A Pilot Randomised Clinical Trial Comparing a Short-Term Perioperative Prophylaxis Regimen to a Long-Term Standard Protocol in Equine Colic Surgery
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Statement
2.2. Study Population
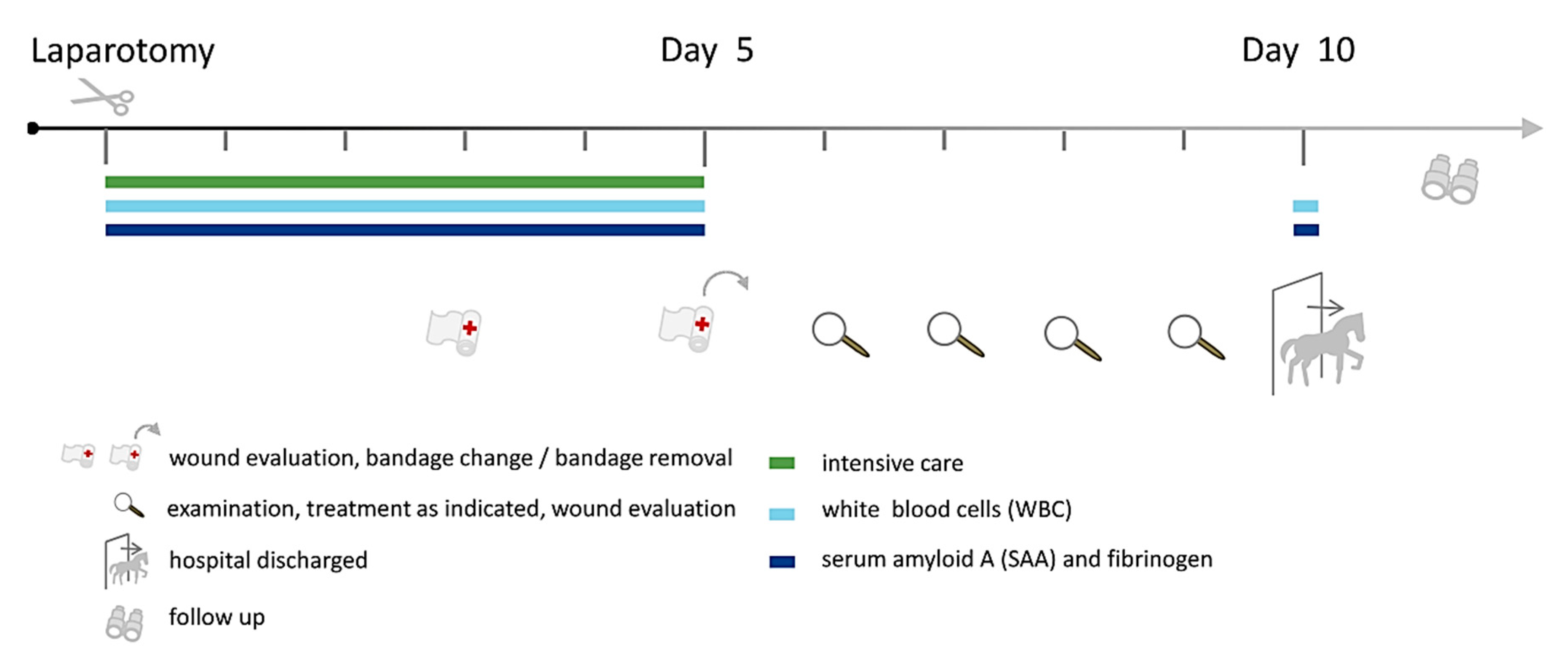
2.3. Clinical Examinations and Diagnostic Laparotomy
2.4. Perioperative Antibiotic Prophylaxis
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Postoperative Events
3.3. Adverse Events Following Colic Surgery Which Were Probably Associated with the PAP Regimen
3.4. Bacteria Associated with SSI
3.5. Laboratory Tests
4. Discussion
4.1. Surgical Site Infections Following Surgery
4.2. Surgery-Associated Factors
4.3. Bacteria Associated with SSI in Horses Receiving Colic Surgery
4.4. Postoperative Colitis
4.5. Haemolytic Anaemia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traub-Dargatz, J.L.; Kopral, C.A.; Seitzinger, A.H.; Garber, L.P.; Forde, K.; White, N.A. Estimate of the national incidence of and operation-level risk factors for colic among horses in the United States, spring 1998 to spring 1999. J. Am. Vet. Med. Assoc. 2001, 219, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Cruse, P.J.; Foord, R. The epidemiology of wound infection: A 10-year prospective study of 62,939 wounds. Surg. Clin. N. Am. 1980, 60, 27–40. [Google Scholar] [CrossRef]
- Bartmann, C.; Bubeck, K.; Georgiadis, S.; Deegen, E. Reduction of complications of the wound healing following ventral median celiotomy in horses. Pferdeheilkunde 2003, 19, 351ff. [Google Scholar] [CrossRef][Green Version]
- French, N.; Smith, J.; Edwards, G.; Proudman, C. Equine surgical colic: Risk factors for postoperative complications. Equine Vet. J. 2002, 34, 444–449. [Google Scholar] [CrossRef]
- Mair, T.; Smith, L. Survival and complication rates in 300 horses undergoing surgical treatment of colic. Part 2: Short-term complications. Equine Vet. J. 2005, 37, 303–309. [Google Scholar] [CrossRef]
- Torfs, S.; Levet, T.; Delesalle, C.; Dewulf, J.; Vlaminck, L.; Pille, F.; Lefere, L.; Martens, A. Risk factors for incisional complications after exploratory celiotomy in horses: Do skin staples increase the risk? Vet. Surg. 2010, 39, 616–620. [Google Scholar] [CrossRef]
- Davis, W.; Fogle, C.; Gerard, M.; Levine, J.; Blikslager, A. Return to use and performance following exploratory celiotomy for colic in horses: 195 cases (2003–2010). Equine Vet. J. 2013, 45, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, M.; Tnibar, A.; Pihl, T.; Andersen, P.; Ekstrøm, C. Sporting activity following colic surgery in horses: A retrospective study. Equine Vet. J. 2011, 43, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Janßen, T.; Gehlen, H.; Vincze, S.; Borchers, K.; Wieler, L.H.; Barton, A.K.; Lübke-Becker, A. Infektionsprävention und Hygiene management in Pferdekliniken. Berliner und Münchener Tierärztliche Wochenschrift 2014, 127, 448–497. [Google Scholar]
