Heterogeneity of Antibiotics Multidrug-Resistance Profile of Uropathogens in Romanian Population
Abstract
1. Introduction
- Probable factors: urinary catheterization, previous hospitalization, previous antibiotic treatment, nursing home resident;
- Possible risk factor: age, previous UTI, male gender;
- Unlikely risk factors: diabetes, recent travel, ethnicity, immunocompromised, female gender.
- “multiple drug-resistant” (MDR)—nonsusceptible to one or more antibiotic agent in three or more antimicrobial categories,
- “extensive” or “extremely” drug-resistant (XDR)—nonsusceptible to one or more antibiotic agents in all but two or less antimicrobial classes, and
- “pan drug-resistant”(PDR)—nonsusceptible to all antimicrobial agents listed.
2. Results
3. Discussion
3.1. MDR Uropathogens in Relation with Patients Age
3.2. Comparison of MDR Escherichia coli Patterns with Other Studies
3.3. Comparison of MDR Klebsiella Patterns with Other Studies
3.4. How MDR Strains Modify Our Daily Practice in UTIs
3.5. Limitations
4. Materials and Methods
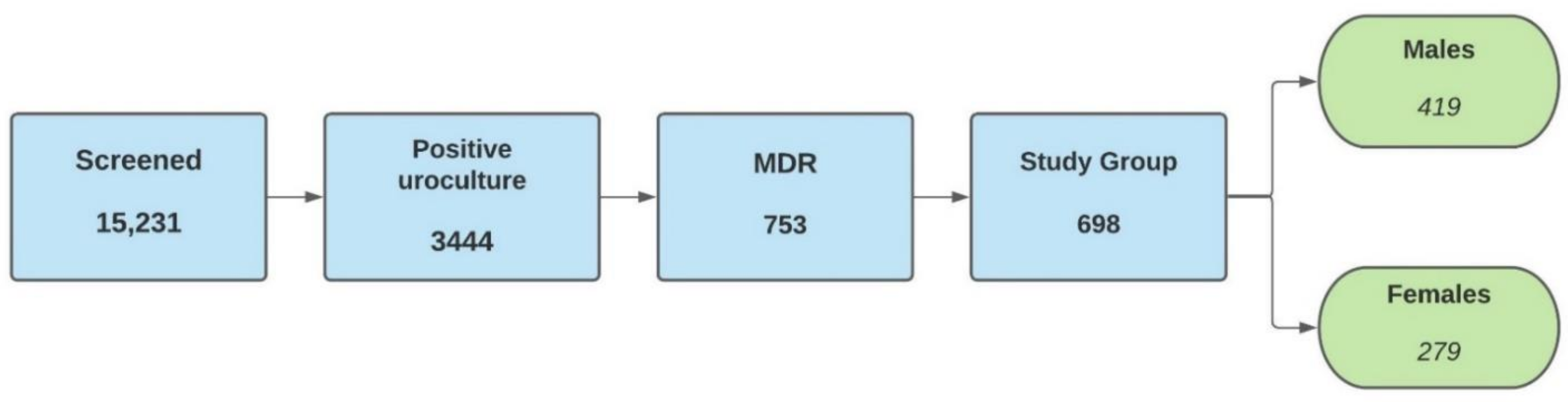
4.1. Study Design and Sample Population
4.2. Inclusion and Exclusion Criteria
- Positive urine culture ≥ 105 CFU/mL;
- Single bacteria strain on urine culture;
- Age ≥ 18 years;
- Standards of MDR-UTIs (nonsusceptible uropathogen to one or more antibiotic agents in three or more antimicrobial categories).
- Urine culture < 105 CFU/mL;
- Two or more bacterial strains on uroculture;
- No requirements of admission into the MDR group;
- Presence of permanent urinary catheter (>1 month).
