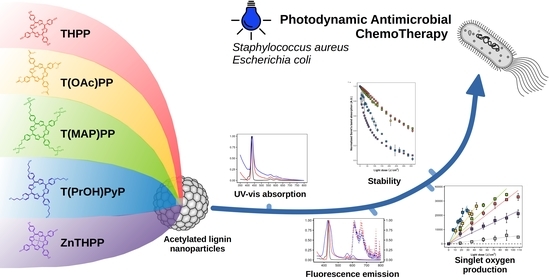
Photophysical and Antibacterial Properties of Porphyrins Encapsulated inside Acetylated Lignin Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
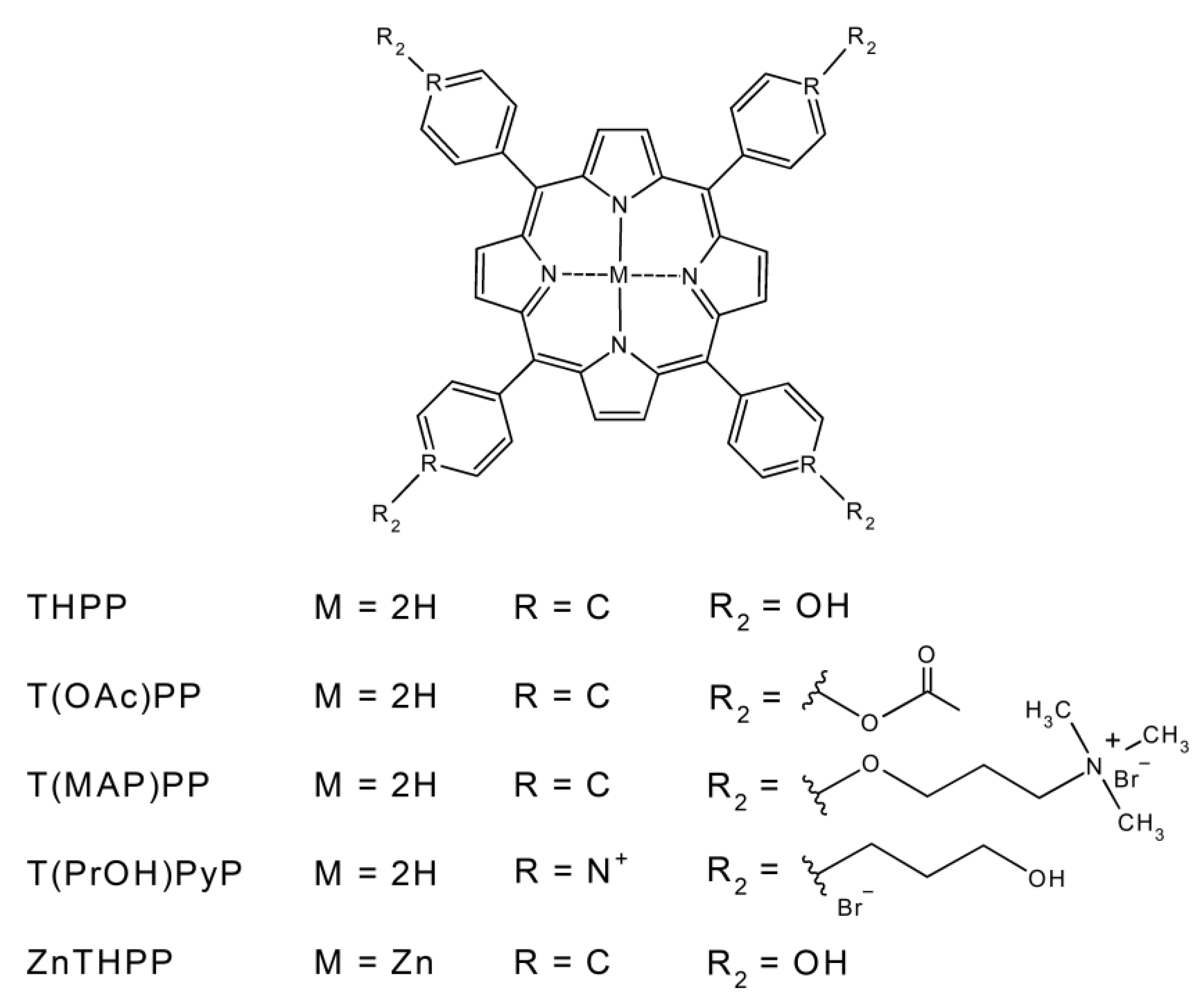
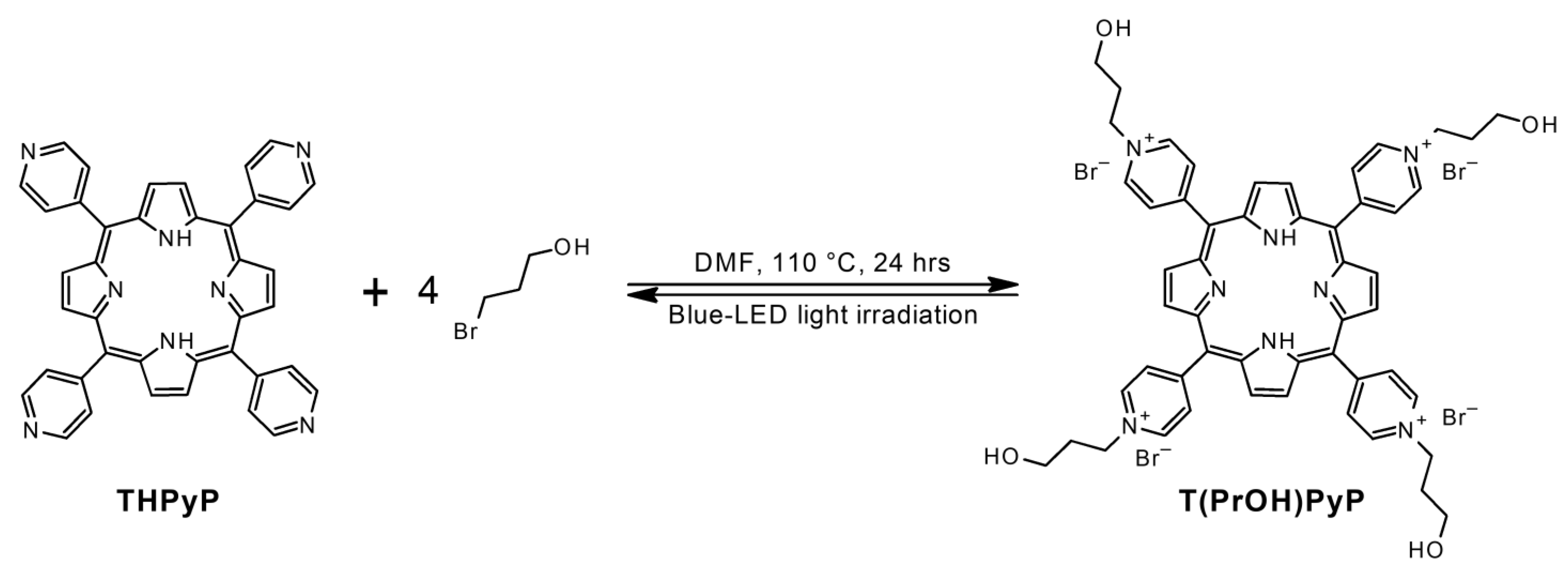
2.1. Porphyrins Synthesis
2.2. Preparation of Acetylated Lignin Nanoparticles
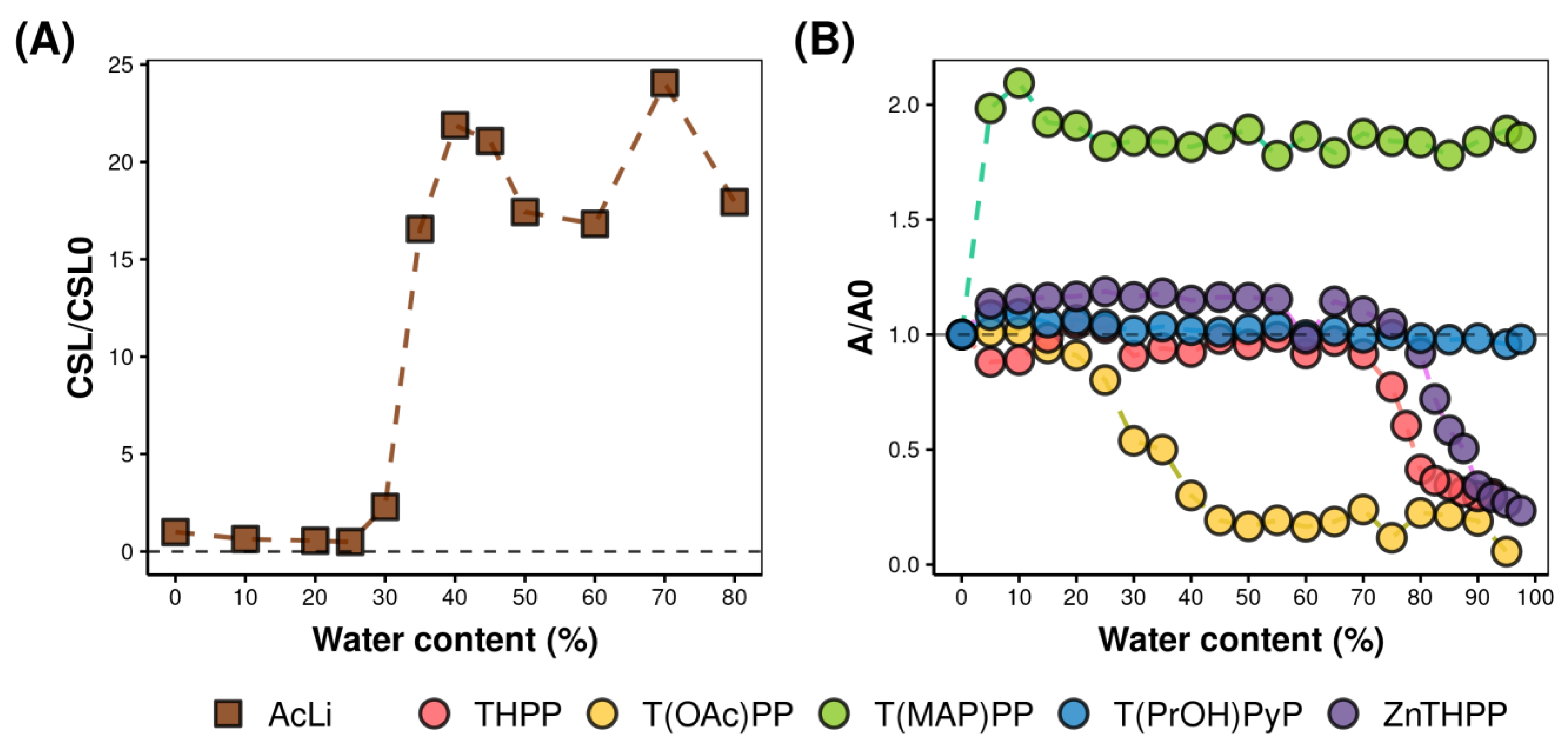
2.3. Physical Properties of Acetylated Lignin Nanoparticles
2.4. Photophysical Properties