- Walther, B.; Tedin, K.; Lübke-Becker, A. Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Vet. Microbiol. 2017, 200, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, H. Umgang mit Antibiotikaresistenzen in Praxis und Klinik. Leipziger Blaue Hefte, 174. In Proceedings of the Leipziger Tierärztekongress, Leipzig, Germany, 16–18 January 2020. [Google Scholar]
- Maddox, T.W.; Clegg, P.D.; Williams, N.J.; Pinchbeck, G.L. Antimicrobial resistance in bacteria from horses: Epidemiology of antimicrobial resistance. Equine Vet. J. 2015, 47, 756–765. [Google Scholar] [CrossRef]
- Ahern, B.J.; Richardson, D.W. Chapter 7-Surgical Site Infection and the Use of Antimicrobials. In Equine Surgery, 4th ed.; Auer, J.A., Richardson, D.W., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 68–74. [Google Scholar]
- Maier, S.; Eckmann, C.; Kramer, A. Perioperative Antibiotikaprophylaxe: Ein Update. Krankenh.hyg. up2date 2015, 10, e1-e1. [Google Scholar] [CrossRef]
- Ebenhoch, M. Abdominaltrauma und Antibiotikaprophylaxe. Trauma und Berufskrankheit 2017, 19, 109–111. [Google Scholar] [CrossRef]
- Teschner, D.; Barton, A.-K.; Klaus, C.; Gehlen, H. Antibiotikaeinsatz bei operierten Kolikpferden in Deutschland. Pferdeheilkunde 2015, 31, 235–240. [Google Scholar] [CrossRef][Green Version]
- Durward-Akhurst, S.A.; Mair, T.S.; Boston, R.; Dunkel, B. Comparison of two antimicrobial regimens on the prevalence of incisional infections after colic surgery. Vet. Rec. 2013, 172, 287. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Houck, P.M. Antimicrobial prophylaxis for surgery: An advisory statement from the National Surgical Infection Prevention Project. Am. J. Surg. 2005, 189, 395–404. [Google Scholar] [CrossRef]
- Rovera, F.; Diurni, M.; Dionigi, G.; Boni, L.; Ferrari, A.; Carcano, G.; Dionigi, R. Antibiotic prophylaxis in colorectal surgery. Expert Rev. Anti-Infect. Ther. 2005, 3, 787–795. [Google Scholar] [CrossRef]
- Anderson, S.L.; Devick, I.; Bracamonte, J.L.; Hendrick, S.; Barber, S.M.; Carmalt, J.L.; Wilson, D.G. Occurrence of Incisional Complications After Closure of Equine Celiotomies With USP 7 Polydioxanone. Vet. Surg. 2015, 44, 521–526. [Google Scholar] [CrossRef]
- Darnaud, S.J.; Southwood, L.L.; Aceto, H.W.; Stefanovski, D.; Tomassone, L.; Zarucco, L. Are horse age and incision length associated with surgical site infection following equine colic surgery? Vet. J. 2016, 217, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Colbath, A.C.; Patipa, L.; Berghaus, R.D.; Parks, A.H. The influence of suture pattern on the incidence of incisional drainage following exploratory laparotomy. Equine Vet. J. 2014, 46, 156–160. [Google Scholar] [CrossRef]
- Tnibar, A.; Grubbe Lin, K.; Thurøe Nielsen, K.; Christophersen, M.T.; Lindegaard, C.; Martinussen, T.; Ekstrøm, C.T. Effect of a stent bandage on the likelihood of incisional infection following exploratory coeliotomy for colic in horses: A comparative retrospective study. Equine Vet. J. 2013, 45, 564–569. [Google Scholar] [CrossRef]
- Dziubinski, N.; Mählmann, K.; Lübke-Becker, A.; Lischer, C. Retrospective Identification of Bacterial Isolates from Emergency Laparotomy Surgical Site Infections in Horses. J. Equine Vet. Sci. 2020, 87, 102927. [Google Scholar] [CrossRef]
