Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling?
Abstract
1. Introduction
2. Data Sources
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
3. Data Analysis
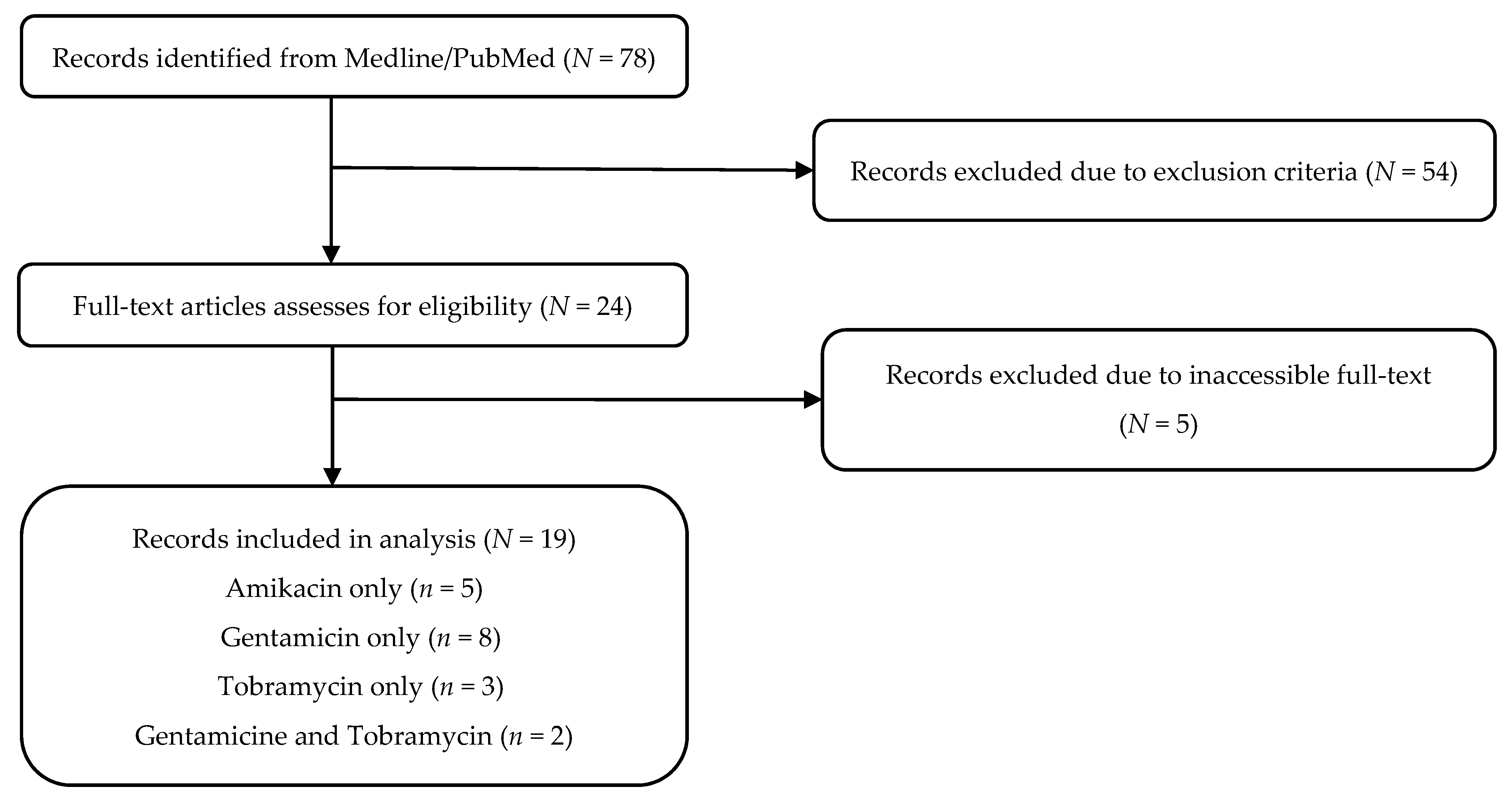
3.1. Study Selection
3.2. Population Characteristics
3.3. Study Designs and Protocols
3.4. Population Pharmacokinetic Analysis
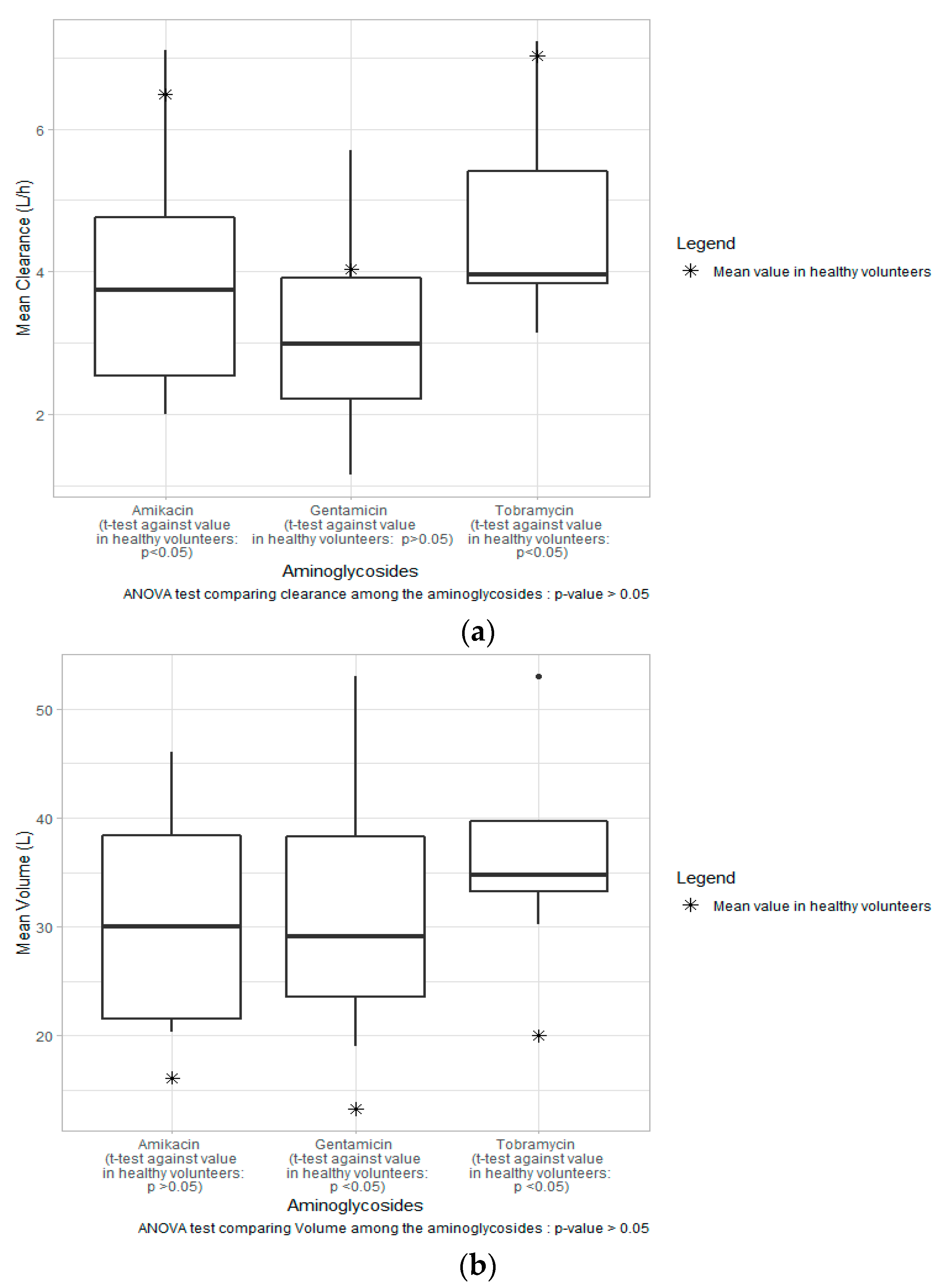
3.5. Estimated Parameters
3.6. Random Effect Modeling
3.7. Inclusion of Covariates
3.8. Simulation of Dosing Regimens
4. Discussion
4.1. Major Covariates
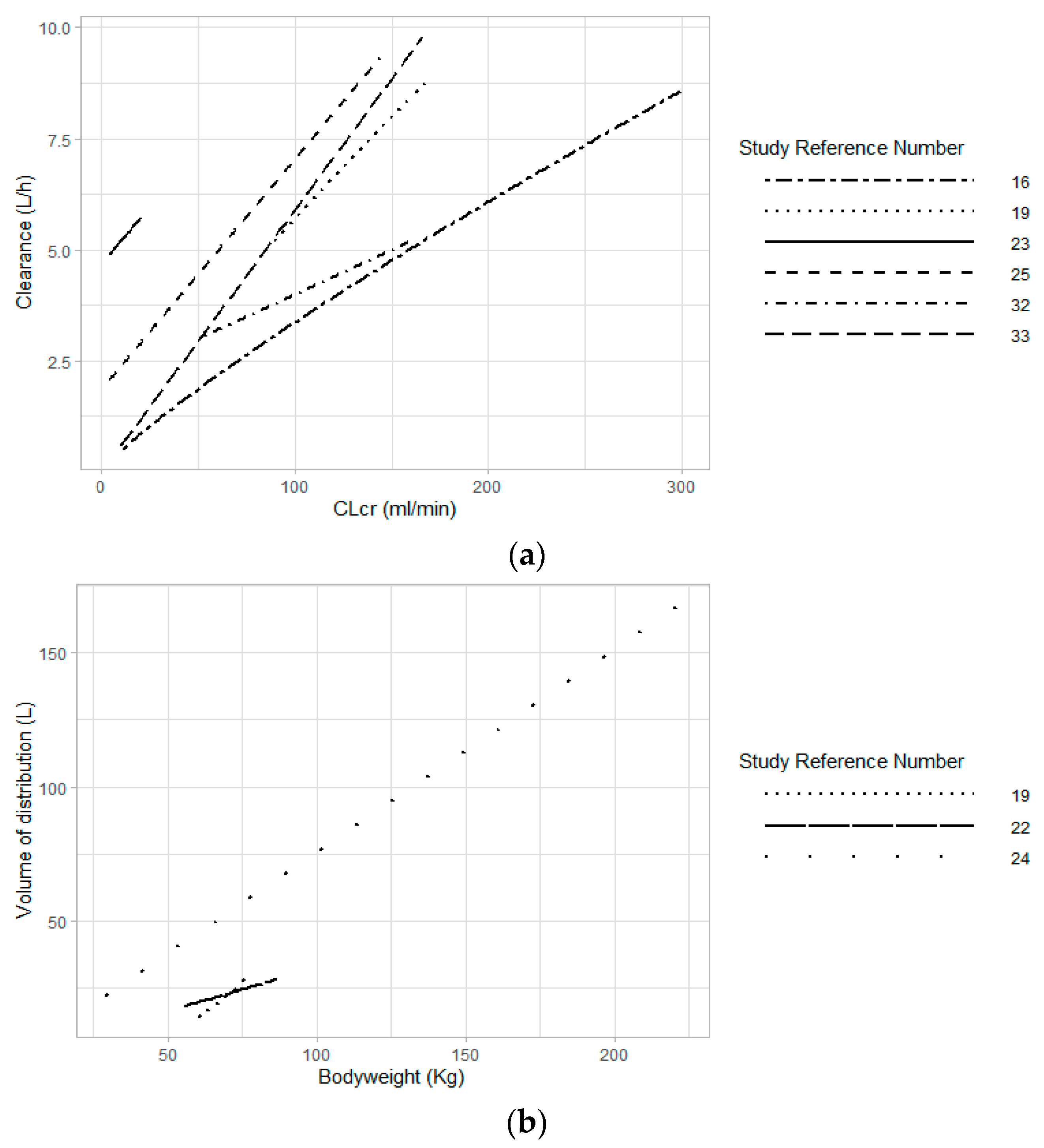
4.1.1. Renal Function
4.1.2. Bodyweight and Body Size
4.2. External Validation and Application
4.3. Simulation of Dosing Regimens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and Resistance. Antimicrob. Agents Chemother. 1999, 43, 727. [Google Scholar] [CrossRef] [PubMed]
- Dombrovskiy, V.Y.; Martin, A.A.; Sunderram, J.; Paz, H.L. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit. Care Med. 2007, 35, 1244–1250. [Google Scholar] [CrossRef]
- Buijk, S.; Mouton, J.; Gyssens, I.; Verbrugh, H.; Bruining, H. Experience with a once-daily dosing program of aminoglycosides in critically ill patients. Intensive Care Med. 2002, 28, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef]
- Bland, C.M.; Pai, M.P.; Lodise, T.P. Reappraisal of Contemporary Pharmacokinetic and Pharmacodynamic Principles for Informing Aminoglycoside Dosing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 1229–1238. [Google Scholar] [CrossRef]
- Craig, W.A. Optimizing Aminoglycoside Use. Crit. Care Clin. 2011, 27, 107–121. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Drusano, G.L.; Ambrose, P.G.; Bhavnani, S.M.; Bertino, J.S.; Nafziger, A.N.; Louie, A. Back to the Future: Using Aminoglycosides Again and How to Dose Them Optimally. Clin. Infect. Dis. 2007, 45, 753–760. [Google Scholar] [CrossRef]
- Germovsek, E.; Barker, C.I.; Sharland, M. What do I need to know about aminoglycoside antibiotics? Arch. Dis. Child.-Educ. Pract. Ed. 2017, 102, 89. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- de Velde, F.; Mouton, J.W.; de Winter, B.C.M.; van Gelder, T.; Koch, B.C.P. Clinical applications of population pharmacokinetic models of antibiotics: Challenges and perspectives. Pharmacol. Res. 2018, 134, 280–288. [Google Scholar] [CrossRef]
- Lovern, M.; Sargentini-Maier, M.-L.; Otoul, C.; Watelet, J.-B. Population pharmacokinetic and pharmacodynamic analysis in allergic diseases. Drug Metab. Rev. 2009, 41, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Hayes, M.; Ling, A.; Shamseer, L.; Chant, C.; Edwards, D.J.; Edwards, S.; Ensom, M.H.H.; Foster, D.R.; Hardy, B.; et al. Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clin. Pharmacokinet. 2015, 54, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Marsot, A.; Guilhaumou, R.; Riff, C.; Blin, O. Amikacin in Critically Ill Patients: A Review of Population Pharmacokinetic Studies. Clin. Pharmacokinet. 2017, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Boidin, C.; Bourguignon, L.; Cohen, S.; Roger, C.; Lefrant, J.-Y.; Roberts, J.A.; Allaouchiche, B.; Lepape, A.; Friggeri, A.; Goutelle, S. Amikacin Initial Dose in Critically Ill Patients: A Nonparametric Approach To Optimize a Priori Pharmacokinetic/Pharmacodynamic Target Attainments in Individual Patients. Antimicrob. Agents Chemother. 2019, 63, e00993-19. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Wallis, S.C.; Muller, L.; Saissi, G.; Lipman, J.; Lefrant, J.-Y.; Roberts, J.A. Influence of Renal Replacement Modalities on Amikacin Population Pharmacokinetics in Critically Ill Patients on Continuous Renal Replacement Therapy. Antimicrob. Agents Chemother. 2016, 60, 4901. [Google Scholar] [CrossRef]