2.4.1. UV-Vis Absorption Characterization
2.4.2. Fluorescence Quantum Yield
2.4.3. Singlet Oxygen Production
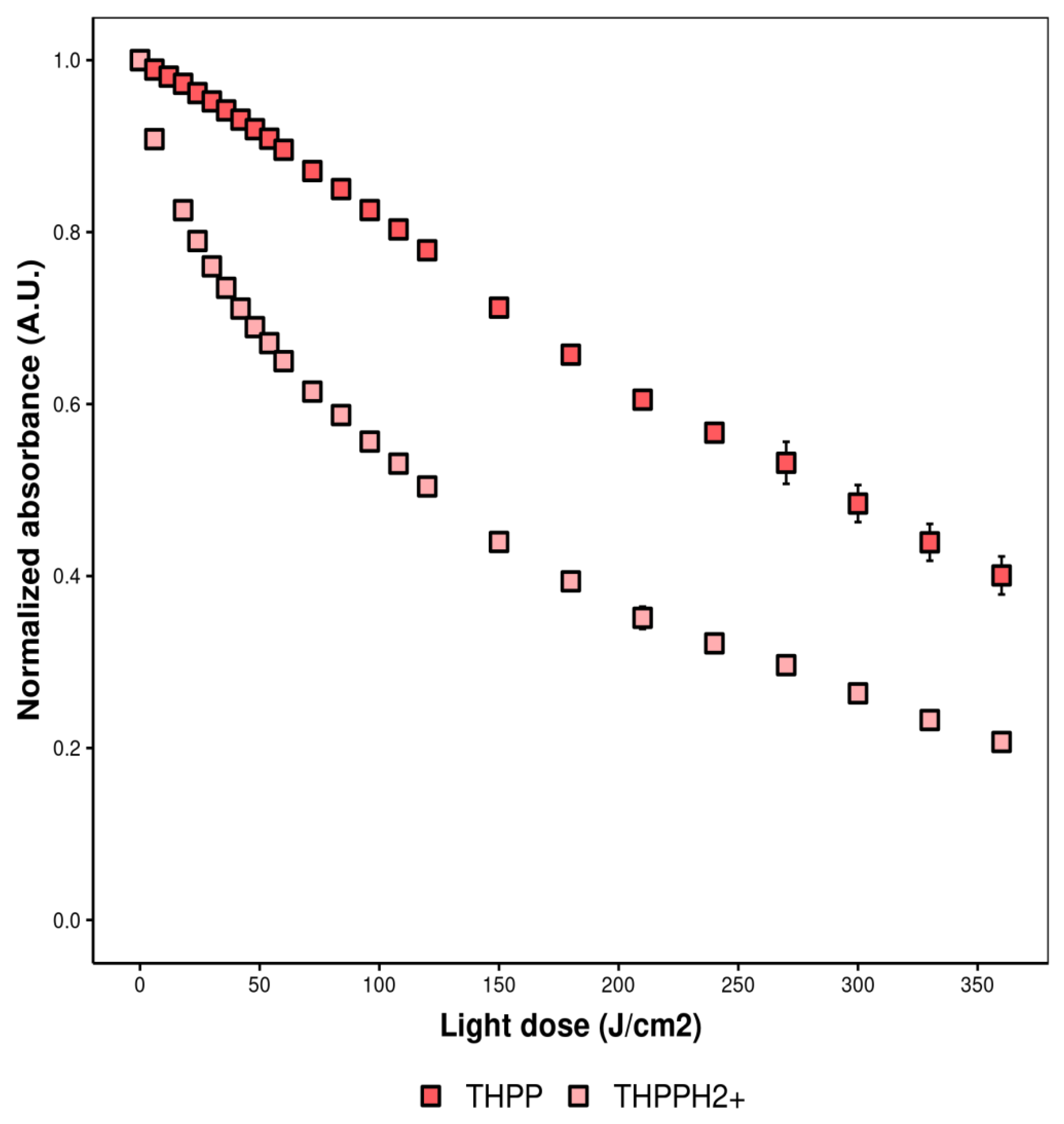
2.4.4. Photobleaching
2.5. Effect of the Aqueous Media on Porphyrins and Porphyrin Loaded Nanoparticles
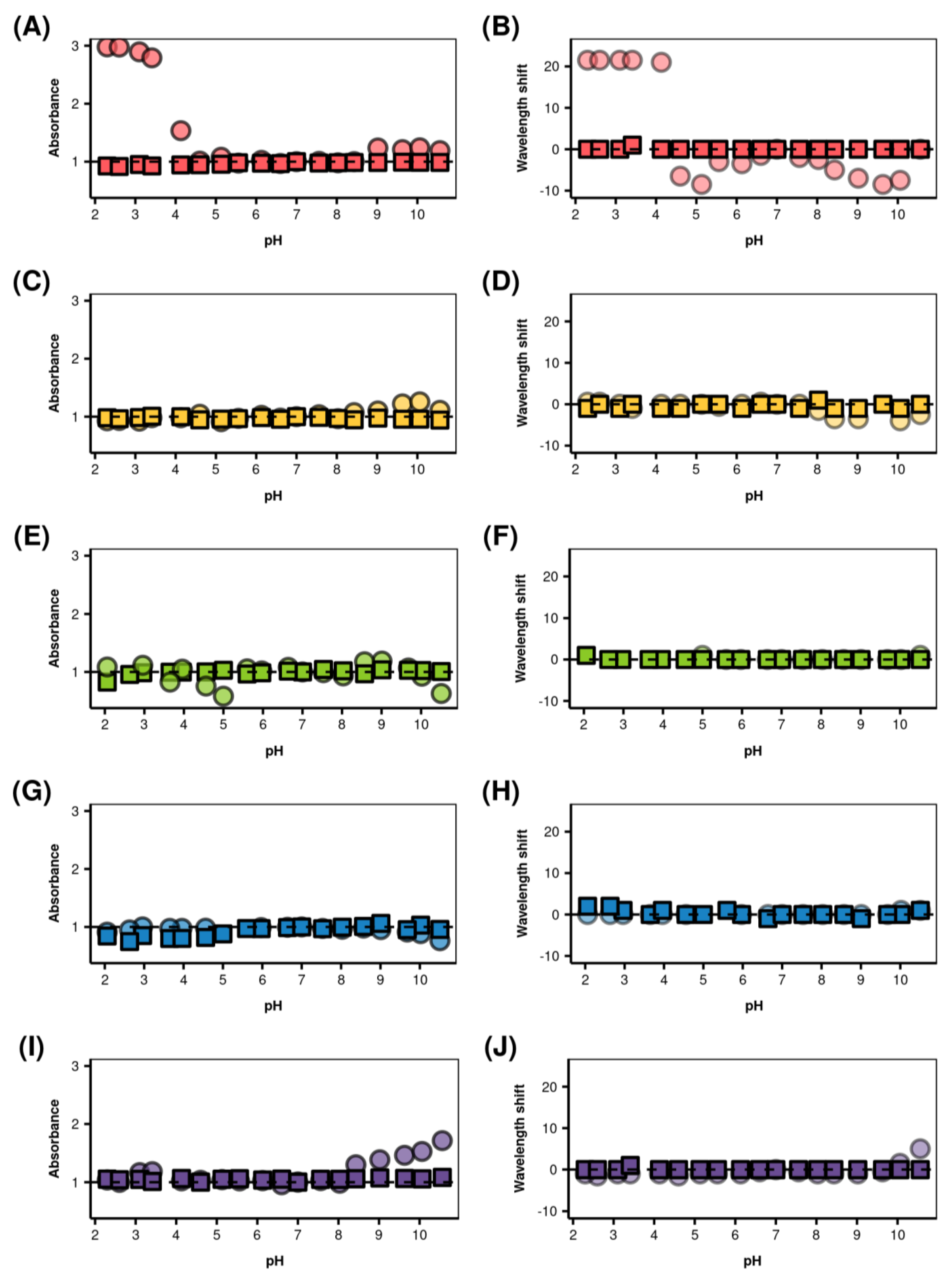
2.5.1. Effect of Fluctuations of pH into the Medium
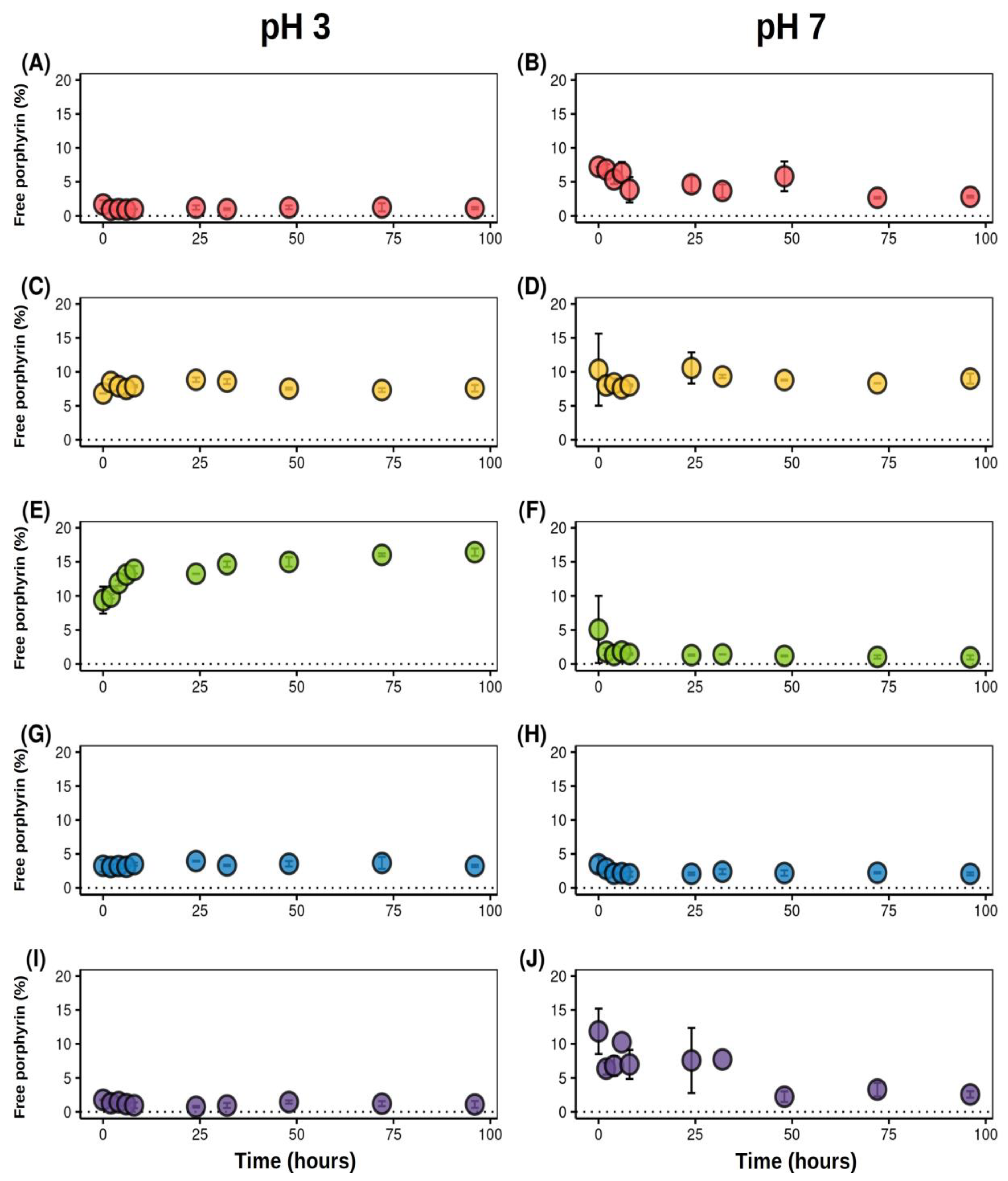
2.5.2. pH Driven Release
2.6. Photodynamic Antimicrobial Chemotherapy Effect
2.6.1. Bacteriostatic Effect
2.6.2. Bactericidal Effect
3. Conclusions
4. Materials and Methods
4.1. Materials, Equipment and Microbiological Strains
4.2. Synthesis of Porphyrins
4.2.1. 5,10,15,20-Tetrakis (4-acetyloxyphenyl)-21H,23H-porphine
4.2.2. 5,10,15,20-Tetrakis(4-(3-N,N,N-trimethylammoniumpropoxy)-phenyl)-21H,23H-porphine bromine
4.2.3. 5,10,15,20-Tetrakis (4-(3-hydroxy)propyloxypyridyl)-21H,23H-porphine bromine
4.2.4. Zinc (II) 5,10,15,20-Tetrakis (4-hydroxyphenyl)-21H,23H-porphine
4.3. Preparation and Quantification of Porphyrin-Loaded Acetylated Lignin Nanoparticles
4.4. Physical Characterization
4.5. Photophysical Characterization
4.6. Influence of pH for the Nanoparticles Behaviour and Stability
4.6.1. pH Buffers
4.6.2. pH Driven Release
4.7. Photodynamic Antimicrobial Chemotherapy
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/332081/9789240005587-eng.pdf (accessed on 15 March 2021).
