually, there are no effective treatments available. Pathogens with a low probability of developing diseases in human organisms. Pathogens that may cause pathology in humans and be a potential hazard for workers; it is unlikely that they can be spread in the community; usually, there are effective treatments | Ebola virus, Lassa virus, Smallpox virus. Nonpathogenic strains of Escherichia Measles virus, Salmonella, Legionella |
| Activity as Environmental pH Increases | Classes of Disinfectants | Mechanisms |
|---|---|---|
| Decreased activity | Phenols and organic acids | Increase in the degree of dissociation of the molecules |
| Hypochlorites | Undissociated hypochlorous acid is the most fast-acting species | |
| Iodine | At low pH, iodine, the most powerful antimicrobial species, is the dominating one | |
| Increased activity | Quaternary ammonium compounds (QACs) | Increase in the degree of ionization of bacterial surface groups leading to an increase in binding |
| Chlorine Type | Use Condition | Advantages | Disadvantages | |
|---|---|---|---|---|
| Clean Condition | Dirty Condition | |||
| Sodium hypochlorite solution (5% available chlorine) | 20 mL/L | 100 mL/L | Can be local (stabilized form) Can be on-side (no stabilized form Does not clog pipes | Shorter shelf life Difficult to ship Low stability (no stabilized form) |
| High-test hypochlorite (70% available chlorine) | 1.4 g/L | 7.0 g/L | Easy to ship Long shelf life | Explosive |
| Sodium dichloroisocyanurate powder (60% available chlorine) | 1.7 g/L | 8.5 g/L | Easy to ship Long shelf life Does not clog pipes | Smell |
| Sodium dichloroisocyanurate tablets (1.5 g available chlorine per tablet) | 1 tablet per L | 4 tablets per L | Easy to ship Long shelf life Does not clog pipes | Smell |
| Disinfectant | Mechanism of Action | Cellular Effect | Antimicrobial Effect | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Chlorine compounds | Oxidation of side chains amino acids in proteins | Unfolding tertiary structure and protein aggregation | Bactericidal, fungicidal, virucidal sporicidal | -Not flammable -Fast-acting -Low-cost -Resistant to water hardness -Relatively stable | -Salt residues -Corrosive to metals -Affected by organic matter -Fabric discoloration -Potential production of trihalomethane -Irritating odor at high concentrations |
| Iodine compounds | Oxidation of thiol groups to disulfides in proteins | Modification of structural protein and/or alterations in enzyme activities | Bactericidal, virucidal | -Not flammable | -Limited spectrum of activity -Degradation of silicone catheters -Staining for surfaces |
| Alcohols | Denaturation and precipitations of cytoplasmic and membrane proteins | Alteration in metabolic processes, membrane damage | Bactericidal, fungicidal, virucidal | -Fast-acting -Noncorrosive -Nonstaining -Suitable for small surfaces disinfection | -Not sporicidal -Affected by organic matter -No cleaning properties -Deterioration of some instruments -Flammable -Rapid evaporation |
| Phenols | Denaturation of cytoplasmic and membrane proteins | Leakage of essential metabolites, release of K+, membrane damage, cytoplasmic coagulation | Bactericidal, fungicidal, virucidal | -Low costs -Not flammable -Nonstaining | -Rapid absorption by porous materials and irritate tissues -Potential depigmentation of skin -Hyperbilirubinemia in infants |
| Quaternary ammonium compounds | Binding to phosphates and fatty acid chains in phospholipids of cell membrane and DNA | Depolarization, membrane damage, cytoplasmic coagulation | Bactericidal, fungicidal, virucidal (enveloped viruses) | -Good cleaning agents -Surface compatible -Long antimicrobial activity -Low costs | -Not sporicidal -Affected by water hardness -Asthma after benzalkonium chloride exposure -Affected by organic matter |
| Hydrogen peroxide and peracids | Oxidation of thiol groups to disulfides in proteins | Modification of structural protein and/or alterations in enzyme activities | Bactericidal, fungicidal, virucidal | -Fast-acting -Safe for workers -Non-toxic by-products -Surface compatible -Nonstaining -Odorless -Not flammable | -More expensive compared to other disinfectants -Not sporicidal at low concentrations |
| Ozone | Oxidation of thiol groups in proteins and interaction with purine and pyrimidine bases | Modification of structural protein, alterations in enzyme activities, and/or DNA damages | Bactericidal, moldicidal, virucidal, protozocidal | -Fast-acting | -Gaseous form not safe -Low stability solutions form -Reacted with organic matter |
| UV light | chemical modifications of nucleotides caused by photon energy emitted | DNA damages (photohydration, photosplitting, photodimerization) | bacteria, fungi, viruses, spores | -Absence of residues or by-products -Fast-acting | -No microbiocidal effect -Eyes and skin damages for UV irradiation at 254-nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artasensi, A.; Mazzotta, S.; Fumagalli, L. Back to Basics: Choosing the Appropriate Surface Disinfectant. Antibiotics 2021, 10, 613. https://doi.org/10.3390/antibiotics10060613
Artasensi A, Mazzotta S, Fumagalli L. Back to Basics: Choosing the Appropriate Surface Disinfectant. Antibiotics. 2021; 10(6):613. https://doi.org/10.3390/antibiotics10060613
Chicago/Turabian StyleArtasensi, Angelica, Sarah Mazzotta, and Laura Fumagalli. 2021. "Back to Basics: Choosing the Appropriate Surface Disinfectant" Antibiotics 10, no. 6: 613. https://doi.org/10.3390/antibiotics10060613
APA StyleArtasensi, A., Mazzotta, S., & Fumagalli, L. (2021). Back to Basics: Choosing the Appropriate Surface Disinfectant. Antibiotics, 10(6), 613. https://doi.org/10.3390/antibiotics10060613