4.3. Sample Collection, Bacterial Culture, Identification of Uropathogens, and Antibiotic Susceptibility Test
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open Forum Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Stamm, W.E. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 2001, 135, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chibelean, C.B.; Petca, R.-C.; Mareș, C.; Popescu, R.-I.; Enikő, B.; Mehedințu, C.; Petca, A. A clinical perspective on the antimicrobial resistance spectrum of uropathogens in a Romanian male population. Microorganisms 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Petca, R.-C.; Mareș, C.; Petca, A.; Negoiță, S.; Popescu, R.-I.; Boț, M.; Barabás, E.; Chibelean, C.B. Spectrum and Antibiotic Resistance of Uropathogens in Romanian Females. Antibiotics 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.V.; Master, R.N.; Karlowsky, J.A.; Bordon, J.M. In vitro antimicrobial resistance of urinary Escherichia coli isolates among US outpatients from 2000 to 2010. Antimicrob. Agents Chemother. 2012, 56, 2181–2183. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Furuya, E.; Lowy, F. Antimicrobial-resistant bacteria in the community setting. Nat. Rev. Microbiol. 2006, 4, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tenney, J.; Hudson, N.; Alnifaidy, H.; Li, J.T.C.; Fung, K.H. Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: A systematic literature review. Saudi Pharm. J. 2018, 26, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Bartoletti, R.; Bruyere, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F. EAU Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2018. [Google Scholar]
- Petca, R.C.; Popescu, R.I.; Mares, C.; Petca, A.; Mehedintu, C.; Sandu, I.; Maru, N. Antibiotic resistance profile of common uropathogens implicated in urinary tract infections in Romania. Farmacia 2019, 67, 994–1004. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E. Comparative epidemiology and resistance trends of Proteae in urinary tract infections of inpatients and outpatients: A 10-year retrospective study. Antibiotics 2019, 8, 91. [Google Scholar] [CrossRef]
- Rowe, T.A.; Juthani-Mehta, M. Urinary tract infection in older adults. Aging Health 2013, 9, 519–528. [Google Scholar] [CrossRef]
- Alpay, Y.; Aykin, N.; Korkmaz, P.; Gulduren, H.M.; Caglan, F.C. Urinary tract infections in the geriatric patients. Pak. J. Med. Sci. 2018, 34, 67–72. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L. Urinary tract infections in the elderly: A review of disease characteristics and current treatment options. Drugs Context 2020, 9. [Google Scholar] [CrossRef]
- Hu, K.K.; Boyko, E.J.; Scholes, D.; Normand, E.; Chen, C.L.; Grafton, J.; Fihn, S.D. Risk factors for urinary tract infections in postmenopausal women. Arch. Intern. Med. 2004, 164, 989–993. [Google Scholar] [CrossRef]
- Brown, J.S.; Vittinghoff, E.; Kanaya, A.M.; Agarwal, S.K.; Hulley, S.; Foxman, B. Heart and Estrogen/Progestin Replacement Study Research Group. Urinary tract infections in postmenopausal women: Effect of hormone therapy and risk factors. Obstet. Gynecol. 2001, 98, 1045–1052. [Google Scholar] [CrossRef]
- Mody, L.; Juthani-Mehta, M. Urinary tract infections in older women: A clinical review. JAMA 2014, 311, 844–854. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113 (Suppl. 1A), 5S–13S. [Google Scholar] [CrossRef]
- Chu, C.M.; Lowder, J.L. Diagnosis and treatment of urinary tract infections across age groups. Am. J. Obstet. Gynecol. 2018, 219, 40–51. [Google Scholar] [CrossRef]
- Lema, V.M.; Lema, A.P.V. Sexual activity and the risk of acute uncomplicated urinary tract infection in premenopausal women: Implications for reproductive health programming. Obstet. Gynecol. Int. J. 2018, 9, 00303. [Google Scholar] [CrossRef][Green Version]
- Baral, P.; Neupane, S.; Marasini, B.P.; Ghimire, K.R.; Lekhak, B.; Shrestha, B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res. Notes 2012, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Dehbanipour, R.; Rastaghi, S.; Sedighi, M.; Maleki, N.; Faghri, J. High prevalence of multidrug-resistance uropathogenic Escherichia coli strains, Isfahan, Iran. J. Nat. Sci. Biol. Med. 2016, 7, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Linhares, I.; Raposo, T.; Rodrigues, A.; Almeida, A. Incidence and diversity of antimicrobial multidrug resistance profiles of uropathogenic bacteria. Biomed Res. Int. 2015, 2015, 354084. [Google Scholar] [CrossRef] [PubMed]