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Murray, A.K. The Novel Coronavirus COVID-19 Outbreak: Global Implications for Antimicrobial Resistance. Front. Microbiol. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Khoshfetrat, M.; Keykha, A.; Sedaghatkia, M.; Farahmandrad, R.; Behnampour, M. Determination of Antibiotic Resistance Pattern of Organisms Isolated from Endotracheal Tube Cultures of Patients Admitted to Intensive Care Unit. Arch. Anesth. Crit. Care 2020, 6, 125–132. [Google Scholar] [CrossRef]
- Sarda, C.; Fazal, F.; Rello, J. Management of ventilator-associated pneumonia (VAP) caused by resistant gram-negative bacteria: Which is the best strategy to treat? Expert Rev. Respir. Med. 2019, 13, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Cunha, B.; Fonseca, L.P.; Calado, C.R. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photoantimicrobials and PACT: What’s in an abbreviation? Photochem. Photobiol. Sci. 2019, 18, 12–14. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Bahador, A.; Pourhajibagher, M.; Alikhani, M.Y. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Bacterial Infections. J. Lasers Med. Sci. 2018, 9, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Vinagreiro, C.S.; Zangirolami, A.; Schaberle, F.A.; Nunes, S.C.C.; Blanco, K.C.; Inada, N.M.; da Silva, G.J.; Pais, A.A.C.C.; Bagnato, V.S.; Arnaut, L.G.; et al. Antibacterial Photodynamic Inactivation of Antibiotic-Resistant Bacteria and Biofilms with Nanomolar Photosensitizer Concentrations. ACS Infect. Dis. 2020, 6, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Calvete, M.J.F.; Pucelik, B.; Dubin, G.; Arnaut, L.G.; Pereira, M.M.; Dąbrowski, J.M. Photoinactivation of microorganisms with sub-micromolar concentrations of imidazolium metallophthalocyanine salts. Eur. J. Med. Chem. 2019, 184, 111740. [Google Scholar] [CrossRef]
- Khaldi, Z.; Nzambe Takeki, J.K.; Ouk, T.-S.; Lucas, R.; Zerrouki, R. Synthesis and photo-bactericidal properties of a cationic porphyrin grafted onto kraft pulp fibers. J. Porphyr. Phthalocyanines 2019, 23, 489–496. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. ChemSusChem 2019, 12, 2039–2054. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops. Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Lauberts, M.; Dizhbite, T.; Lauberte, L.; Jurkjane, V.; Telysheva, G. Antioxidant activity of various lignins and lignin-related phenylpropanoid units with high and low molecular weight. Holzforschung 2015, 69, 795–805. [Google Scholar] [CrossRef]
- Rocca, D.M.; Vanegas, J.P.; Fournier, K.; Becerra, M.C.; Scaiano, J.C.; Lanterna, A.E. Biocompatibility and photo-induced antibacterial activity of lignin-stabilized noble metal nanoparticles. RSC Adv. 2018, 8, 40454–40463. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Marchand, G.; Villandier, N.; Ouk, T.-S.; Pereira, M.M.; Calvete, M.J.F.; Calliste, C.A.; Żak, A.; Piksa, M.; Pawlik, K.J.; et al. Porphyrin-Loaded Lignin Nanoparticles Against Bacteria: A Photodynamic Antimicrobial Chemotherapy Application. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Twyman, L.J. Probing Dense Packed Limits of a Hyperbranched Polymer through Ligand Binding and Size Selective Catalysis. Macromolecules 2013, 46, 7055–7074. [Google Scholar] [CrossRef]
- Majumder, R.; Roy, S.; Okamoto, K.; Nagao, S.; Matsuo, T.; Parui, P.P. Porphyrin-Based Probe for Simultaneous Detection of Interface Acidity and Polarity during Lipid-Phase Transition of Vesicles. Langmuir 2020, 36, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, H.; Chang, H.; Qiu, S.; Deng, B.; Liao, J. Covalent and Non-covalent Chemical Modification of Multi-walled Carbon Nanotubes with Tetra-(4-hydroxylphenyl)porphyrin and Its Complexes. Chin. J. Chem. 2011, 29, 1901–1905. [Google Scholar] [CrossRef]
