Point Prevalence Survey of Antimicrobial Use in a Malaysian Tertiary Care University Hospital
Abstract
1. Introduction
2. Results
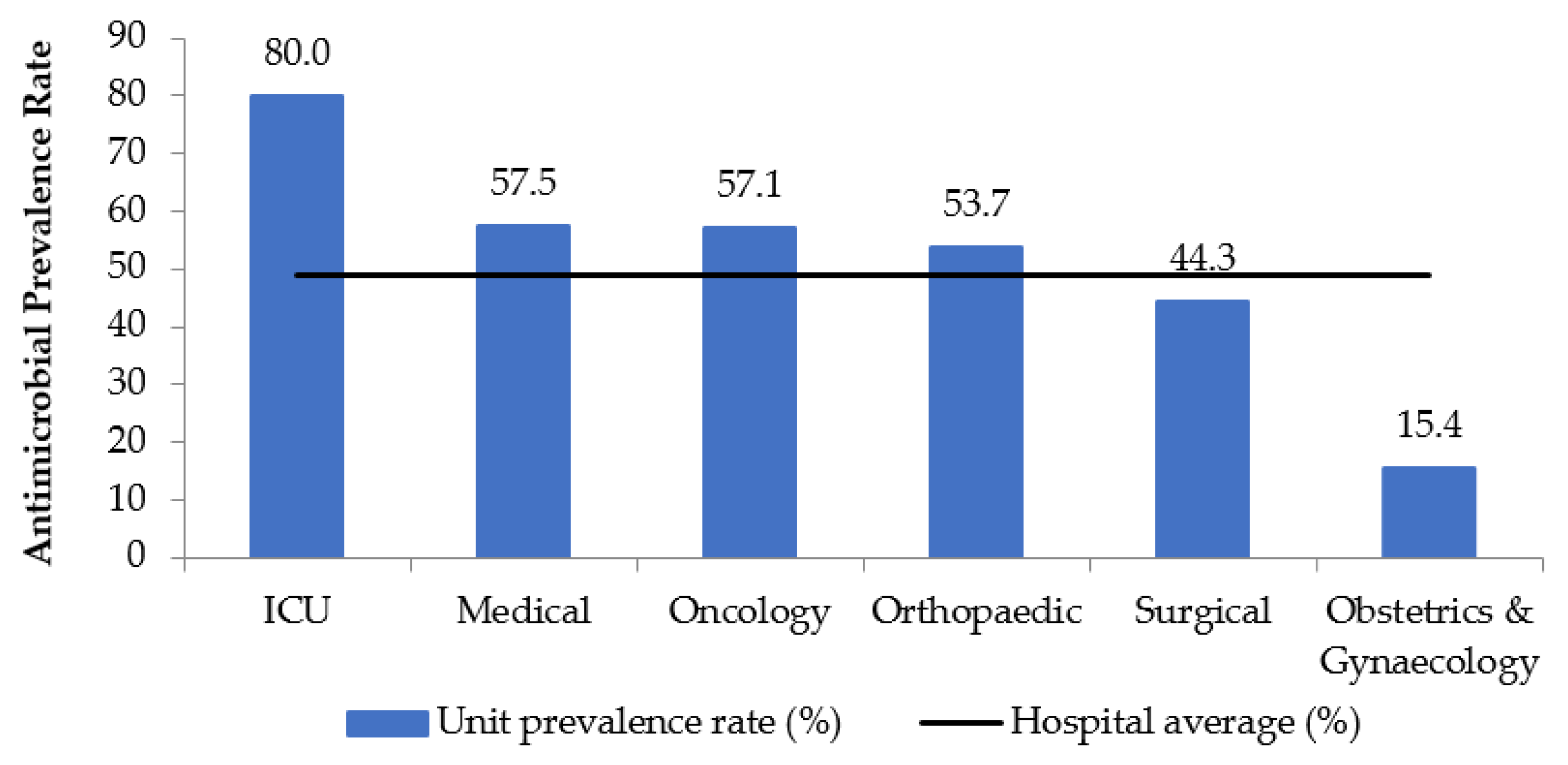
2.1. Demographics and Prevalence
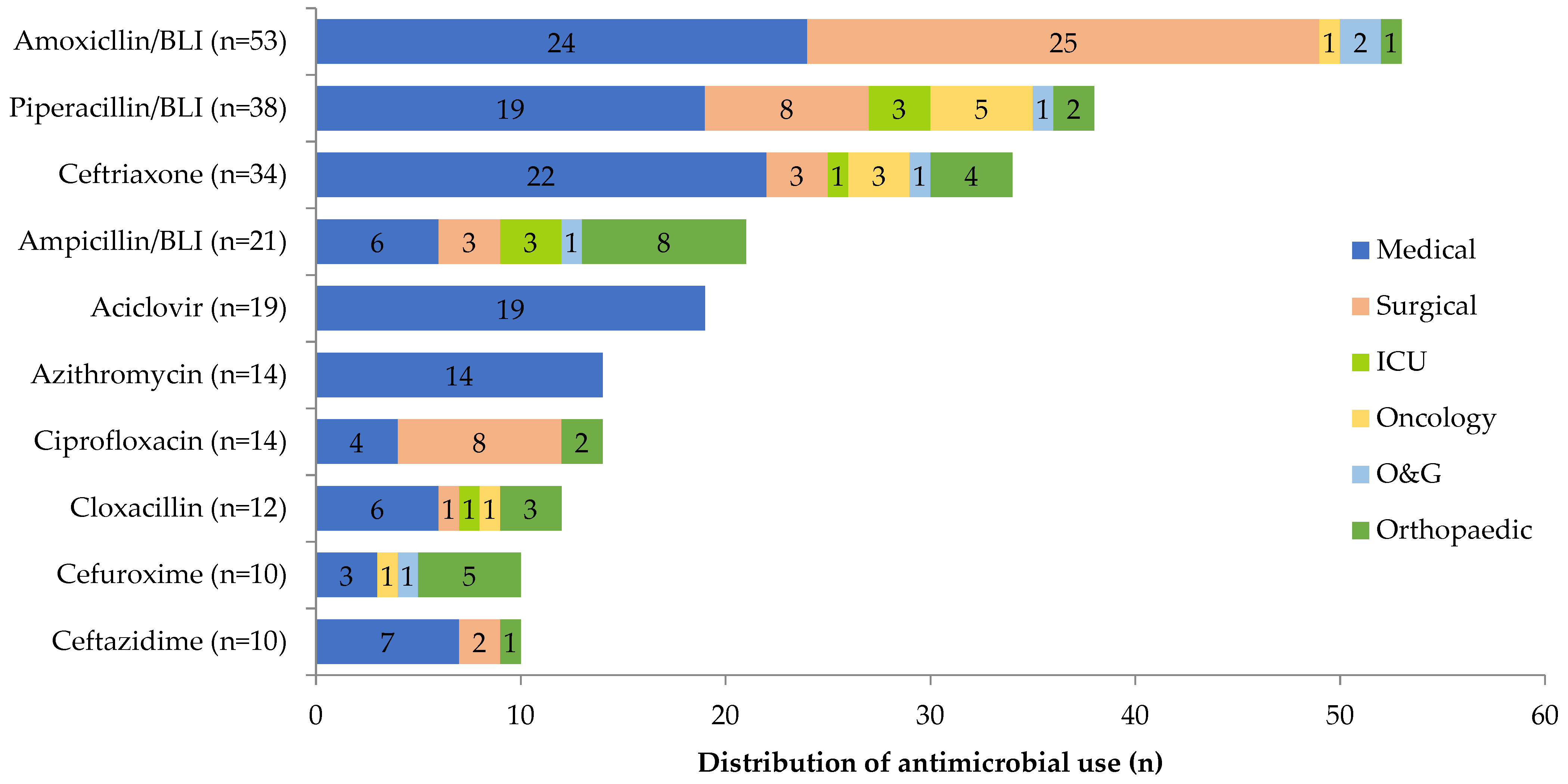
2.2. Prescription Rate and Antibiotic Use
2.3. Factors Influencing Compliance
3. Discussion
4. Materials and Methods
4.1. Study Design and Settings
4.2. Definition
4.2.1. Empiric/Directed/Prophylaxis
4.2.2. Compliance
4.3. Data Analysis
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Achievements in Public Health, 1900–1999: Control of Infectious Diseases. MMWR Morb. Mortal. Wkly. Rep. 1999, 48, 621. [Google Scholar]
- Hulscher, M.E.; Grol, R.P.; van der Meer, J.W. Antibiotic prescribing in hospitals: A social and behavioural scientific approach. Lancet Infect. Dis. 2010, 10, 167–175. [Google Scholar] [CrossRef]
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: http://apps.who.int/iris/handle/10665/193736 (accessed on 11 November 2020).
- Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs. 2014. Available online: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html (accessed on 11 November 2020).
- Ministry of Health Malaysia. Malaysian Action Plan on Antimicrobial Resistance (MyAP-AMR) 2017–2021. Minist. Heal. Malaysia 2017, 2017–2021. Available online: https://www.moh.gov.my/moh/resources/Penerbitan/Garis%20Panduan/Garis%20panduan%20Umum%20(Awam)/National_Action_Plan_-_FINAL_29_june.pdf (accessed on 7 March 2019).
- Cai, Y.; Venkatachalam, I.; Tee, N.W.; Yen Tan, T.; Kurup, A.; Wong, S.Y.; Low, C.Y.; Wang, Y.; Lee, W.; Liew, Y.X.; et al. Prevalence of healthcare-associated infections and antimicrobial use among adult inpatients in Singapore acute-care hospitals: Results from the first national point prevalence survey. Clin. Infect. Dis. 2017, 64, S61–S67. [Google Scholar] [CrossRef] [PubMed]
- Ingram, P.R.; Seet, J.M.; Budgeon, C.A.; Murray, R. Point-prevalence study of inappropriate antibiotic use at a tertiary Australian hospital. Intern. Med. J. 2012, 42, 719–721. [Google Scholar] [CrossRef]
- Xie, D.; Xiang, L.; Li, R.; Hu, Q.; Luo, Q.; Xiong, W. A multicenter point-prevalence survey of antibiotic use in 13 Chinese hospitals. J. Infect. Public Health 2015, 8, 55–61. [Google Scholar] [CrossRef]
- Plachouras, D.; Kärki, T.; Hansen, S.; Hopkins, S.; Lyytikäinen, O.; Moro, M.L.; Reilly, J.; Zarb, P.; Zingg, W.; Kinross, P.; et al. Antimicrobial use in european acute care hospitals: Results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Leung, V.; Li, M.; Wu, J.H.C.; Langford, B.; Zvonar, R.; Powis, J.; Longpre, J.; Béïque, L.; Gill, S.; Ho, G.; et al. Evaluating antimicrobial use and spectrum of activity in Ontario hospitals: Feasibility of a multicentered point prevalence study. Open Forum Infect. Dis. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Oduyebo, O.; Olayinka, A.; Iregbu, K.; Versporten, A.; Goossens, H.; Nwajiobi-Princewill, P.; Jimoh, O.; Ige, T.; Aigbe, A.; Ola-Bello, O.; et al. A point prevalence survey of antimicrobial prescribing in four Nigerian Tertiary Hospitals. Ann. Trop. Pathol. 2017, 8, 42. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Versporten, A.; Godman, B.; Hashmi, F.K.; Goossens, H.; Saleem, F. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: Findings and implications. Expert Rev. Anti. Infect. Ther. 2019, 17, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.P.M.; Goossens, H.; Versporten, A.; Costa, S.F. Global point prevalence survey of antimicrobial consumption in Brazilian hospitals. J. Hosp. Infect. 2020, 104, 165–171. [Google Scholar] [CrossRef]
- World Health Organization. Health Care-associated Infections. Fact Sheet. Available online: http://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf (accessed on 26 April 2020).