- van Spijk, J.N.; Schmitt, S.; Schoster, A. Infections caused by multidrug-resistant bacteria in an equine hospital (2012–2015). Equine Vet. Educ. 2017, 653–658. [Google Scholar] [CrossRef]
- Walther, B.; Luebke-Becker, A.; Stamm, I.; Gehlen, H.; Barton, A.K.; Janssen, T.; Wieler, L.H.; Guenther, S. Suspected nosocomial infections with multi-drug resistant E. coli, including extended-spectrum beta-lactamase (ESBL)-producing strains, in an equine clinic. Berliner und Munchener tierarztliche Wochenschrift 2014, 127, 421–427. [Google Scholar] [PubMed]
- Vincze, S.; Stamm, I.; Kopp, P.A.; Hermes, J.; Adlhoch, C.; Semmler, T.; Wieler, L.H.; Lübke-Becker, A.; Walther, B. Alarming proportions of methicillin-resistant Staphylococcus aureus (MRSA) in wound samples from companion animals, Germany 2010–2012. PLoS ONE 2014, 9, e85656. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Klein, K.-S.; Barton, A.-K.; Semmler, T.; Huber, C.; Merle, R.; Tedin, K.; Mitrach, F.; Lübke-Becker, A.; Gehlen, H. Equine methicillin-resistant sequence type 398 Staphylococcus aureus (MRSA) harbor mobile genetic elements promoting host adaptation. Front. Microbiol. 2018, 9, 2516. [Google Scholar] [CrossRef]
- Walther, B.; Klein, K.S.; Barton, A.K.; Semmler, T.; Huber, C.; Wolf, S.A.; Tedin, K.; Merle, R.; Mitrach, F.; Guenther, S.; et al. Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Acinetobacter baumannii among horses entering a veterinary teaching hospital: The contemporary “Trojan Horse”. PLoS ONE 2018, 13, e0191873. [Google Scholar] [CrossRef]
- Klohnen, A. New perspectives in postoperative complications after abdominal surgery. Vet. Clin. Equine Pract. 2009, 25, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, L.D.; Pascoe, J.; Jang, S.S.; Willits, N.H.; Greenman, S.L. Evaluation of iodophor skin preparation techniques and factors influencing drainage from ventral midline incisions in horses. J. Am. Vet. Med. Assoc. 1999, 215, 963–969. [Google Scholar] [PubMed]
- Fürst, A.; Kummer, M.; Kümmerle, J.; Bettschart Wolfensberger, R.; Schwarzwald, C. Mögliche Komplikationen in der Kolikchirurgie. Pferdeheilkunde 2012, 5, 522–530. [Google Scholar] [CrossRef][Green Version]
- Smith, L.; Mellor, D.; Marr, C.; Reid, S.; Mair, T. Incisional complications following exploratory celiotomy: Does an abdominal bandage reduce the risk? Equine Vet. J. 2007, 39, 277–283. [Google Scholar] [CrossRef]
- Wilson, D.A.; Baker, G.J.; Boero, M.J. Complications of celiotomy incisions in horses. Vet. Surg. 1995, 24, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ingle-Fehr, J.E.; Baxter, G.M.; Howard, R.D.; Trotter, G.W.; Stashak, T.S. Bacterial culturing of ventral median celiotomies for prediction of postoperative incisional complications in horses. Vet. Surg. 1997, 26, 7–13. [Google Scholar] [CrossRef]
- Kobluk, C.N.; Ducharme, N.G.; Lumsden, J.H.; Pascoe, P.J.; Livesey, M.A.; Hurtig, M.; Horney, F.D.; Arighi, M. Factors affecting incisional complication rates associated with colic surgery in horses: 78 cases (1983–1985). J. Am. Vet. Med. Assoc. 1989, 195, 639–642. [Google Scholar] [PubMed]