- Manciuc, C.; Mihai, I.F.; Filip-Ciubotaru, F.; Lacatusu, G.A. Resistance profile of multidrug-resistant urinary tract infections and their susceptibility to carbapenems. Farmacia 2020, 68, 715–721. [Google Scholar] [CrossRef]
- Sultan, A.; Rizvi, M.; Khan, F.; Sami, H.; Shukla, I.; Khan, H.M. Increasing antimicrobial resistance among uropathogens: Is fosfomycin the answer? Urol. Ann. 2015, 7, 26–30. [Google Scholar] [CrossRef]
- Mishra, M.P.; Debata, N.K.; Padhy, R.N. Surveillance of multidrug resistant uropathogenic bacteria in hospitalized patients in Indian. Asian Pac. J. Trop. Biomed. 2013, 3, 315–324. [Google Scholar] [CrossRef]
- Padmini, N.; Ajilda, A.A.K.; Sivakumar, N.; Selvakumar, G. Extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae: Critical tools for antibiotic resistance pattern. J. Basic Microbiol. 2017, 57, 460–470. [Google Scholar] [CrossRef]
- Martínez-Martínez, L.; Pascual, A.; García, I.; Tran, J.; Jacoby, G. Interaction of plasmid and host quinolone resistance. J. Antimicrob. Chemother. 2003, 51, 1037–1039. [Google Scholar] [CrossRef][Green Version]
- Shahbazi, S.; Asadi Karam, M.R.; Habibi, M.; Talebi, A.; Bouzari, S. Distribution of extended-spectrum β-lactam, quinolone and carbapenem resistance genes, and genetic diversity among uropathogenic Escherichia coli isolates in Tehran, Iran. J. Glob. Antimicrob. Resist. 2018, 14, 118–125. [Google Scholar] [CrossRef]
- Toner, L.; Papa, N.; Aliyu, S.H.; Dev, H.; Lawrentschuk, N.; Al-Hayek, S. Extended-spectrum beta-lactamase producing Enterobacteriaceae in hospital urinary tract infections: Incidence and antibiotic susceptibility profile over 9 years. World J. Urol. 2016, 34, 1031–1037. [Google Scholar] [CrossRef]
- Sbiti, M.; Lahmadi, K.; Louzi, L. Profil épidémiologique des entérobactéries uropathogènes productrices de bêta lactamases à spectre élargi. Pan. Afr. Med. J. 2017, 28, 29. [Google Scholar] [CrossRef]
- Grude, N.; Tveten, Y.; Kristiansen, B.E. Urinary tract infections in Norway: Bacterial aetiology and susceptibility. A retrospective study of clinical isolates. Clin. Microbiol. Infect. 2001, 7, 543–547. [Google Scholar] [CrossRef]
- Corkill, J.E.; Anson, J.J.; Hart, C.A. High prevalence of the plasmid-mediated quinolone resistance determinant qnrA in multidrug-resistant Enterobacteriaceae from blood cultures in Liverpool, UK. J. Antimicrob. Chemother. 2005, 56, 1115–1117. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Walsh, K.E.; Mills, D.M.; Walker, V.J.; Oh, H.; Robicsek, A.; Hooper, D.C. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 2006, 50, 1178–1182. [Google Scholar] [CrossRef]
- Bischoff, S.; Walter, T.; Gerigk, M.; Ebert, M.; Vogelmann, R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect. Dis. 2018, 18, 56. [Google Scholar] [CrossRef]
- Gasperini, B.; Cherubini, A.; Lucarelli, M.; Espinosa, E.; Prospero, E. Multidrug-resistant bacterial infections in geriatric hospitalized patients before and after the COVID-19 outbreak: Results from a retrospective observational study in two geriatric wards. Antibiotics 2021, 10, 95. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Stewardson, A.J.; Abbott, I.J.; Peleg, A.Y. Nitrofurantoin and fosfomycin for resistant urinary tract infections: Old drugs for emerging problems. Aust. Prescr. 2019, 42, 14–19. [Google Scholar] [CrossRef]
- Benea, E.O.; Gavriliu, L.C.; Popescu, C.; Popescu, G.A. Ghidul Angelescu. Terapie Antimicrobiana 2018, 3rd ed.; Editura Bucuresti: Bucharest, Romania, 2018; pp. 181–192. [Google Scholar]
- World Health Organization. Guidelines for the Collection of Clinical Specimens during Field Investigation of Outbreaks; World Health Organization: Geneva, Switzerland, 2000; pp. 1–51. [Google Scholar]
- Clinical and Laboratory Standards Institute® (CLSI). M 100 Performance Standards for Antimicrobial Susceptibility Testing, 28th ed. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 10 May 2020).