- Azmi, S.; Aljunid, S.M.; Maimaiti, N.; Ali, A.A.; Muhammad Nur, A.; De Rosas-Valera, M.; Encluna, J.; Mohamed, R.; Wibowo, B.; Komaryani, K.; et al. Assessing the burden of pneumonia using administrative data from Malaysia, Indonesia, and the Philippines. Int. J. Infect. Dis. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- National Centre for Antimicrobial Stewardship and Australian Commission on Safety and Quality in Health Care. Antimicrobial Prescribing Practice in Australian Hospitals: Results of the 2017 Hospital National Antimicrobial Prescribing Survey; ACSQHC: Sidney, Australia, 2018.
- Ansari, F.; Erntell, M.; Goossens, H.; Davey, P.; Ii, E.; Care, H.; Group, S. The European Surveillance of Antimicrobial Consumption ( ESAC ) Point-Prevalence Survey of Antibacterial Use in 20 European Hospitals in 2006. Clin. Infect. Dis. 2009, 49, 1496–1504. [Google Scholar] [CrossRef]
- Singh, S.K.; Sengupta, S.; Antony, R.; Bhattacharya, S.; Mukhopadhyay, C.; Ramasubramanian, V.; Sharma, A.; Sahu, S.; Nirkhiwale, S.; Gupta, S.; et al. Variations in antibiotic use across India: Multi-centre study through Global Point Prevalence survey. J. Hosp. Infect. 2019, 103, 280–283. [Google Scholar] [CrossRef]
- Metsini, A.; Vazquez, M.; Sommerstein, R.; Marschall, J.; Voide, C.; Troillet, N.; Gardiol, C.; Pittet, D.; Zingg, W. The Swissnoso Network Point prevalence of healthcare-associated infections and antibiotic use in three large Swiss acute-care hospitals. Swiss Med. Wkly. 2018, 148, w14617. [Google Scholar] [CrossRef]
- Gul, Y.A.; Lian, L.H.; Jabar, F.M.; Moissinac, K. Antibiotic prophylaxis in elective colorectal surgery. ANZ J. Surg. 2002, 72, 275–278. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- Vandael, E.; Latour, K.; Goossens, H.; Magerman, K.; Drapier, N.; Catry, B.; Versporten, A. Point prevalence survey of antimicrobial use and healthcare-associated infections in Belgian acute care hospitals: Results of the Global-PPS and ECDC-PPS 2017. Antimicrob. Resist. Infect. Control 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Umeokonkwo, C.D.; Madubueze, U.C.; Onah, C.K.; Okedo-Alex, I.N.; Adeke, A.S.; Versporten, A.; Goossens, H.; Igwe-Okomiso, D.; Okeke, K.; Azuogu, B.N.; et al. Point prevalence survey of antimicrobial prescription in a tertiary hospital in South East Nigeria: A call for improved antibiotic stewardship. J. Glob. Antimicrob. Resist. 2019, 17, 291–295. [Google Scholar] [CrossRef]
- van Spreuwel, P.C.J.M.; Blok, H.; Langelaar, M.; Kullberg, B.J.; Mouton, J.W.; Natsch, S. Identifying targets for quality improvement in hospital antibiotic prescribing. Neth. J. Med. 2015, 73, 161–168. [Google Scholar]
- Li, H.K.; Agweyu, A.; English, M.; Bejon, P. An Unsupported Preference for Intravenous Antibiotics. PLoS Med. 2015, 12, e1001825. [Google Scholar] [CrossRef]
- Broom, J.; Broom, A.; Adams, K.; Plage, S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J. Antimicrob. Chemother. 2016, 71, 2295–2299. [Google Scholar] [CrossRef]
- Pollack, L.A.; Plachouras, D.; Sinkowitz-Cochran, R.; Gruhler, H.; Monnet, D.L.; Weber, J.T. A concise set of structure and process indicators to assess and compare antimicrobial stewardship programs among EU and US Hospitals: Results from a multinational expert panel. Infect. Control Hosp. Epidemiol. 2016, 37, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Shrayteh, Z.M.; Rahal, M.K.; Malaeb, D.N. Practice of switch from intravenous to oral antibiotics. Springerplus 2014, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zarb, P.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC). Drugs 2011, 71, 745–755. [Google Scholar] [CrossRef]
- Health Protection Scotland. Information Services Division. Scottish Antimicrobial Prescribing Group (SAPG) Report on Antimicrobial Use and Resistance in Humans in 2009. 2011. Available online: http://www.scottishmedicines.org.uk/SAPG/Scottish_Prescribing_Group__SAPG_ (accessed on 29 April 2020).