- Coomer, R.; Mair, T.; Edwards, G.; Proudman, C. Do subcutaneous sutures increase risk of laparotomy wound suppuration? Equine Vet. J. 2007, 39, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Honnas, C.; Cohen, N. Risk factors for wound infection following celiotomy in horses. J. Am. Vet. Med. Assoc. 1997, 210, 78–81. [Google Scholar]
- Macdonald, M.H.; Pascoe, J.R.; Stover, S.M.; Meagher, D.M. Survival after small intestine resection and anastomosis in horses. Vet. Surg. 1990, 18, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.; Walmsley, J. Retrospective analysis of the results of 151 exploratory laparotomies in horses with gastrointestinal disease. Equine Vet. J. 1993, 25, 427–431. [Google Scholar] [CrossRef]
- Gibson, K.T.; Curtis, C.R.; Turner, A.S.; McIlwraith, C.W.; Aanes, W.A.; Stashak, T.S. Incisional hernias in the horse incidence and predisposing factors. Vet. Surg. 1989, 18, 360–366. [Google Scholar] [CrossRef]
- Packer, M.; German, A.; Hunter, L.; Trayhurn, P.; Proudman, C. Adipose tissue-derived adiponectin expression is significantly associated with increased post operative mortality in horses undergoing emergency abdominal surgery. Equine Vet. J. 2011, 43, 26–33. [Google Scholar] [CrossRef]
- Wieler, L.H.; Ewers, C.; Guenther, S.; Walther, B.; Lübke-Becker, A. Methicillin-resistant staphylococci (MRS) and extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in companion animals: Nosocomial infections as one reason for the rising prevalence of these potential zoonotic pathogens in clinical samples. Int. J. Med. Microbiol. 2011, 301, 635–641. [Google Scholar]
- Walther, B.; Lübke-Becker, A.; Brunnberg, L.; Kohn, B.; Wieler, L.H. Einsatz von Antibiotika in der Kleintiermedizin: Quo vadis? Der praktische Tierarzt 2011, 1, 1047–1049. [Google Scholar]
- Weese, J.S.; Rousseau, J.; Willey, B.M.; Archambault, M.; McGeer, A.; Low, D.E. Methicillin-resistant Staphylococcus aureus in horses at a veterinary teaching hospital: Frequency, characterization, and association with clinical disease. J. Vet. Intern. Med. 2006, 20, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. A review of post-operative infections in veterinary orthopaedic surgery. Vet. Comp. Orthop. Traumatol. 2008, 21, 99–105. [Google Scholar] [CrossRef]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef]
- Benz, R.; Selke, S.; Holländer, R. Der Hygieneplan: Ein Wegweiser durch den Alltag der Krankenhaushygiene; Kohlhammer: Stuttgart, Germany, 1998. [Google Scholar]
- Bergström, K.; Aspan, A.; Landén, A.; Johnston, C.; Grönlund-Andersson, U. The first nosocomial outbreak of methicillin-resistant Staphylococcus aureus in horses in Sweden. Acta Vet. Scand. 2012, 54, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dallap Schaer, B.L.; Linton, J.K.; Aceto, H. Antimicrobial Use in Horses Undergoing Colic Surgery. J. Vet. Intern. Med. 2012, 26, 1449–1456. [Google Scholar] [CrossRef]