- Carrié, C.; Delzor, F.; Roure, S.; Dubuisson, V.; Petit, L.; Molimard, M.; Breilh, D.; Biais, M. Population Pharmacokinetic Study of the Suitability of Standard Dosing Regimens of Amikacin in Critically Ill Patients with Open-Abdomen and Negative-Pressure Wound Therapy. Antimicrob. Agents Chemother. 2020, 64, e02098-19. [Google Scholar] [CrossRef]
- Aréchiga-Alvarado, N.A.; Medellín-Garibay, S.E.; Milán-Segovia, R.d.C.; Ortiz-Álvarez, A.; Magaña-Aquino, M.; Romano-Moreno, S. Population Pharmacokinetics of Amikacin Administered Once Daily in Patients with Different Renal Functions. Antimicrob. Agents Chemother. 2020, 64, e02178-19. [Google Scholar] [CrossRef]
- Petitcollin, A.; Dequin, P.F.; Darrouzain, F.; Vecellio, L.; Boulain, T.; Garot, D.; Paintaud, G.; Ternant, D.; Ehrmann, S. Pharmacokinetics of high-dose nebulized amikacin in ventilated critically ill patients. J. Antimicrob. Chemother. 2016, 71, 3482–3486. [Google Scholar] [CrossRef]
- French, M.A.; Cerra, F.B.; Plaut, M.E.; Schentag, J.J. Amikacin and gentamicin accumulation pharmacokinetics and nephrotoxicity in critically ill patients. Antimicrob. Agents Chemother. 1981, 19, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hodiamont, C.J.; Juffermans, N.P.; Bouman, C.S.C.; de Jong, M.D.; Mathôt, R.A.A.; van Hest, R.M. Determinants of gentamicin concentrations in critically ill patients: A population pharmacokinetic analysis. Int. J. Antimicrob. Agents 2017, 49, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Teigen, M.M.B.; Duffull, S.; Dang, L.; Johnson, D.W. Dosing of Gentamicin in Patients with End-Stage Renal Disease Receiving Hemodialysis. J. Clin. Pharmacol. 2006, 46, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Rea, R.S.; Capitano, B.; Bies, R.; Bigos, K.L.; Smith, R.; Lee, H. Suboptimal Aminoglycoside Dosing in Critically Ill Patients. Ther. Drug Monit. 2008, 30, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.C.; Prins, J.M.; Mistício, M.C.; Nunguiane, G.; Lang, C.N.; Beirão, J.C.; Mathôt, R.A.A.; van Hest, R.M. Population Pharmacokinetics with Monte Carlo Simulations of Gentamicin in a Population of Severely Ill Adult Patients from Sub-Saharan Africa. Antimicrob. Agents Chemother. 2019, 63, e02328-18. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; Janssen, J.M.; de Jong, M.D.; Mathôt, R.A.; Juffermans, N.P.; van Hest, R.M. Therapeutic Drug Monitoring of Gentamicin Peak Concentrations in Critically Ill Patients. Ther. Drug Monit. 2017, 39, 522–530. [Google Scholar] [CrossRef]
- Roberts, J.A.; Field, J.; Visser, A.; Whitbread, R.; Tallot, M.; Lipman, J.; Kirkpatrick, C.M.J. Using population pharmacokinetics to determine gentamicin dosing during extended daily diafiltration in critically ill patients with acute kidney injury. Antimicrob. Agents Chemother. 2010, 54, 3635–3640. [Google Scholar] [CrossRef]