- Qiu, N.; Li, Y.; Li, Y.; Wang, H.; Duan, Q.; Kakuchi, T. A photo- and thermo-responsive star-shaped diblock copolymer with a porphyrin core prepared via consecutive ATRPs. RSC Adv. 2016, 6, 47912–47918. [Google Scholar] [CrossRef]
- Tesakova, M.V.; Semeikin, A.S.; Parfenyuk, V.I. Electrochemical determination of antioxidant properties of a series of tetraphenylporphyrin derivatives and their zinc complexes. J. Porphyr. Phthalocyanines 2015, 19, 1032–1038. [Google Scholar] [CrossRef]
- Caminos, D.A.; Durantini, E.N. Synthesis of asymmetrically meso-substituted porphyrins bearing amino groups as potential cationic photodynamic agents. J. Porphyr. Phthalocyanines 2005, 9, 334–342. [Google Scholar] [CrossRef]
- Poli, E.; Ouk, T.S.; Barrière, G.; Lévèque, G.; Sol, V.; Denes, E. Does low hydroxyl group surface density explain less bacterial adhesion on porous alumina? Orthop. Traumatol. Surg. Res. 2019, 105, 473–477. [Google Scholar] [CrossRef]
- Beyle, A. Physical and Biochemical Risk Phenomena in Nanotechnology. In Nanotechnolgy Safety; Elsevier: Amsterdam, The Netherlands, 2013; pp. 219–231. [Google Scholar]
- Zannotti, M.; Giovannetti, R.; Minofar, B.; Řeha, D.; Plačková, L.; D’Amato, C.A.; Rommozzi, E.; Dudko, H.V.; Kari, N.; Minicucci, M. Aggregation and metal-complexation behaviour of THPP porphyrin in ethanol/water solutions as function of pH. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 235–248. [Google Scholar] [CrossRef]
- Leroy-Lhez, S.; Rezazgui, O.; Issawi, M.; Elhabiri, M.; Calliste, C.A.; Riou, C. Why are the anionic porphyrins so efficient to induce plant cell death? A structure-activity relationship study to solve the puzzle. J. Photochem. Photobiol. A Chem. 2019, 368, 276–289. [Google Scholar] [CrossRef]
- Marchand, G.; Fabre, G.; Maldonado-Carmona, N.; Villandier, N.; Leroy-Lhez, S. Acetylated lignin nanoparticles as a possible vehicle for photosensitizing molecules. Nanoscale Adv. 2020, 2, 5648–5658. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Ouk, T.-S.S.; Calvete, M.J.F.F.; Pereira, M.M.; Villandier, N.; Leroy-Lhez, S. Conjugating biomaterials with photosensitizers: Advances and perspectives for photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2020, 19, 445–461. [Google Scholar] [CrossRef]
- Pineiro, M.; Carvalho, A.L.; Pereira, M.M.; Gonsalves, A.D.; Arnaut, L.G.; Formosinho, S.J. Photoacoustic Measurements of Porphyrin Triplet-State Quantum Yields and Singlet-Oxygen Efficiencies. Chem. A Eur. J. 1998, 4, 2299–2307. [Google Scholar] [CrossRef]
- Callaghan, S.; Senge, M.O. The good, the bad, and the ugly—Controlling singlet oxygen through design of photosensitizers and delivery systems for photodynamic therapy. Photochem. Photobiol. Sci. 2018, 17, 1490–1514. [Google Scholar] [CrossRef]
- Riou, C.; Calliste, C.A.; Da Silva, A.; Guillaumot, D.; Rezazgui, O.; Sol, V.; Leroy-Lhez, S. Anionic porphyrin as a new powerful cell death inducer of Tobacco Bright Yellow-2 cells. Photochem. Photobiol. Sci. 2014, 13, 621. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Tanielian, C.; Dunsbach, R.; Wolff, C. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. J. Photochem. Photobiol. A Chem. 1994, 79, 11–17. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Effects of substituents on the photophysical properties of symmetrical porphyrins. Dyes Pigments 2013, 96, 440–448. [Google Scholar] [CrossRef]
- Caminos, D.A.; Spesia, M.B.; Durantini, E.N. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N,N,N-trimethylammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem. Photobiol. Sci. 2006, 5, 56–65. [Google Scholar] [CrossRef]