| Isolated Bacteria | BCH | EUH | MCH | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Gram negative | 226 | 86.25 | 266 | 95.68 | 152 | 96.20 | 644 | 92.26 |
| Escherichia coli | 75 | 28.62 | 109 | 39.20 | 99 | 62.65 | 283 | 40.54 |
| Klebsiella spp. | 114 | 43.51 | 111 | 39.92 | 35 | 22.15 | 260 | 37.24 |
| Pseudomonas aeruginosa | 20 | 7.63 | 29 | 10.43 | 11 | 6.96 | 60 | 8.59 |
| Proteus spp. | 17 | 6.48 | 17 | 6.11 | 7 | 4.43 | 41 | 5.87 |
| Gram positive | 36 | 13.74 | 12 | 4.31 | 6 | 3.79 | 54 | 7.73 |
| Enterococcus spp. | 13 | 4.96 | 11 | 3.95 | 6 | 3.79 | 30 | 4.29 |
| Staphylococcus spp. | 23 | 8.77 | 1 | 0.35 | - | - | 24 | 3.43 |
| Age Groups (Years) | Females | Males | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 18–29 | 18 | 6.45 | 28 | 6.68 | 46 | 6.59 |
| 30–39 | 14 | 5.01 | 9 | 2.14 | 23 | 3.29 |
| 40–49 | 16 | 5.73 | 15 | 3.57 | 31 | 4.44 |
| 50–59 | 33 | 11.82 | 33 | 7.87 | 66 | 9.45 |
| 60–69 | 69 | 24.73 | 126 | 30.07 | 195 | 27.93 |
| ≥70 | 129 | 46.23 | 208 | 49.64 | 337 | 48.28 |