- Barletta, J.F.; Johnson, S.B.; Nix, D.E.; Nix, L.C.; Erstad; Brian, L. Population Pharmacokinetics of Aminoglycosides in Critically Ill Trauma Patients on Once-Daily Regimens. J. Trauma Acute Care Surg. 2000, 49, 869–872. [Google Scholar] [CrossRef]
- Gomes, A.; van der Wijk, L.; Proost, J.H.; Sinha, B.; Touw, D.J. Pharmacokinetic modeling of gentamicin in treatment of infective endocarditis: Model development and validation of existing models. PLoS ONE 2017, 12, e0177324. [Google Scholar] [CrossRef]
- Watling, S.M.; Kisor, D.F. Population Pharmacokinetics: Development of a Medical Intensive Care Unit-Specific Gentamicin Dosing Nomogram. Ann. Pharmacother. 1993, 27, 151–154. [Google Scholar] [CrossRef]
- Kisor, D.F.; Watling, S.M.; Zarowitz, B.J.; Jelliffe, R.W. Population Pharmacokinetics of Gentamicin. Clin. Pharmacokinet. 1992, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Conil, J.-M.; Georges, B.; Ruiz, S.; Rival, T.; Seguin, T.; Cougot, P.; Fourcade, O.; Pharmd, G.H.; Saivin, S. Tobramycin disposition in ICU patients receiving a once daily regimen: Population approach and dosage simulations. Br. J. Clin. Pharmacol. 2011, 71, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Aarons, L.; Vozeh, S.; Wenk, M.; Weiss, P.; Follath, F. Population pharmacokinetics of tobramycin. Br. J. Clin. Pharm. 1989, 28, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hennig, S.; Standing, J.F.; Staatz, C.E.; Thomson, A.H. Population Pharmacokinetics of Tobramycin in Patients with and without Cystic Fibrosis. Clin. Pharmacokinet. 2013, 52, 289–301. [Google Scholar] [CrossRef]
- Bilbao-Meseguer, I.; Rodríguez-Gascón, A.; Barrasa, H.; Isla, A.; Solinís, M.Á. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic Acute Kidney Injury in Critically Ill Patients: Clinical Characteristics and Outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431. [Google Scholar] [CrossRef] [PubMed]
- Lode, H.; Grunert, K.; Koeppe, P.; Langmaack, H. Pharmacokinetic and Clinical Studies with Amikacin, a New Aminoglycoside Antibiotic. J. Infect. Dis. 1976, 134, S316–S322. [Google Scholar] [CrossRef]
- Demczar, D.J.; Nafziger, A.N.; Bertino, J.S., Jr. Pharmacokinetics of gentamicin at traditional versus high doses: Implications for once-daily aminoglycoside dosing. Antimicrob. Agents Chemother. 1997, 41, 1115–1119. [Google Scholar] [CrossRef]
- Garraffo, R.; Drugeon, H.B.; Dellamonica, P.; Bernard, E.; Lapalus, P. Determination of optimal dosage regimen for amikacin in healthy volunteers by study of pharmacokinetics and bactericidal activity. Antimicrob. Agents Chemother. 1990, 34, 614–621. [Google Scholar] [CrossRef]
- Green, T.P.; Mirkin, B.L.; Peterson, P.K.; Sinaiko, A.R.; Ramsay, N.K.C.; O’Dea, R.F. Tobramycin Serum Level Monitoring in Young Patients with Normal Renal Function. Clin. Pharmacokinet. 1984, 9, 457–468. [Google Scholar] [CrossRef]
- Lim, A.K.H.; Mathanasenarajah, G.; Larmour, I. Assessment of aminoglycoside dosing and estimated glomerular filtration rate in determining gentamicin and tobramycin area under the curve and clearance. Intern. Med. J. 2015, 45, 319–329. [Google Scholar] [CrossRef]
- Chin, P.K.L.; Florkowski, C.M.; Begg, E.J. The performances of the Cockcroft-Gault, Modification of Diet in Renal Disease Study and Chronic Kidney Disease Epidemiology Collaboration equations in predicting gentamicin clearance. Ann. Clin. Biochem. 2013, 50, 546–557. [Google Scholar] [CrossRef]