- James, R.; Upjohn, L.; Cotta, M.; Luu, S.; Marshall, C.; Buising, K.; Thursky, K. Measuring antimicrobial prescribing quality in Australian hospitals: Development and evaluation of a national antimicrobial prescribing survey tool. J. Antimicrob. Chemother. 2014, 70, 1912–1918. [Google Scholar] [CrossRef]
- Al Matar, M.; Enani, M.; Binsaleh, G.; Roushdy, H.; Alokaili, D.; Al Bannai, A.; Khidir, Y.; Al-Abdely, H. Point prevalence survey of antibiotic use in 26 Saudi hospitals in 2016. J. Infect. Public Health 2019, 12, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Zarb, P.; Amadeo, B.; Muller, A.; Drapier, N.; Vankerckhoven, V.; Davey, P.; Goossens, H.; Metz-Gercek, S.; Jansens, H.; Markova, B.; et al. Identification of targets for quality improvement in antimicrobial prescribing: The web-based ESAC point prevalence survey 2009. J. Antimicrob. Chemother. 2011, 66, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Skodvin, B.; Aase, K.; Charani, E.; Holmes, A.; Smith, I. An antimicrobial stewardship program initiative: A qualitative study on prescribing practices among hospital doctors. Antimicrob. Resist. Infect. Control 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Pulcini, C.; Botelho-Nevers, E.; Dyar, O.J.; Harbarth, S. The impact of infectious disease specialists on antibiotic prescribing in hospitals. Clin. Microbiol. Infect. 2014, 20, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Grtler, N.; Erba, A.; Giehl, C.; Tschudin-Sutter, S.; Bassetti, S.; Osthoff, M. Appropriateness of antimicrobial prescribing in a Swiss tertiary care hospital: A repeated point prevalence survey. Swiss Med. Wkly. 2019, 149, w20135. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n | % |
|---|---|---|
| Age (year) | ||
| Mean (SD) | 59.96 (17.25) | |
| Age group | ||
| 16–29 | 15 | 6.4 |
| 30–49 | 43 | 18.4 |
| 50–64 | 66 | 28.2 |
| 65–79 | 84 | 35.9 |
| ≥80 | 26 | 11.1 |
| Gender | ||
| Male | 132 | 56.4 |
| Treating specialities | ||
| Medical | 122 | 52.1 |
| Surgical | 51 | 21.8 |
| Orthopaedic | 29 | 12.4 |
| Obstetrics & Gynaecology | 8 | 3.4 |
| Intensive care unit | 12 | 5.1 |
| Oncology | 12 | 5.1 |
| Renal replacement therapy/dialysis | ||
| Yes | 7 | 3.0 |
| Directed antimicrobial therapy | ||
| Yes | 42 | 18.0 |
| Number of antimicrobial prescribed | ||
| 1 | 162 | 69.2 |
| 2 | 45 | 19.2 |
| ≥3 | 27 | 11.5 |
| Prescription by Specialities, n (%) | ||||||
|---|---|---|---|---|---|---|