| Antibiotics | Gram-Negative Organisms Isolated | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Klebsiella spp. | Pseudomonas aeruginosa | Proteus spp. | Total | |||||||||||
| R | S | NA | R | S | NA | R | S | NA | R | S | NA | R | S | NA | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n % | n % | |
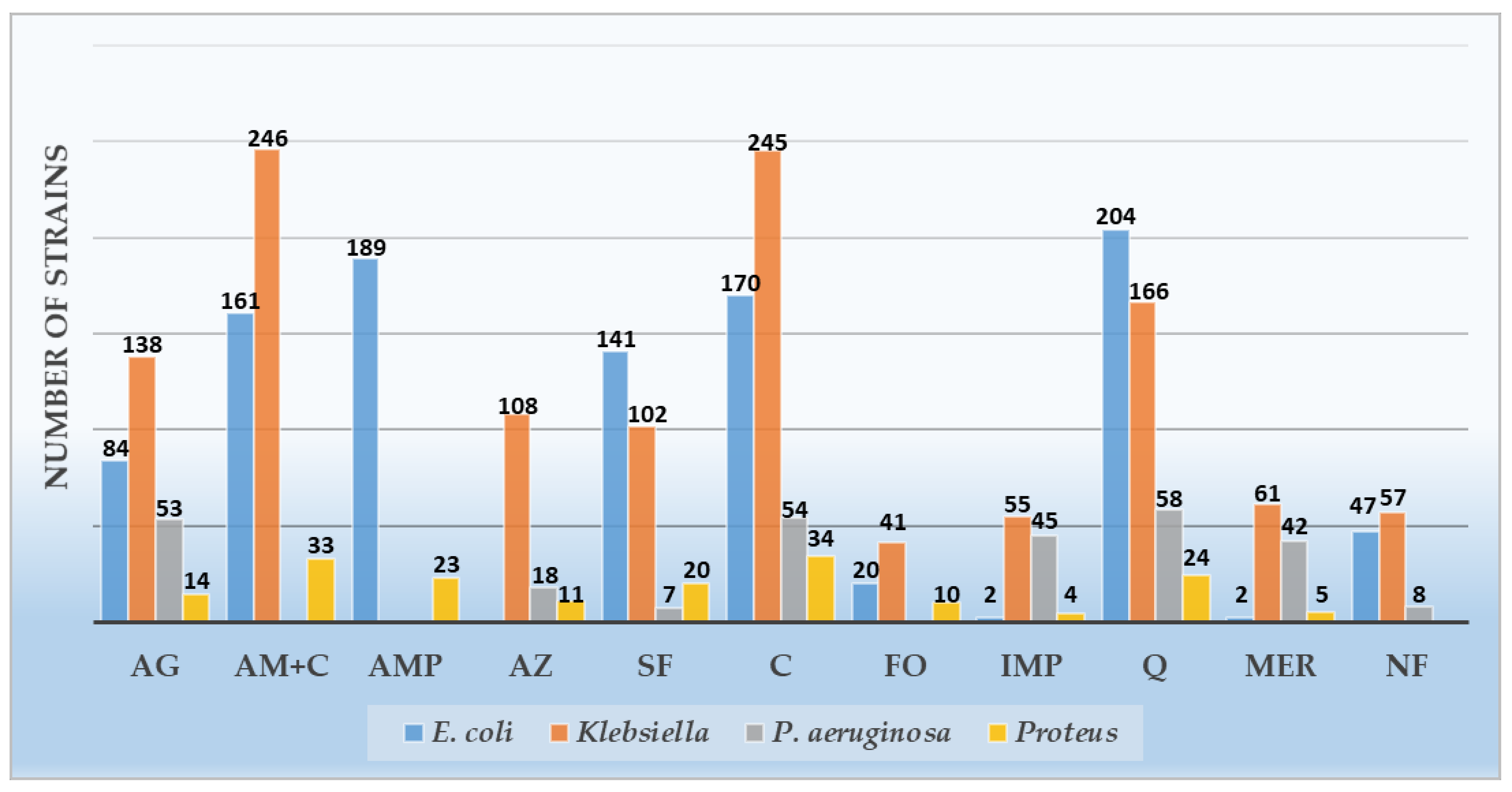
| Amikacin | 84 (29.68) | 189 (66.78) | 10 (3.53) | 138 (53.07) | 120 (46.15) | 2 (0.76) | 53 (88.33) | 6 (10.0) | 1 (1.66) | 14 (34.14) | 27 (65.85) | - | 289 (44.87) | 342 (53.1) | 13 (2.01) |
| Amoxicillin- Clavulanic ac. | 161 (56.89) | 114 (40.28) | 8 (2.82) | 246 (94.61) | 11 (4.23) | 3 (1.15) | - | - | - | 33 (80.48) | 7 (17.07) | 1 (2.43) | 440 (75.34) | 132 (22.6) | 12 (2.05) |
| Ampicillin | 189 (66.78) | 6 (2.12) | 88 (31.09) | - | - | - | - | - | - | 23 (56.09) | 2 (4.87) | 16 (39.02) | 212 (65.43) | 8 (2.46) | 104 (32.09) |
| Aztreonam | - | - | - | 108 (41.53) | 3 (1.15) | 149 (57.3) | 18 (30.0) | 2 (3.33) | 40 (66.66) | 11 (26.82) | 6 (14.63) | 24 (58.53) | 137 (37.95) | 11 (3.04) | 213 (59.0) |