- Pai, M.P.; Nafziger, A.N.; Bertino, J.S. Simplified Estimation of Aminoglycoside Pharmacokinetics in Underweight and Obese Adult Patients. Antimicrob. Agents Chemother. 2011, 55, 4006. [Google Scholar] [CrossRef] [PubMed]
- Sunder, S.; Jayaraman, R.; Mahapatra, H.S.; Sathi, S.; Ramanan, V.; Kanchi, P.; Gupta, A.; Daksh, S.K.; Ram, P. Estimation of renal function in the intensive care unit: The covert concepts brought to light. J. Intensive Care 2014, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Minville, V.; Asehnoune, K.; Virtos, M.; Georges, B.; Fourcade, O.; Conil, J.-M. Screening of patients with augmented renal clearance in ICU: Taking into account the CKD-EPI equation, the age, and the cause of admission. Ann. Intensive Care 2015, 5, 49. [Google Scholar] [CrossRef]
- Bragadottir, G.; Redfors, B.; Ricksten, S.-E. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury—True GFR versus urinary creatinine clearance and estimating equations. Crit. Care 2013, 17, R108. [Google Scholar] [CrossRef]
- Grootaert, V.; Willems, L.; Debaveye, Y.; Meyfroidt, G.; Spriet, I. Augmented Renal Clearance in the Critically Ill: How to Assess Kidney Function. Ann. Pharmacother. 2012, 46, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G.; Lancaster, C.S.; Schlatter, E.; Franke, R.M.; Sprowl, J.A.; Pavenstädt, H.; Massmann, V.; Guckel, D.; Mathijssen, R.H.J.; Yang, W.; et al. Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin. Cancer Res. 2012, 18, 1101–1108. [Google Scholar] [CrossRef]
- Zarowitz, B.J.; Robert, S.; Peterson, E.L. Prediction of Glomerular Filtration Rate Using Aminoglycoside Clearance in Critically Ill Medical Patients. Ann. Pharmacother. 1992, 26, 1205–1210. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Jackson, S.H.D. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharm. 2004, 57, 6–14. [Google Scholar] [CrossRef]
- Triggs, E.; Charles, B. Pharmacokinetics and Therapeutic Drug Monitoring of Gentamicin in the Elderly. Clin. Pharmacokinet. 1999, 37, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.A.; Blouin, R.A. Gentamicin Pharmacokinetics. J. Am. Geriatr. Soc. 1982, 30, 309–311. [Google Scholar] [CrossRef]
- Matzke, G.R.; Jameson, J.J.; Halstenson, C.E. Gentamicin Disposition in Young and Elderly Patients with Various Degrees of Renal Function. J. Clin. Pharmacol. 1987, 27, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Velissaris, D.; Karamouzos, V.; Marangos, M.; Pierrakos, C.; Karanikolas, M. Pharmacokinetic changes and dosing modification of aminoglycosides in critically ill obese patients: A literature review. J. Clin. Med. Res. 2014, 6, 227–233. [Google Scholar] [CrossRef]
- Pai, M.P. Anti-infective Dosing for Obese Adult Patients: A Focus on Newer Drugs to Treat Methicillin-resistant Staphylococcus aureus Acute Bacterial Skin and Skin Structure Infections. Clin. Ther. 2016, 38, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.G.; Baldwin, I.; Freitag, E.; Glassford, N.; Bellomo, R. Estimation of fluid status changes in critically ill patients: Fluid balance chart or electronic bed weight? J. Crit. Care 2012, 27, 745.e7–745.e712. [Google Scholar] [CrossRef]
- Alghanem, S.S.; Touw, D.J.; Thomson, A.H. Pharmacokinetic/pharmacodynamic analysis of weight- and height-scaled tobramycin dosage regimens for patients with cystic fibrosis. J. Antimicrob. Chemother. 2019, 74, 2311–2317. [Google Scholar] [CrossRef]