- Nardi, G.; Manet, I.; Monti, S.; Miranda, M.A.; Lhiaubet-Vallet, V. Scope and limitations of the TEMPO/EPR method for singlet oxygen detection: The misleading role of electron transfer. Free Radic. Biol. Med. 2014, 77, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Marchand, G.; Calliste, C.A.; Williams, R.M.; McLure, C.; Leroy-Lhez, S.; Villandier, N. Acetylated Lignins: A Potential Bio-Sourced Photosensitizer. Chem. Select. 2018, 3, 5512–5516. [Google Scholar] [CrossRef]
- Figueiredo, P.; Ferro, C.; Kemell, M.; Liu, Z.; Kiriazis, A.; Lintinen, K.; Florindo, H.F.; Yli-Kauhaluoma, J.; Hirvonen, J.; Kostiainen, M.A.; et al. Functionalization of carboxylated lignin nanoparticles for targeted and pH-responsive delivery of anticancer drugs. Nanomedicine 2017, 12, 2581–2596. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, D.; Tao, Y.; Li, Y.; Wang, L.; Liu, J.; He, J.; Lei, J. Self-assembled pH-responsive polymeric nanoparticles based on lignin-histidine conjugate with small particle size for efficient delivery of anti-tumor drugs. Biochem. Eng. J. 2020, 156, 107526. [Google Scholar] [CrossRef]
- Cui, J.-G.; Mo, D.-M.; Jiang, Y.; Gan, C.-F.; Li, W.-G.; Wu, A.; Li, X.-Y.; Xiao, J.-A.; Hu, Q.; Yuan, H.-Y.; et al. Fabrication, Characterization, and Insecticidal Activity Evaluation of Emamectin Benzoate–Sodium Lignosulfonate Nanoformulation with pH-Responsivity. Ind. Eng. Chem. Res. 2019, 58, 19741–19751. [Google Scholar] [CrossRef]
- Godard, J.; Chapron, D.; Bregier, F.; Rosilio, V.; Sol, V. Synthesis and supramolecular arrangement of new stearoyl acid-based phenalenone derivatives. Colloids Sur. A Physicochem. Eng. Asp. 2021, 612, 125988. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Le Guern, F.; Ouk, T.-S.; Yerzhan, I.; Nurlykyz, Y.; Arnoux, P.; Frochot, C.; Leroy-Lhez, S.; Sol, V. Photophysical and Bactericidal Properties of Pyridinium and Imidazolium Porphyrins for Photodynamic Antimicrobial Chemotherapy. Molecules 2021, 26, 1122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, Q.; Yu, S.; Yan, M.; Berglund, L. Optically Transparent Wood from a Nanoporous Cellulosic Template: Combining Functional and Structural Performance. Biomacromolecules 2016, 17, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
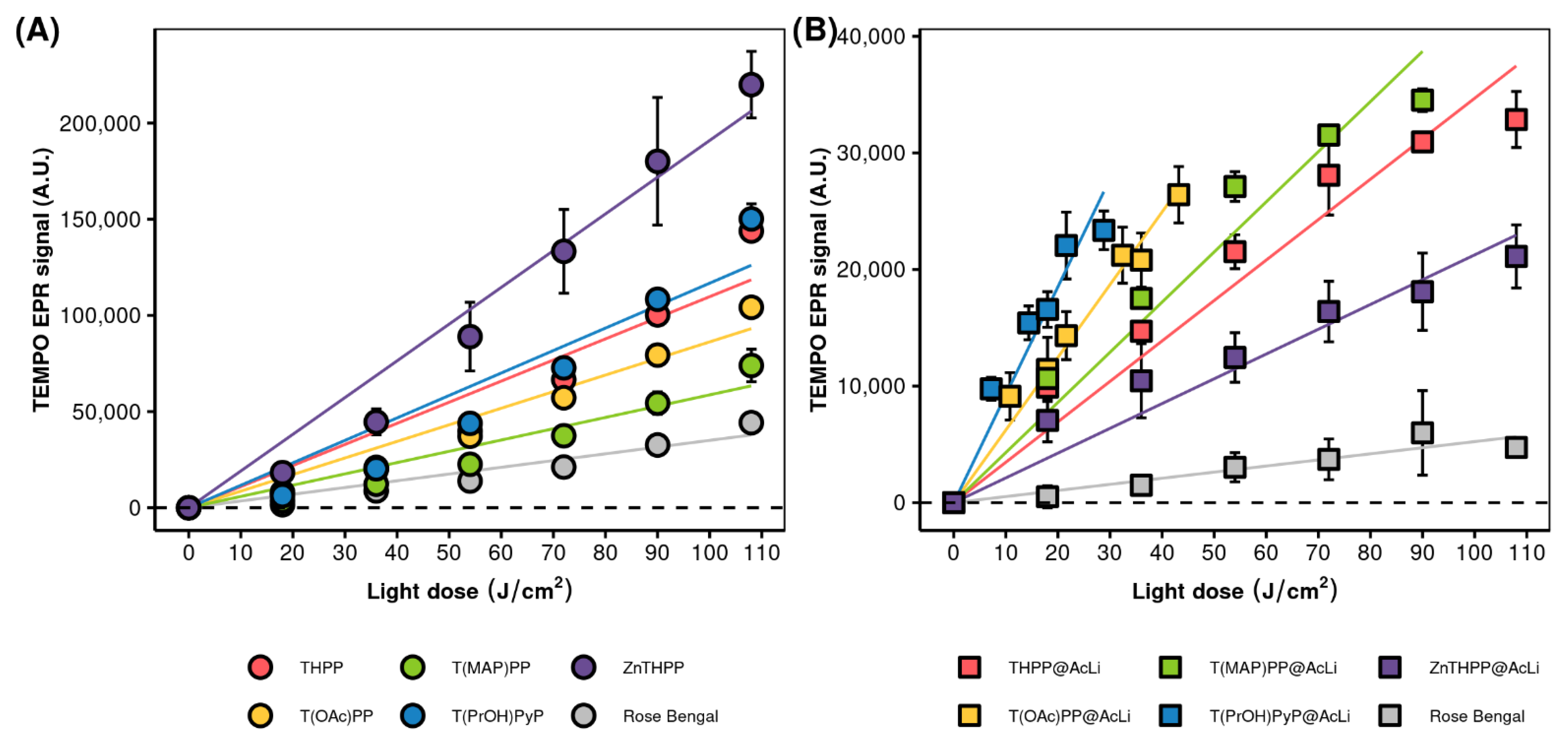
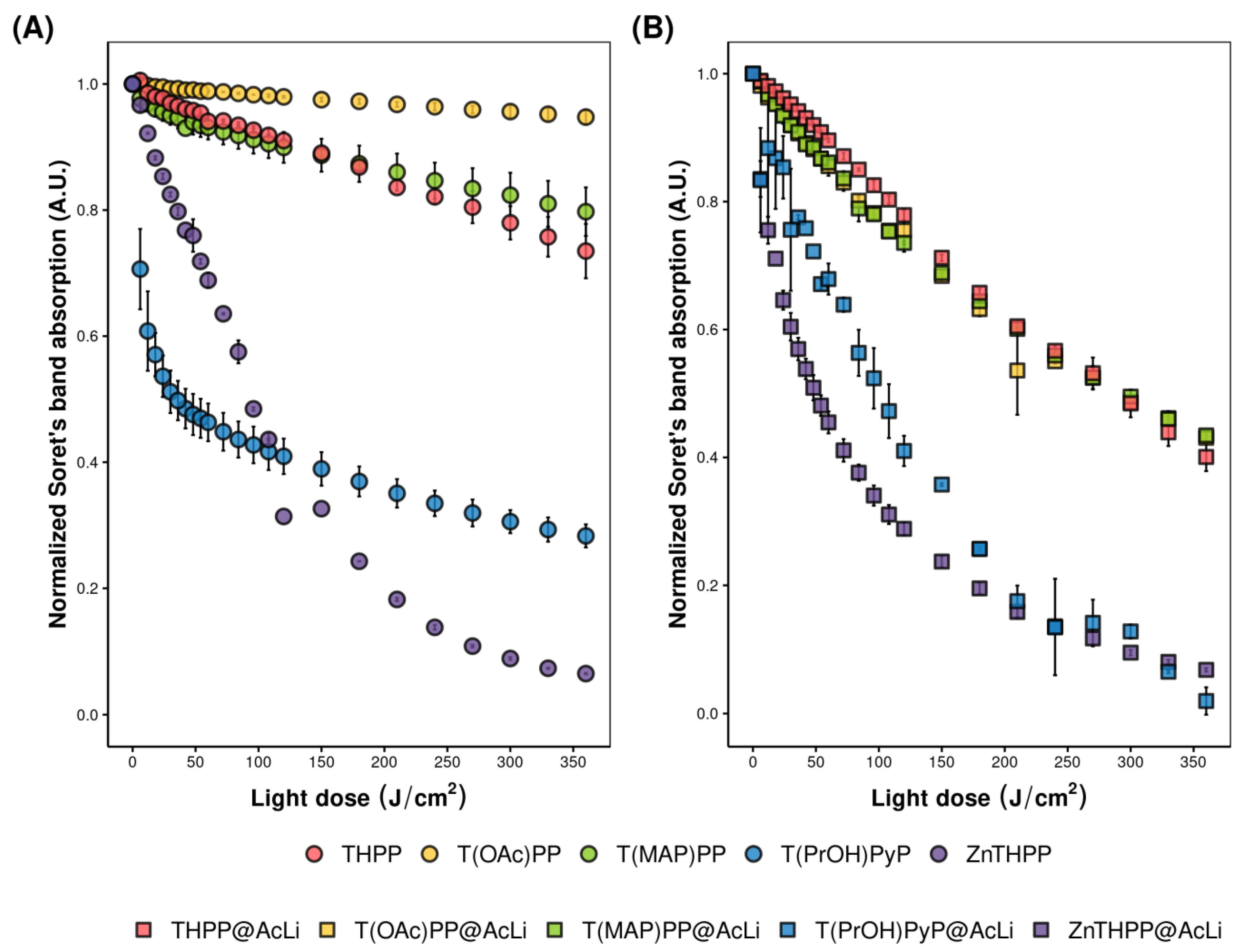
| Nanoparticles | DLS | PDI | Zeta Potential (mV) b | |||
|---|---|---|---|---|---|---|
| Mean Size (nm) | Range (D95) a | R2 Gaussian Model Fitting | Normality of Residuals (p Value) | |||
| @AcLi | 184.3 | 57.16–311.44 | 0.7517 | 0.2232 | 0.183 ± 0.026 | −22.1 ± 2.041 |
| THPP@AcLi | 160.4 | 51.29–262.98 | 0.8112 | 0.4243 | 0.126 ± 0.028 | −20.8 ± 0.474 |
| T(OAc)PP@AcLi | 199.6 | 78.92–320.28 | 0.8535 | 0.1327 | 0.122 ± 0.117 | −21.180 ± 0.887 |
| T(MAP)PP@AcLi | 886.2 | 597.8–1174.6 | 0.8931 | 0.8561 | 0.653 ± 0.145 | −2.808 ± 1.461 |
| T(PrOH)PyP@AcLi | 1348 | 880.6–1815.4 | 0.6612 | 0.9621 | 0.457 ± 0.016 | −9.962 ± 1.301 |
| ZnTHPP@AcLi | 208.2 | 77.88–338.52 | 0.8721 | 0.2714 | 0.117 ± 0.020 | −24.140 ± 1.618 |
| Porphyrin | Free in DMF | Free in PB pH 7 a | Free in PB pH 7 vs. Free in DMF | Encapsulated in PB pH 7 | Encapsulated vs. Free in DMF | Encapsulated vs. Free in PB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε (M−1 cm−1) | λ (nm) | ε (M−1 cm−1) | λ (nm) | εPB/εDMF | Δλ (nm) | ε (M−1 cm−1) | λ (nm) | ε/εFree | Δλ (nm) | ε/εFree | Δλ (nm) | |
| THPP | 35.0668 × 104 | 424 | 5.9361 × 104 | 440 | 0.169 | 16 | 12.9984 × 104 | 434 | 0.3707 | 10 | 2.1897 | −6 |
| T(OAc)PP | 36.1354 × 104 | 419 | 4.4123 × 104 | 429 | 0.122 | 10 | 12.0609 × 104 | 426 | 0.3338 | 7 | 2.7335 | −3 |
| T(MAP)PP | 20.1871 × 104 | 422 | 15.7138 × 104 | 425 | 0.778 | 3 | 20.7684 × 104 | 432 | 1.0288 | 10 | 1.3217 | −7 |
| T(PrOH)PyP | 13.2376 × 104 | 427 | 13.2908 × 104 | 419 | 1.004 | −8 | 1.3472 × 104 | 448 | 0.1018 | 21 | 0.1014 | −19 |
| ZnTHPP | 47.7749 × 104 | 430 | 5.9163 × 104 | 426 | 0.124 | −4 | 18.5219 × 104 | 435 | 0.3877 | 5 | 3.1307 | −9 |