| Indicators (Number of Prescriptions, n) | Medical | Surgical | ICU | Oncology | O&G | Orthopaedic |
| 202 (56.5%) | 70 (19.6%) | 21 (5.9%) | 15 (4.2%) | 13 (3.6%) | 36 (10.1%) | |
| Treatment | ||||||
| Empiric treatment (234) | 151 (64.5) | 41 (17.5) | 6 (2.5) | 13 (5.5%) | 3 (1.3) | 20 (8.5) |
| Directed therapy (58) | 17 (29.0) | 15 (26.0) | 15 (26.0) | 0 | 2 (3.4) | 9 (15.5) |
| Prophylaxis (65) | 34 (52.3) | 14 (21.5) | 0 | 2 (3.0) | 8 (12.3) | 7 (10.7) |
| Medical (41) | 34 (82.9) | 0 | 0 | 2 (4.8) | 5 (12.2) | 0 |
| Surgical (24) | 0 | 14 (58.3) | 0 | 0 | 3 (12.5) | 7 (29.1) |
| Route of administration | ||||||
| Intravenous (224) | 109 (48.7) | 48 (21.4) | 16 (7.1) | 13 (5.8) | 8 (3.6) | 30 (13.4) |
| Oral (108) | 82 (76.0) | 11 (10.2) | 5 (4.6) | 2 (1.9) | 4 (3.7) | 4 (3.7) |
| Other (25) | 11 (44.0) | 11 (44.0) | 0 | 0 | 1 (4.0) | 2 (8.0) |
| Reason for use documented | ||||||
| Yes (287) | 172 (59.9) | 42 (14.6) | 20 (7.0) | 14 (4.9) | 8 (2.8) | 31 (10.8) |
| No (70) | 30 (42.9) | 28 (40.0) | 1 (1.4) | 1 (1.4) | 5 (7.1) | 5 (7.1) |
| Stop/review date documented | ||||||
| Yes (119) | 82 (68.9) | 8 (6.7) | 10 (8.4) | 1 (0.8) | 9 (7.6) | 9 (7.6) |
| No (238) | 120 (50.4) | 62 (26.0) | 11 (4.6) | 14 (5.9) | 4 (1.7) | 27 (11.3) |
| Compliance with guideline | ||||||
| Yes (141) | 105 (74.5) | 21 (14.9) | 3 (2.1) | 5 (3.5) | 1 (0.7) | 6 (4.3) |
| No (139) | 69 (49.6) | 31 (22.3) | 3 (2.2) | 9 (6.5) | 9 (6.5) | 18 (12.9) |
| Not applicable (77) * | 28 (36.4) | 18 (23.4) | 15 (19.5) | 1 (1.2) | 3 (3.9) | 12 (15.6) |
| Compliant with Guideline | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Factors | Compliant, Number of Prescriptions (%) | Non-Compliant, Number of Prescriptions (%) | Crude Odd Ratio for Non-Compliant (95% Confidence Interval) | p-Value | Adjusted Odd Ratio for Non-Compliant (95% Confidence Interval) | p-Value |
| Specialities | 0.231 | |||||
| Medical | 105 (60.3) | 69 (39.7) | 1.00 (Reference) | <0.001 b | 1.00 (Reference) | |
| Surgical | 21 (40.4) | 31 (59.6) | 2.25 (1.19–4.23) | 1.18 (0.57–2.46) | 0.661 | |
| ICU | 3 (50.0) | 3 (50.0) | 1.52 (0.30–7.76) | 0.85 (0.15–4.77) | 0.856 | |
| Oncology | 5 (35.7) | 9 (64.3) | 2.74 (0.88–8.52) | 1.72 (0.51–5.75) | 0.379 | |
| O&G | 1 (10.0) | 9 (90.0) | 13.70 (1.70–110.53) | 9.54 (1.10–82.89) | 0.041 | |
| Orthopaedic | 6 (25.0) | 18 (75.0) | 4.57 (1.73–12.07) | 2.26 (0.80–6.39) | 0.123 | |
| Route | 0.001 | |||||
| Oral | 73 (80.2) | 18 (19.8) | 1.00 (Reference) | <0.001 a | 1.00 (Reference) | |