- Crass, R.L.; Pai, M.P. Optimizing Estimated Glomerular Filtration Rate to Support Adult to Pediatric Pharmacokinetic Bridging Studies in Patients with Cystic Fibrosis. Clin. Pharmacokinet. 2019, 58, 1323–1332. [Google Scholar] [CrossRef]
- Maskin, L.P.; Attie, S.; Setten, M.; Rodriguez, P.O.; Bonelli, I.; Stryjewski, M.E.; Valentini, R. Accuracy of Weight and Height Estimation in an Intensive Care Unit. Anaesth. Intensive Care 2010, 38, 930–934. [Google Scholar] [CrossRef]
- Bloomfield, R.; Steel, E.; MacLennan, G.; Noble, D.W. Accuracy of weight and height estimation in an intensive care unit: Implications for clinical practice and research. Crit. Care Med. 2006, 34, 2153–2157. [Google Scholar] [CrossRef]
- Brendel, K.; Comets, E.; Laffont, C.; Mentré, F. Evaluation of different tests based on observations for external model evaluation of population analyses. J. Pharm. Pharm. 2010, 37, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; van Hest, R.M.; Roggeveen, L.F.; Fleuren, L.M.; Thoral, P.J.; Bosman, R.J.; van der Voort, P.H.J.; Girbes, A.R.J.; Mathot, R.A.A.; Elbers, P.W.G. External Evaluation of Population Pharmacokinetic Models of Vancomycin in Large Cohorts of Intensive Care Unit Patients. Antimicrob. Agents Chemother. 2019, 63, e02543-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Guilhaumou, R.; Blin, O.; Velly, L.; Marsot, A. External evaluation of population pharmacokinetic models for continuous administration of meropenem in critically ill adult patients. Eur. J. Clin. Pharmacol. 2020, 76, 1281–1289. [Google Scholar] [CrossRef]
- Bukkems, L.H.; Roger, C.; Hodiamont, C.J.; Lefrant, J.-Y.; Juffermans, N.P.; Roberts, J.A.; van Hest, R.M. Predictive performance of a gentamicin population pharmacokinetic model in two western populations of critically ill patients. Int. J. Antimicrob. Agents 2018, 52, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Y.; Guan, M.-X. Mitochondrial DNA mutations associated with aminoglycoside induced ototoxicity. J. Otol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
| Drug | Study | Year | Study Type | Population | Aminoglycoside Administration | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Characteristics | N (Male/Female) | Age (Years) a | Body Weight (kg) a | Height (cm) a | BMI (kg/m2) a | Dosage Regimen | Administered Dose (mg/kg) a | Samples per Patient | Total Samples | Sample Frequency (h) | ||||
| Amikacin | Boidin C [16] | 2019 | Retrospective (TDM) | Critically ill with sepsis | 166 (108/58) | 65 (19–85) b | 76.5 (41.5–137.5) b | 170 (137–190) b | 25.6 (16–46) b | Administered Daily | 23.4 (11–39.7) [20.0–27.0] b | NR | 395 | Peak and trough |
| Roger C [17] | 2016 | Observational pharmacokinetic study | Critically ill undergoing CVVH (n = 9) and CVVHDF (n = 11) | 16 (12/4) | 72 [65–75] b | 80 [73–89] b | 167 [162–178] b | 27 [24–32] b | 15–30 mg/kg every 24 or 36 h | NR | 9 | 261 | Predose, end of infusion (0.5), 1,1.5, 2, 4, 8, 12, and 24 | |
| Carrié C [18] | 2020 | Retrospective (TDM) | Critically ill septic patients treated by OA/NPT | 70 (53/17) | 65 [51–73] b | 80 [65–94] b | NR | 27 [25–32] b | As per medical care by the local Department of Laboratory Medicine | 26 [24–29] b | NR | 179 (non-CRRT: 121, CRRT: 58) | Peak and trough | |