| Trimethoprim/ Sulfamethoxazole | 141 (49.82) | 58 (20.49) | 84 (29.68) | 102 (39.23) | 35 (13.46) | 123 (47.3) | 7 (11.66) | 1 (1.66) | 52 (86.66) | 20 (48.78) | 4 (9.75) | 17 (41.46) | 270 (41.92) | 98 (15.21) | 276 (42.85) |
| Ceftazidime | 170 (60.07) | 101 (35.68) | 12 (4.24) | 245 (94.23) | 14 (5.38) | 1 (0.38) | 54 (90.0) | 6 (10.0) | 0 | 34 (82.92) | 6 (14.63) | 1 (2.43) | 503 (78.10) | 127 (19.72) | 14 (2.17) |
| Fosfomycin | 20 (7.06) | 237 (83.74) | 26 (9.18) | 41 (15.76) | 60 (23.07) | 159 (61.15) | - | - | - | 10 (24.39) | 6 (14.63) | 25 (60.97) | 71 (12.15) | 303 (51.88) | 210 (35.95) |
| Imipenem | 2 (0.7) | 81 (28.62) | 200 (70.67) | 55 (21.15) | 136 (52.3) | 69 (26.53) | 45 (75.0) | 6 (10.0) | 9 (15.0) | 4 (9.75) | 12 (29.26) | 25 (60.97) | 106 (16.45) | 235 (36.49) | 303 (47.04) |
| Levofloxacin | 204 (72.08) | 47 (16.6) | 32 (11.30) | 166 (63.84) | 68 (26.15) | 26 (10.0) | 58 (96.66) | 1 (1.66) | 1 (1.66) | 24 (58.53) | 10 (24.39) | 7 (17.07) | 452 (70.18) | 126 (19.56) | 66 (10.24) |
| Meropenem | 2 (0.7) | 86 (30.38) | 195 (68.90) | 61 (23.46) | 145 (55.76) | 54 (20.76) | 42 (70.0) | 10 (16.66) | 8 (13.33) | 5 (12.19) | 27 (65.85) | 9 (21.95) | 110 (17.08) | 268 (41.61) | 266 (41.3) |