| Intravenous | 64 (37.2) | 108 (62.8) | 6.84 (3.75–12.49) | 2.22 (0.93–5.31) | 0.072 | |
| Other * | 4 (23.5) | 13 (76.5) | 13.18 (3.84–45.26) | 19.05 (4.19–86.60) | <0.001 | |
| Antimicrobial class | 0.149 | |||||
| Penicillin | 41 (39.0) | 64 (61.0) | 1.00 (Reference) | <0.001 b | 1.00 (Reference) | |
| Cephalosporin | 12 (22.2) | 42 (77.8) | 2.24 (1.06–4.76) | 2.18 (0.94–5.06) | 0.071 | |
| Quinolone | 7 (50.0) | 7 (50.0) | 0.64 (0.21–1.96) | 1.42 (0.28–7.620 | 0.673 | |
| Other ** | 29 (59.2) | 20 (40.8) | 0.44 (0.22–0.88) | 0.63 (0.25–1.60) | 0.334 | |
| Antifungal | 13 (68.4) | 6 (31.6) | 0.30 (0.10–0.84) | 1.05 (0.25–4.37) | 0.947 | |
| Antiviral ^ | 25 (100.0) | 0 (0.0) | 0 | NA | NA | |
| Antituberculosis ^ | 14 (100.0) | 0 (0.0) | 0 | NA | NA | |
| Number of prescriptions per patient | 0.001 | |||||
| 1 | 49 (39.5) | 75 (60.5) | 1.00 (Reference) | <0.001 a | 1.00 (Reference) | |
| 2 | 33 (45.2) | 40 (54.8) | 0.79 (0.44–1.42) | 0.99 (0.44–2.24) | 0.975 | |
| ≥3 | 59 (71.1) | 24 (28.9) | 0.27 (0.15–0.48) | 0.21 (0.08–0.53) | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamaluddin, N.A.H.; Periyasamy, P.; Lau, C.L.; Ponnampalavanar, S.; Lai, P.S.M.; Ramli, R.; Tan, T.L.; Kori, N.; Yin, M.K.; Azman, N.J.; et al. Point Prevalence Survey of Antimicrobial Use in a Malaysian Tertiary Care University Hospital. Antibiotics 2021, 10, 531. https://doi.org/10.3390/antibiotics10050531
Jamaluddin NAH, Periyasamy P, Lau CL, Ponnampalavanar S, Lai PSM, Ramli R, Tan TL, Kori N, Yin MK, Azman NJ, et al. Point Prevalence Survey of Antimicrobial Use in a Malaysian Tertiary Care University Hospital. Antibiotics. 2021; 10(5):531. https://doi.org/10.3390/antibiotics10050531
Chicago/Turabian StyleJamaluddin, Nurul Adilla Hayat, Petrick Periyasamy, Chee Lan Lau, Sasheela Ponnampalavanar, Pauline Siew Mei Lai, Ramliza Ramli, Toh Leong Tan, Najma Kori, Mei Kuen Yin, Nur Jannah Azman, and et al. 2021. "Point Prevalence Survey of Antimicrobial Use in a Malaysian Tertiary Care University Hospital" Antibiotics 10, no. 5: 531. https://doi.org/10.3390/antibiotics10050531
APA StyleJamaluddin, N. A. H., Periyasamy, P., Lau, C. L., Ponnampalavanar, S., Lai, P. S. M., Ramli, R., Tan, T. L., Kori, N., Yin, M. K., Azman, N. J., James, R., Thursky, K., & Naina-Mohamed, I. (2021). Point Prevalence Survey of Antimicrobial Use in a Malaysian Tertiary Care University Hospital. Antibiotics, 10(5), 531. https://doi.org/10.3390/antibiotics10050531