| Aréchiga-Alvarado NA [19] | 2020 | Prospective (TDM) | Critically ill mexican patients with suspected or proved infectious under treatment with amikacin | 50 (45/5) | 33.5 (18.0–64.0) b | 70.0 (44.0–138.0) b | 170.1 ± 7.9 | 24.0 (16.0–38.2) b | Once daily IV dosing | 1000 (500–1000) mg c | 2 | 80 | 0.5 and 12 | |
| Petitcollin A [20] | 2016 | Prospective pharmacokinetic study | Ventilated critically ill patients on high-dose nebulized amikacin | 20 (18/2) | 57 (20–80) | 67 (50–84) | NR | NR | 20 mg/kg infusion of amikacin followed by either 3 other infusions or 3 nebulizations of 60 mg/kg amikacin (q24 h) | NR | 33 (11–45) b | 522 | 0.5, 1, 1.5, 2, 3, 4, 6, 10, and 24 | |
| French MA [21] | 1981 | Prospective and retrospective (TDM) | Critically ill patients | 25 (15/10) | 58 ± 14 | NR | NR | NR | 9 to 15 mg/kg per day | 40.60 ± 42.67 | NR | NR | NR | |
| Gentamicin | Hodiamont CJ [22] | 2017 | Retrospective (TDM) | Critically ill patients on or off CVVH | 44 (20/24) | 61 (20–78) | 70.5 (42.0–116) | 170 (154–195) | NR | Starting dose of 4 mg/kg TBW, except for patientstreated for endocarditis due to Gram-positivemicro-organisms who were treated with 3 mg/kg in combination with a cell-wall-targeting antibiotic | 4.0 (2.0–6.6) b | NR | 303 | 0.5 and the second sample was collected the next morning at 06:00 a.m., regardless of the time the first dose was administered |
| Teigen MM [23] | 2006 | Prospective and retrospective (TDM) | Patients on hemodialysis receiving gentamicin to treat a suspected or proven infection | 46 (23/23) | 57.3 ± 17.3 (18–83) | 72.4 ± 17.2 (42.1–100.5) | 164.7 ± 11.6 (135–195) | NR | NR | NR | 4.6 ± 2.2 (1–10) | NR | 0.5, 1 sample at the beginning of dialysis, 1 sample at the end of dialysis, and 1 interdialytic blood sample taken prior to the next dialysis session | |
| Rea RS [24] | 2008 | Retrospective (TDM) | Critically ill patients | 102 (45/57) | 61.4 ± 16.8 (18.4–92.3) | 81.4 ± 30.3 (29.0–222.3) | NR | NR | 7 mg/kg/day | NR | 2.1 (1–9) | 211 | NR | |
| Bos JC [25] | 2019 | Prospective observational pharmacokinetic study | Severally ill non-ICU sub-Saharan African Adult patients | 48 (24/24) | 40 (20–86) | 51 (33–76) | NR | NR | 80 to 160 mg/kg q8 h or 80 to 240 mg/kg q12 or 24 h | NR | NR | 141 | Predose, 30 to 120 min after intravenous administration and two random time points during the dosing interval | |
| Hodiamont CJ [26] | 2017 | Prospective (TDM) | Critically ill patients | 59 (30/29) | 60.9 ± 17.2 | 79.2 ± 22.0 | NR | NR | Fixed first dose of approximately 5 mg/kg. Patients who were treated for endocarditis with 3 mg/kg in combination with a beta-lactam antibiotic | 5.1 ± 1.1 | 6.7 ± 5.9 | 416 | Peak and random timepoint between 6 and 23 h after the administration | |
| Roberts JA [27] | 2010 | Prospective pharmacokinetic study | Critically ill patients with acute kidney injury necessitating extended daily diafiltration | 14 (13/1) | 66.0 (57.0–74.5) b | 92.5 (80.0–111.1) b | NR | NR | NR | NR | NR | 265 | 0, 0.25, 0.5, 1, 2, 3, 5, 8, and 10 | |
| Barletta JF [28] | 2000 | Prospective (TDM) | Critically ill trauma patients | 19 | 40 ± 17 (17–75) | Adjusted (dosing) weight: 73.7 ± 15.9 | NR | NR | NR | Gentamicine: 6.9 ± 0.39 (6–7.2) Tobramycine: 6.6 ± 1.03 (4.9–7.8) | NR | 53 | 4 and 8 | |
| Gomes A [29] | 2017 | Retrospective (TDM) | Endocarditis patients | 65 (21/44) | 69.3 (32–92) | 76.2 (46–121) | 173.9 (149–193) | NR | 3 mg/kg q24 h | NR | NR | 221 | NR | |