| Nitrofurantoin | 47 (16.6) | 111 (39.22) | 125 (44.16) | 57 (21.92) | 38 (14.61) | 165 (63.46) | 8 (13.33) | 5 (8.33) | 47 (78.33) | - | - | - | 112 (18.57) | 154 (25.53) | 337 (55.88) |
| Antibiotics | n (%) |
|---|---|
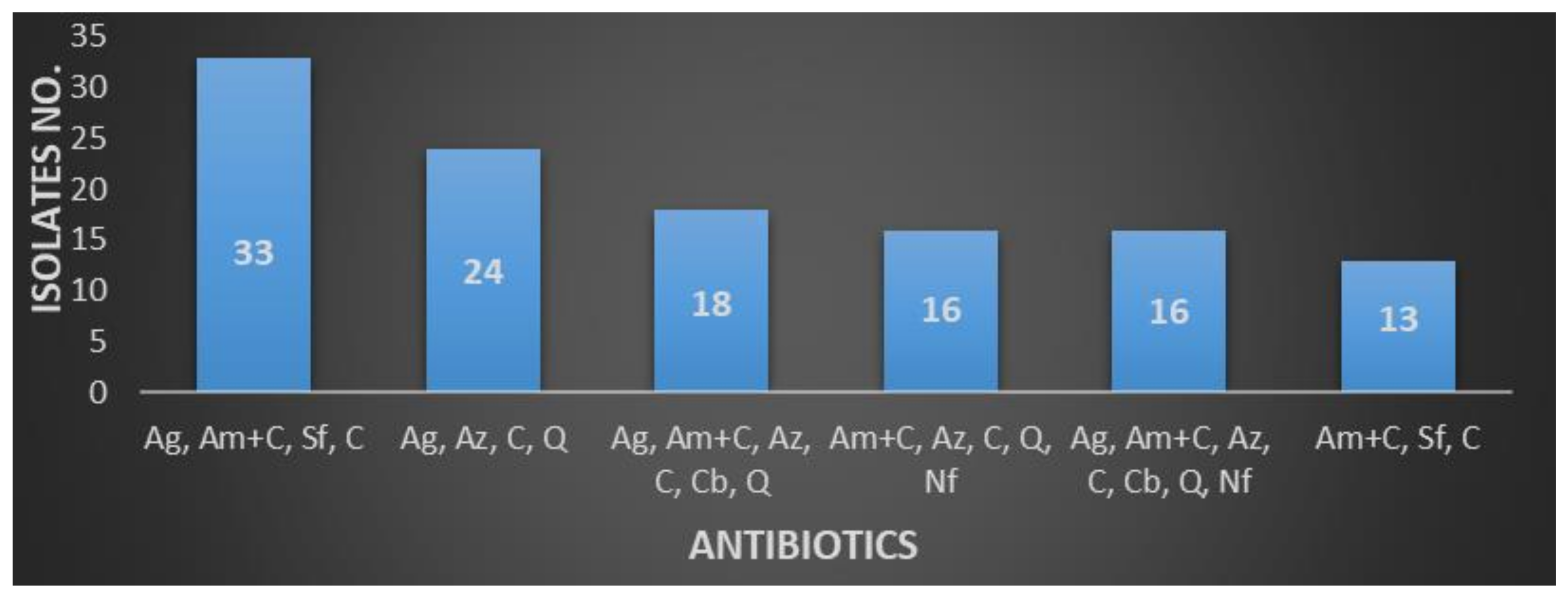
| Amoxicillin + Clavulanate, Aztreonam, Cephalosporins, Quinolones | 56 (8.02) |
| Aminoglycosides, Amoxicillin + Clavulanate, Sulfonamides, Cephalosporins | 35 (5.01) |
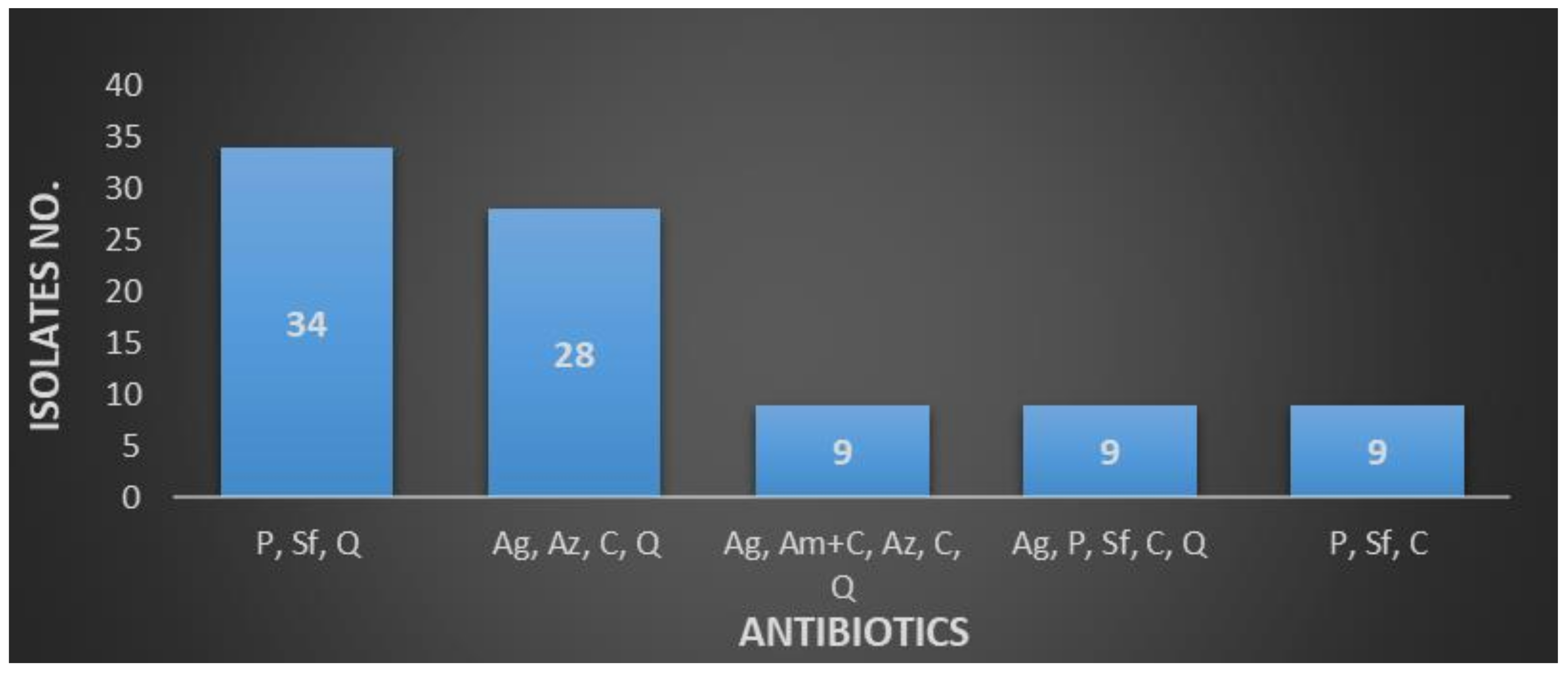
| Penicillin, Sulfonamides, Quinolones | 34 (4.87) |
| Aminoglycosides, Amoxicillin + Clavulanate, Aztreonam, Cephalosporins, Carbapenems, Quinolones | 28 (4.01) |
| Amoxicillin + Clavulanate, Aztreonam, Cephalosporins, Quinolones, Nitrofurantoin | 21 (3.0) |
| Aminoglycosides, Amoxicillin + Clavulanate, Aztreonam, Cephalosporins, Quinolones | 19 (2.72) |
| Aminoglycosides, Penicillin, Cephalosporins | 17 (2.43) |
| Aminoglycosides, Sulfonamides, Cephalosporins | 14 (2.0) |
| Penicillin, Cephalosporins, Quinolones | 12 (1.71) |