- Ernst, N.S.; Hernandez, J.A.; MacKay, R.J.; Brown, M.P.; Gaskin, J.M.; Nguyen, A.D.; Giguere, S.; Colahan, P.T.; Troedsson, M.R.; Haines, G.R. Risk factors associated with fecal Salmonella shedding among hospitalized horses with signs of gastrointestinal tract disease. J. Am. Vet. Med. Assoc. 2004, 225, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hird, D.; Casebolt, D.; Carter, J.; Pappaioanou, M.; Hjerpe, C. Risk factors for salmonellosis in hospitalized horses. J. Am. Vet. Med. Assoc. 1986, 188, 173. [Google Scholar] [PubMed]
- Costa, M.C.; Stämpfli, H.R.; Arroyo, L.G.; Allen-Vercoe, E.; Gomes, R.G.; Weese, J.S. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet. Res. 2015, 11, 19. [Google Scholar] [CrossRef]
- Harlow, B.E.; Lawrence, L.M.; Flythe, M.D. Diarrhea-associated pathogens, lactobacilli and cellulolytic bacteria in equine feces: Responses to antibiotic challenge. Vet. Microbiol. 2013, 166, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Karcher, L.F.; Dill, S.G.; Anderson, W.I.; King, J.M. Right dorsal colitis. J. Vet. Intern. Med. 1990, 4, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Blikslager, A. The effect of nonsteroidal anti-inflammatory drugs on the equine intestine. Equine Vet. J. 2011, 43, 140–144. [Google Scholar] [CrossRef]
- McConnico, R.S.; Morgan, T.W.; Williams, C.C.; Hubert, J.D.; Moore, R.M. Pathophysiologic effects of phenylbutazone on the right dorsal colon in horses. Am. J. Vet. Res. 2008, 69, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Divers, T.; Whitlock, R. Renal clearance and fractional excretion of electrolytes over a 24-hour period in horses. Am. J. Vet. Res. 1984, 45, 2431–2435. [Google Scholar] [PubMed]
- Galvin, N.; Dillon, H.; McGovern, F. Right dorsal colitis in the horse: Minireview and reports on three cases in Ireland. Ir. Vet. J. 2004, 57, 467. [Google Scholar] [CrossRef] [PubMed]
- Parraga, M.E.; Spier, S.J.; Thurmond, M.; Hirsh, D. A clinical trial of probiotic administration for prevention of Salmonella shedding in the postoperative period in horses with colic. J. Vet. Intern. Med. 1997, 11, 36–41. [Google Scholar] [CrossRef]
- Prange, T.; Holcombe, S.J.; Brown, J.A.; Dechant, J.E.; Fubini, S.L.; Embertson, R.M.; Peroni, J.; Rakestraw, P.C.; Hauptman, J.G. Resection and anastomosis of the descending colon in 43 horses. Vet. Surg. 2010, 39, 748–753. [Google Scholar] [CrossRef]
- McConnico, R.; Roberts, M.; Tompkins, M. Penicillin-induced immune-mediated hemolytic anemia in a horse. J. Am. Vet. Med. Assoc. 1992, 201, 1402. [Google Scholar]
- Blue, J.T.; Dinsmore, R.P.; Anderson, K.L. Immune-mediated hemolytic anemia induced by penicillin in horses. Cornell Vet. 1987, 77, 263–276. [Google Scholar]
- Step, D.; Blue, J.; Dill, S. Penicillin-induced hemolytic anemia and acute hepatic failure following treatment of tetanus in a horse. Cornell Vet. 1991, 81, 13–18. [Google Scholar] [PubMed]
- Robbins, R.; Wallace, S.; Brunner, C.; Gardner, T.; DiFranco, B.; Speirs, V. Immune-mediated haemolytic disease after penicillin therapy in a horse. Equine Vet. J. 1993, 25, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.R.; Hinchcliff, K.W. Heparin: A review of its pharmacology and therapeutic use in horses. J. Vet. Intern. Med. 1994, 8, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.G.; Meyers, K.M.; Reed, S.M. Reduction of the red blood cell mass of horses: Toxic effect of heparin anticoagulant therapy. Am. J. Vet. Res. 1983, 44, 2271–2276. [Google Scholar] [PubMed]