| Watling SM [30] | 1993 | Prospective (TDM) | Critically ill patients | 36 (20/16) | 54.7 ± 16.6 | 75.7 ± 16.4 | 172 ± 15 | NR | 3 mg/kg q12 h, q18 h, q24 h, q36 h, or q72 h | NR | 2.8 ± 1.6 | 102 | 1 h and at the dosing interval midpoint | |
| Kisor DF [31] | 1992 | Retrospective (TDM) | Patients with indicators of malnutrition (bodyweight less than ideal bodyweight, low serum ALB) | 17 (16/1) | 73.8 ± 11.8 | 54.3 ± 9.9 | NR | NR | NR | NR | 8.0 ± 1.2 | 72 | NR | |
| French MA [21] | 1981 | Prospective and retrospective (TDM) | Critically ill patients | 25 (15/10) | 62 ± 15 | NR | NR | NR | 3 to 5 mg/kg per day | 31.73 ± 27.26 | NR | NR | NR | |
| Tobramycin | Conil JM [32] | 2011 | Retrospective (TDM) | Critically ill patients | 32 (27/5) | 62.5 ± 15.3 | 77.5 ± 18.8 | NR | NR | 5 mg/kg q24 h for 3–5 days | NR | NR | NR | Peak and trough |
| Aarons L [33] | 1989 | Retrospective (TDM) | Unselected poplation of patients treated with tobramycin | 97 (52/45) | 50.6 ± 19.0 (51.0;16–85) c | 66.5 ± 12.5 (66.8; 42–120) c | NR | NR | NR | NR | (1–9) | 322 | 2, 6 h after the dose for patients with normal renal function 2, 6, 12, and 24 h for patients with impaired renal function | |
| Hennig S [34] | 2013 | Retrospective (TDM) | Patients with or without cystic fibrosis | 208 (109/99) | 31.7 (18.0–85.0) | 58.0 (37.0–120.0) | NR | NR | NR | 5.2 (0.9–12.0) per day | NR | CF: 4514 No CF: 1095 | NR | |
| Drug | Study | Modeling | Simulation | |||
|---|---|---|---|---|---|---|
| Software | Model | Evaluation | Optimal Dosing Regimen | Target | ||
| Amikacin | Boidin C [16] | NPAG (Pmetrics) | 2 compartments | Advanced internal | Optimal initial amikacin dose for Cmax: 3.5 g Optimal initial amikacin dose for AUC0–24: 3.8 g Optimal doses were based on an MIC of 8 mg/L | Cmax/MIC ≥ 8, AUC0–24/MIC ≥ 75 and Cmin ≤ 2.5 mg/L |
| Roger C [17] | NPAG (Pmetrics) | 2 compartments | Advanced internal (bootstrap, n = 1000) | 25 mg/kg every 48 h in critically ill patients receiving CRRT based on an MIC of 8 mg/L | Cmax/MIC ≥ 8 and Cmin ≤ 2.5 mg/L | |
| Carrié C [18] | Monolix | 2 compartments | Advanced internal (NPDE) | 25–30 mg/kg every 36–48 h based on an MIC of 8 mg/L | Cmax/MIC ≥ 8, AUC0–24/MIC ≥ 75 and Cmin ≤ 2.5 mg/L | |
| Aréchiga-Alvarado NA [19] | NONMEM 7.3 | 1 compartment | Advanced internal (bootstrap, n = 1000) and external (13 patients) | Based on an MIC of 8 mg/L and a dose of 30 mg/kg, the probability of having Cmax/MIC ≥ 8 was above 75% for creatinine clearance ranging from 60 mL/min to 200 mL/min a | Cmax/MIC ≥ 8 and AUC0–24/MIC ≥ 75 | |
| Petitcollin A [20] | Monolix 4.2.3 | 2 compartments | Advanced internal (NPDE) | – | – | |
| French MA [21] | NONLIN | 2 compartments | NR | – | – | |
| Gentamicin | Hodiamont CJ [22] | NONMEM 7.1.2 | 2 compartments | Advanced internal (bootstrap, n = 1000) | – | – |
| Teigen MM [23] | NONMEM 5 | 1 compartment | Basic internal | Predialysis administration of 300 mg, 240 mg, and 220 mg as first, second, and third dose, respectively, for patients who dialyze 3 times a week | Cmax ≥ 8 mg/L AUCmin,48h ≥ 140 AUCmax,48h ≤ 240 | |
| Rea RS [24] | NONMEM 5.1 | 1 compartment | Advanced internal (bootstrap, n = 1000) | Initial doses of 7 mg/kg of either gentamicin or tobramycin. Then, it is recommended to verify Cmax after the first dose and determining MIC for the pathogen(s) with adjustment of subsequent doses to achieve the PD target b | Cmax/MIC ≥ 10 | |