| Aminoglycosides, Cephalosporins, Carbapenems, Quinolones | 11 (1.57) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petca, R.-C.; Negoiță, S.; Mareș, C.; Petca, A.; Popescu, R.-I.; Chibelean, C.B. Heterogeneity of Antibiotics Multidrug-Resistance Profile of Uropathogens in Romanian Population. Antibiotics 2021, 10, 523. https://doi.org/10.3390/antibiotics10050523
Petca R-C, Negoiță S, Mareș C, Petca A, Popescu R-I, Chibelean CB. Heterogeneity of Antibiotics Multidrug-Resistance Profile of Uropathogens in Romanian Population. Antibiotics. 2021; 10(5):523. https://doi.org/10.3390/antibiotics10050523
Chicago/Turabian StylePetca, Răzvan-Cosmin, Silvius Negoiță, Cristian Mareș, Aida Petca, Răzvan-Ionuț Popescu, and Călin Bogdan Chibelean. 2021. "Heterogeneity of Antibiotics Multidrug-Resistance Profile of Uropathogens in Romanian Population" Antibiotics 10, no. 5: 523. https://doi.org/10.3390/antibiotics10050523
APA StylePetca, R.-C., Negoiță, S., Mareș, C., Petca, A., Popescu, R.-I., & Chibelean, C. B. (2021). Heterogeneity of Antibiotics Multidrug-Resistance Profile of Uropathogens in Romanian Population. Antibiotics, 10(5), 523. https://doi.org/10.3390/antibiotics10050523