- Meyers, K.; Duncan, S.; Reed, S. Research in anticoagulation in equine gastrointestinal disease. In Proceedings of the Equine Colic Research Symposium, Athens, GA, USA, 15 September 1982; pp. 121–129. [Google Scholar]
- Moore, J.; Mahaffey, E.; Zboran, M. Heparin-induced agglutination of erythrocytes in horses. Am. J. Vet. Res. 1987, 48, 68–71. [Google Scholar] [PubMed]
- Trowbridge, A.A.; Caraveo, J.; Green, J.B., III; Amaral, B.; Stone, M.J. Heparin-related immune thrombocytopenia: Studies of antibody-heparin specificity. Am. J. Med. 1978, 65, 277–283. [Google Scholar] [CrossRef]
- Gerhards, H. Low dose calcium heparin in horses: Plasma heparin concentrations, effects on red blood cell mass and on coagulation variables. Equine Vet. J. 1991, 23, 37–43. [Google Scholar] [CrossRef]
| Surgical Site Infections (SSI) | ||||||
|---|---|---|---|---|---|---|
| Group | ID | Diagnosis during Surgery | Adverse Event after Surgery * | 10 Days | 30 Days | Results of Microbiological Cultures from SSI |
| SSG | 10 | Torsio coli | SSI | − | + | E. faecium |
| 13 | Ileal obstipation | SSI | + | + | E. cloacae (ESBL) | |
| 19 | Ileal obstipation | SSI | + | + | MRSA, E. coli, Fusobacterium spp., B. fragiles | |
| 23a | Caecal obstipation | Colitis | − | − | - | |
| 28 | Nephrosplenic entrapment | SSI | − | + | E. coli, K. pneumoniae, MRSA, E. faecium | |
| 29 | Lipoma pendulans | SSI | + | + | E. coli, E. cloacae, E. faecium | |
| 46 | Obstipation of the ascending colon | SSI | − | + | n.a. | |
| 66 | Lipoma pendulans | SSI | + | + | E. cloacae | |
| 5DG | 12 | Obstipation of the ascending colon | Colitis | − | − | - |
| 18 | Incarceration of the ascending colon | Haemolytic anaemia | − | − | - | |
| 33 | Torsio caeci with incarcerated jejunum | Colitis | − | − | - | |
| 41 | Lipoma pendulans | SSI | + | + | E. aerogenes (ESBL), MRSA | |
| 56 a | Meteorism of the colon | Colitis | − | − | - | |
| 60 | Diaphragmatic hernia | Haemolytic anaemia | − | − | - | |
| 62 | Lipoma pendulans | Colitis, Haemolytic anaemia | − | + | n.a. | |
| Single-Shot Group | 5-Day Group | ||||
|---|---|---|---|---|---|
| n = 30 | n = 37 | p | |||
| n | % | n | % | ||
| Surgical site infections (SSI) | |||||
| SI within 10 days after surgery | 4 | 13 | 1 | 3 | 0.17 |
| total SSI | 7 | 23 | 2 | 5 | 0.07 |
| Classification of surgery | |||||
| clean | 12 | 40 | 9 | 24 | 0.13 |
| clean-contaminated | 18 | 60 | 28 | 76 | 0.13 |
| contaminated | 0 | 0 | 0 | 0 | |
| Colitis | 1 | 3 | 4 | 11 | 0.37 |
| Haemolytic anaemia | 0 | 0 | 3 | 8 | 0.25 |
| SSG | 5DG | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Day | Time | n | Mean ± Standard Deviation or Median (Min–Max) | n | Mean ± Standard Deviation or Median (Min–Max) | p |
| WBC (G/L) | 0 | variable | 30 | 8.57 ± 2.36 | 37 | 9.22 ± 3.68 | 0.399 |