| Bos JC [25] | NONMEM 7.1.2 | 1 compartment | Advanced internal (bootstrap, n = 1000) | 7 mg/kg/day considering an MIC of 2 mg/L | Cmax/MIC ≥ 8 | |
| Hodiamont CJ [26] | NONMEM 7.2 | 2 compartments | Advanced internal (bootstrap, n = 1000) | 6 mg/kg as starting dose | Cmax therapeutic range of 15–20 mg/L | |
| Roberts JA [27] | NONMEM 6.1 | 2 compartments | Advanced internal (bootstrap, n = 1000) | 6 mg/kg every 48 h before the commencement of EDD-f | Cmax > 10 mg/L and 70 mg·h/L ≤ AUC0–24 ≤ 120 mg·h/L | |
| Barletta JF [28] | Nonlinear mixed effect modelling | 1 compartment | NR | – | – | |
| Gomes A [29] | MwPharm | 1 compartment | Advanced internal (bootstrap, n = 1000) and external (14 patients) | – | – | |
| Watling SM [30] | NPEM c | 1 compartment | External of dosing nomogram only (15 patients) | – | – | |
| Kisor DF [31] | NPEM | 1 compartment | NR | – | – | |
| French MA [21] | NONLIN | 2 compartments | NR | – | – | |
| Tobramycin | Conil JM [32] | NONMEM 5 | 2 compartments | Advanced internal (NPDE and bootstrap, n = 1000) and external (17 patients) | Peak and AUC pharmacodynamic targets could not be reached simultaneously in more than 45% of the ICU patient population. Combination therapy in addition to TDM are required to manage efficacy and toxicity | Cmax/MIC > 10, Cmin ≤ 1 mg/L AUC between 80 and 125 mg·h/L for MIC ≤ 1 mg/L |
| Aarons L [33] | NONMEM | 2 compartments | External (34 patients) | First 48 h: 100 mg Q8 h and Maintenance dose: 120 mg Q8 h, patient with CLcr > 100 mL/min First 48 h: 80 mg Q8 h and Maintenance dose: 90 mg Q8 h, patient with CLcr = 75 mL/min First 48 h: 93 mg Q12 h and Maintenance dose: 90 mg Q12 h, patient with CLcr = 50 mL/min First 48 h: 60 mg Q12 and Maintenance dose: 54 mg Q12 h, patient with CLcr = 30 mL/min First 48 h: 80 mg Q24 and Maintenance dose: 70 mg Q24 h, patient with CLcr = 20 mL/min First 48 h: 67 mg Q24 and Maintenance dose: 54 mg Q24 h, patient with CLcr = 15 mL/min First 48 h: 60 mg Q24 and Maintenance dose: 35 mg Q24 h, patient with CLcr = 10 mL/min | Cmax = 6 mg/L and average concentrations within a dosing interval ≤ 4 mg/L | |
| Hennig S [34] | NONMEM 7.2 | 2 compartments | Advanced internal (bootstrap, n = 300) | 11 mg/kg/day for Cystic Fibrosis patients | Cmax = 20 mg/L (relating to a 1-h peak/MIC ratios of 20/2) and Cmin < 1 mg/L | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, A.; Simard, C.; Wang, Y.L.; Williamson, D.; Marsot, A. Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling? Antibiotics 2021, 10, 507. https://doi.org/10.3390/antibiotics10050507
Duong A, Simard C, Wang YL, Williamson D, Marsot A. Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling? Antibiotics. 2021; 10(5):507. https://doi.org/10.3390/antibiotics10050507
Chicago/Turabian StyleDuong, Alexandre, Chantale Simard, Yi Le Wang, David Williamson, and Amélie Marsot. 2021. "Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling?" Antibiotics 10, no. 5: 507. https://doi.org/10.3390/antibiotics10050507
APA StyleDuong, A., Simard, C., Wang, Y. L., Williamson, D., & Marsot, A. (2021). Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling? Antibiotics, 10(5), 507. https://doi.org/10.3390/antibiotics10050507