| Porphyrin | Fluorescent Quantum Yield (ΦF) | ΦF encapsulated/ΦF free in PB (PB pH 7) | ||
|---|---|---|---|---|
| Free Porphyrin (DMF) | Free Porphyrin (PB pH 7) | Encapsulated Porphyrin (PB pH 7) | ||
| THPP | 0.1696 | 0.0034 | 0.0103 | 3.029 |
| T(OAc)PP | 0.1176 | 0.0089 | 0.0690 | 7.753 |
| T(MAP)PP | 0.1341 | 0.1349 | 0.0310 | 0.230 |
| T(PrOH)PyP a | 0.1933 | 0.4219 | 3.5835 a | 8.494 a |
| ZnTHPP | 0.0648 | 0.0063 | 0.0101 | 1.603 |
| Porphyrin | Singlet Oxygen Quantum (ΦΔ) | ||
|---|---|---|---|
| Free Porphyrin a | Literature b | ||
| THPP | 0.5900 | 0.57 (DMA oxidation, DMF, air, λEx = 401 nm) | [37] |
| T(OAc)PP | 0.6560 | ||
| T(MAP)PP | 0.6321 | 0.51 (DMA oxidation, DMF, air, λEx = 420 nm) | [38] |
| T(PrOH)PyP | 0.5804 | ||
| ZnTHPP | 0.7320 | ||
| Porphyrin | S. aureus | E. coli | ||
|---|---|---|---|---|
| Light | Dark | Light | Dark | |
| THPP | 0.78 µM | >50 µM | >50 µM | >50 µM |
| THPP@AcLi | >25 µM | >50 µM | >50 µM | >50 µM |
| T(OAc)PP | >50 µM | >50 µM | >50 µM | >50 µM |
| T(OAc)PP@AcLi | >50 µM | >50 µM | >50 µM | >50 µM |
| T(MAP)PP | 1.56 µM | 3.13 µM | 1.56 µM | >50 µM |
| T(MAP)PP@AcLi | >50 µM | >50 µM | 50 µM | 50 µM |
| T(PrOH)PyP | 6.25 µM | 50 µM | 1.56 µM | >50 µM |
| T(PrOH)PyP@AcLi | >50 µM | >50 µM | 50 µM | 50 µM |
| ZnTHPP | 0.78 µM | 3.13 µM | >50 µM | >50 µM |
| ZnTHPP@AcLi | 50 µM | 50 µM | 50 µM | 50 µM |
| Porphyrin | S. aureus | E. coli | ||
|---|---|---|---|---|
| Light | Dark | Light | Dark | |
| THPP | 0.0488 µM (16) | 0.1953 µM | >50 µM | >50 µM |
| THPP@AcLi | 0.7813 µM (32) | >1.5625 µM | >50 µM | >50 µM |
| T(OAc)PP | >50 µM | >50 µM | >50 µM | >50 µM |
| T(OAc)PP@AcLi | >50 µM | >50 µM | >50 µM | >50 µM |
| T(MAP)PP | 0.0500 µM (32) | >0.2 µM | 0.4 µM (4) | >0.4 µM |
| T(MAP)PP@AcLi | >50 µM | >50 µM | >50 µM | >50 µM |
| T(PrOH)PyP | 0.200 µM (32) | >0.2 µM | 0.2 µM (8) | >0.2 µM |
| T(PrOH)PyP@AcLi | 1.0 µM (50) | >2 µM | 6.25 µM (8) | >50 µM |
| ZnTHPP | 0.0977 µM (8) | >0.1953 µM | >50 µM | >50 µM |
| ZnTHPP@AcLi | 6.25 µM (8) | >50 µM | >50 µM | >50 µM |
| Porphyrin | Solvent or Solvents Mixtures |
|---|---|
| THPP | Acetone |
| T(OAc)PP | Acetone:DMF 9:1 |
| T(MAP)PP | Acetone:DMSO 9:1 |
| T(PrOH)PyP | Acetone:DMSO 9:1 |
| ZnTHPP | THF |
| Porphyrin | Excitation Wavelength | Emission Wavelength |
|---|---|---|
| THPP | 419 nm | 652 nm |
| T(OAc)PP | 415 nm | 648 nm |
| T(MAP)PP | 418 nm | 652 nm |
| T(PrOH)PyP | 427 nm | 650 nm |
| ZnTHPP | 424 nm | 607 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Carmona, N.; Ouk, T.-S.; Villandier, N.; Calliste, C.A.; Calvete, M.J.F.; Pereira, M.M.; Leroy-Lhez, S. Photophysical and Antibacterial Properties of Porphyrins Encapsulated inside Acetylated Lignin Nanoparticles. Antibiotics 2021, 10, 513. https://doi.org/10.3390/antibiotics10050513
Maldonado-Carmona N, Ouk T-S, Villandier N, Calliste CA, Calvete MJF, Pereira MM, Leroy-Lhez S. Photophysical and Antibacterial Properties of Porphyrins Encapsulated inside Acetylated Lignin Nanoparticles. Antibiotics. 2021; 10(5):513. https://doi.org/10.3390/antibiotics10050513
Chicago/Turabian StyleMaldonado-Carmona, Nidia, Tan-Sothea Ouk, Nicolas Villandier, Claude Alain Calliste, Mário J. F. Calvete, Mariette M. Pereira, and Stéphanie Leroy-Lhez. 2021. "Photophysical and Antibacterial Properties of Porphyrins Encapsulated inside Acetylated Lignin Nanoparticles" Antibiotics 10, no. 5: 513. https://doi.org/10.3390/antibiotics10050513
APA StyleMaldonado-Carmona, N., Ouk, T.-S., Villandier, N., Calliste, C. A., Calvete, M. J. F., Pereira, M. M., & Leroy-Lhez, S. (2021). Photophysical and Antibacterial Properties of Porphyrins Encapsulated inside Acetylated Lignin Nanoparticles. Antibiotics, 10(5), 513. https://doi.org/10.3390/antibiotics10050513