| 1 | morning | 30 | 6.81 ± 4.21 | 37 | 5.77 ± 2.23 | 0.202 | |
| evening | 30 | 6.36 ± 3.94 | 37 | 6.34 ± 2.61 | 0.957 | ||
| 2 | morning | 30 | 5.36 ± 3.45 | 37 | 5.47 ± 2.64 | 0.886 | |
| evening | 29 | 4.96 ± 3.24 | 33 | 5.24 ± 2.4 | 0.692 | ||
| 3 | morning | 29 | 5 ± 3.06 | 37 | 5.17 ± 2.47 | 0.796 | |
| evening | 26 | 5.15 ± 2.9 | 34 | 5.48 ± 2.51 | 0.637 | ||
| 4 | morning | 27 | 5.45 ± 2.72 | 36 | 5.36 ± 2.05 | 0.873 | |
| evening | 25 | 6.19 ± 2.79 | 32 | 5.69 ±1.68 | 0.407 | ||
| 5 | morning | 27 | 6.91 ± 2.36 | 35 | 6.94 ± 2.14 | 0.953 | |
| evening | 25 | 7.71 ± 2.17 | 29 | 8.01 ± 20.8 | 0.603 | ||
| 10 | morning | 25 | 10.59 ± 3.78 | 29 | 10.3 ± 3.74 | 0.774 | |
| SAA (µg/mL) | 0 | variable | 22 | 13.3 (3.5–748.68) | 30 | 18.4 (3.5–649.36) | 0.541 |
| 1 | morning | 28 | 598.81 ± 163.11 | 37 | 602.73 ± 204.1 | 0.934 | |
| 2 | morning | 28 | 743.71 ± 121.51 | 33 | 750.1 ± 196.57 | 0.882 | |
| 3 | morning | 28 | 781.98 ± 137.87 | 35 | 759.57 ± 136.4 | 0.521 | |
| 4 | morning | 26 | 705.72 ± 167.22 | 32 | 732.48 ± 196.82 | 0.557 | |
| 5 | morning | 26 | 632.55 ± 197.15 | 29 | 629.34 ± 226.04 | 0.956 | |
| 10 | morning | 25 | 110.82 (5.43–783.05) | 26 | 64.93 (3.5–707.62) | 0.356 | |
| Fibrinogen (mg/dL) | 0 | variable | 27 | 188.63 ± 70.52 | 31 | 199.26 ± 51.34 | 0.51 |
| 1 | morning | 30 | 225.48 ± 72.112 | 35 | 231.1 ± 60.35 | 0.733 | |
| 2 | morning | 30 | 564.86 ± 59.45 | 34 | 274.35 ± 63.43 | 0.54 | |
| 3 | morning | 29 | 270.51 ± 81.81 | 34 | 319.19 ± 167.87 | 0.16 | |
| 4 | morning | 27 | 288.43 ± 72.37 | 33 | 306.48 ± 65.79 | 0.316 | |
| 5 | morning | 26 | 288.29 ± 69.15 | 32 | 309.64 ± 76.1 | 0.273 | |
| 10 | morning | 25 | 285.55 ± 79.77 | 25 | 265.36 ± 89.87 | 0.405 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stöckle, S.D.; Kannapin, D.A.; Kauter, A.M.L.; Lübke-Becker, A.; Walther, B.; Merle, R.; Gehlen, H. A Pilot Randomised Clinical Trial Comparing a Short-Term Perioperative Prophylaxis Regimen to a Long-Term Standard Protocol in Equine Colic Surgery. Antibiotics 2021, 10, 587. https://doi.org/10.3390/antibiotics10050587
Stöckle SD, Kannapin DA, Kauter AML, Lübke-Becker A, Walther B, Merle R, Gehlen H. A Pilot Randomised Clinical Trial Comparing a Short-Term Perioperative Prophylaxis Regimen to a Long-Term Standard Protocol in Equine Colic Surgery. Antibiotics. 2021; 10(5):587. https://doi.org/10.3390/antibiotics10050587
Chicago/Turabian StyleStöckle, Sabita Diana, Dania A. Kannapin, Anne M. L. Kauter, Antina Lübke-Becker, Birgit Walther, Roswitha Merle, and Heidrun Gehlen. 2021. "A Pilot Randomised Clinical Trial Comparing a Short-Term Perioperative Prophylaxis Regimen to a Long-Term Standard Protocol in Equine Colic Surgery" Antibiotics 10, no. 5: 587. https://doi.org/10.3390/antibiotics10050587
APA StyleStöckle, S. D., Kannapin, D. A., Kauter, A. M. L., Lübke-Becker, A., Walther, B., Merle, R., & Gehlen, H. (2021). A Pilot Randomised Clinical Trial Comparing a Short-Term Perioperative Prophylaxis Regimen to a Long-Term Standard Protocol in Equine Colic Surgery. Antibiotics, 10(5), 587. https://doi.org/10.3390/antibiotics10050587







