Anaerobic Digestion and Removal of Sulfamethoxazole, Enrofloxacin, Ciprofloxacin and Their Antibiotic Resistance Genes in a Full-Scale Biogas Plant
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sampling of Anaerobic Digestor
4.2. Chemicals and Reagents
4.3. Analytical Determination of SMX, CIP and ENR
4.4. Total Microbial Abundance
4.5. DNA Extraction
4.6. Quantification of ARGs and intI1 Sequences by qPCR
4.7. Antibiotic and ARG Removal
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Samadder, S. Performance evaluation of anaerobic digestion technology for energy recovery from organic fraction of municipal solid waste: A review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A review of biochemical process of anaerobic digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.R.; Merlin Christy, P. A review on current aspects and diverse prospects for enhancing biogas pro-duction in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient recovery from digestate of anaerobic digestion of livestock manure: A review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Mitchell, R.; Gu, J.D. Environmental Microbiology, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; p. 363. [Google Scholar]
- Iocoli, G.A.; Zabaloy, M.C.; Pasdevicelli, G.; Gómez, M.A. Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: Characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci. Total Environ. 2019, 647, 11–19. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Visca, A.; Massini, G.; Patrolecco, L.; Mazzurco Miritana, V.; Grenni, P. Environmental fate of antibiotics and resistance genes in livestock waste and digestate from Biogas plants. Environ. Sci. Pollut. Res. Manag. 2020, 2020, 1. [Google Scholar] [CrossRef]
- Checcucci, A.; Trevisi, P.; Luise, D.; Modesto, M.; Blasioli, S.; Braschi, I.; Mattarelli, P. Exploring the animal waste resistome: The spread of antimicrobial resistance genes through the use of livestock manure. Front. Microbiol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Lou, E.G.; Harb, M.; Smith, A.L.; Stadler, L.B. Livestock manure improved antibiotic resistance gene removal during co-treatment of domestic wastewater in an anaerobic membrane bioreactor. Environ. Sci. Water Res. Technol. 2020, 6, 2832–2842. [Google Scholar] [CrossRef]
- Congilosi, J.L.; Aga, D.S. Review on the fate of antimicrobials, antimicrobial resistance genes, and other micropollutants in manure during enhanced anaerobic digestion and composting. J. Hazard. Mater. 2021, 405, 123634. [Google Scholar] [CrossRef]
- Sanz, C.; Casado, M.; Navarro-Martin, L.; Tadić, D.; Parera, J.; Tugues, J.; Bayona, J.M.; Piña, B. Antibiotic and antibiotic-resistant gene loads in swine slurries and their digestates: Implications for their use as fertilizers in agriculture. Environ. Res. 2021, 194, 110513. [Google Scholar] [CrossRef]
- Barnes, K.K.; Christenson, S.C.; Kolpin, D.W.; Focazio, M.J.; Furlong, E.T.; Zaugg, S.D.; Meyer, M.T.; Barber, L.B. Pharmaceuticals and other organic waste water contaminants within a leachate plume downgradient of a municipal landfill. Groundw. Monit. Remediat. 2004, 24, 119–126. [Google Scholar] [CrossRef]
- Ferro, G.; Polo-López, M.I.; Martínez-Piernas, A.B.; Fernández-Ibáñez, P.; Agüera, A.; Rizzo, L. Cross-contamination of residual emerging contaminants and antibiotic resistant bacteria in lettuce crops and soil irrigated with wastewater treated by sunlight/H2O2. Environ. Sci. Technol. 2015, 49, 11096–11104. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.D.; Cassada, D.A.; Larsen, M.L.; Mware, N.A.; Li, X.; D’Alessio, M.; Zhang, Y.; Sallach, J.B. Detection, occurrence and fate of emerging contaminants in agricultural environments. Water Environ. Res. 2017, 89, 897–920. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Johnson, P.; Smith, E.J.; Sinclair, C.J.; Stutt, E.; Levy, L.S. Uptake of veterinary medicines from soils into plants. J. Agric. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K.; Manley-Harris, M. Sorption of selected veterinary antibiotics onto dairy farming soils of con-trasting nature. Sci. Total Environ. 2014, 472, 695–703. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Zhang, H.; Shen, G.; Yuan, Z.; Zhang, W. Degradation kinetics and mechanism of sulfadiazine and sulfa-methoxazole in an agricultural soil system with manure application. Sci. Total Environ. 2017, 607, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.; Guo, W.; Chang, S.; Nguyen, D.; Kumar, S.M.; Du, B.; Wei, Q.; Wei, D. Problematic effects of antibiotics on anaerobic treatment of swine wastewater. Bioresour. Technol. 2018, 263, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, J.; Barra Caracciolo, A.; Ademollo, N.; Cardoni, M.; Di Lenola, M.; Gaze, W.; Stanton, I.; Grenni, P.; Pescatore, T.; Spataro, F.; et al. Dissipation of the antibiotic sulfamethoxazole in a soil amended with anaerobically digested cattle manure. J. Hazard. Mater. 2019, 378, 120769. [Google Scholar] [CrossRef] [PubMed]
- Nowara, A.; Burhenne, A.J.; Spiteller, M. Binding of fluoroquinolone carboxylic acid derivatives to clay minerals. J. Agric. Food Chem. 1997, 45, 1459–1463. [Google Scholar] [CrossRef]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef]
- Vasudevan, D.; Bruland, G.L.; Torrance, B.S.; Upchurch, V.G.; MacKay, A.A. pH-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption. Geoderma 2009, 151, 68–76. [Google Scholar] [CrossRef]
- Rosendahl, I.; Siemens, J.; Kindler, R.; Groeneweg, J.; Zimmermann, J.; Czerwinski, S.; Lamshöft, M.; Laabs, V.; Wilke, B.-M.; Vereecken, H.; et al. Persistence of the fluoroquinolone antibiotic difloxacin in soil and lacking effects on nitrogen turnover. J. Environ. Qual. 2012, 41, 1275–1283. [Google Scholar] [CrossRef]
- Andriamalala, A.; Vieublé-Gonod, L.; Dumeny, V.; Cambier, P. Fate of sulfamethoxazole, its main metabolite N-ac-sulfamethoxazole and ciprofloxacin in agricultural soils amended or not by organic waste products. Chemosphere 2018, 191, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Cetecioglu, Z.; Ince, B.; Orhon, D.; Ince, O. Acute inhibitory impact of antimicrobials on acetoclastic methanogenic activity. Bioresour. Technol. 2012, 114, 109–116. [Google Scholar] [CrossRef]
- Aydin, S.; Ince, B.; Cetecioglu, Z.; Arikan, O.; Ozbayram, E.G.; Shahi, A.; Ince, O. Combined effect of erythromycin, tetracycline and sulfamethoxazole on performance of anaerobic sequencing batch reactors. Bioresour. Technol. 2015, 186, 207–214. [Google Scholar] [CrossRef]
- Wang, S.; Du, K.; Yuan, R.; Chen, H.; Wang, F.; Zhou, B. Effects of sulfonamide antibiotics on digestion performance and microbial community during swine manure anaerobic digestion. Environ. Eng. Res. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Mohring, S.A.I.; Strzysch, I.; Fernandes, M.R.; Kiffmeyer, T.K.; Tuerk, J.; Hamscher, G. Degradation and elimination of various sulfonamides during anaerobic fermentation: A promising step on the way to sustainable pharmacy? Environ. Sci. Technol. 2009, 43, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Cetecioglu, Z.; Ince, B.; Gros, M.; Rodriguez-Mozaz, S.; Barceló, D.; Ince, O.; Orhon, D. Biodegradation and reversible inhibitory impact of sulfamethoxazole on the utilization of volatile fatty acids during anaerobic treatment of pharmaceutical industry wastewater. Sci. Total Environ. 2015, 536, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Mazzurco Miritana, V.; Massini, G.; Visca, A.; Grenni, P.; Patrolecco, L.; Spataro, F.; Rauseo, J.; Garbini, G.L.; Signorini, A.; Rosa, S.; et al. Effects of sulfamethoxazole on the microbial community dynamics during the anaerobic digestion process. Front. Microbiol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Silva, A.R.; Gomes, J.C.; Salvador, A.F.; Martins, G.; Alves, M.M.; Pereira, L. Ciprofloxacin, diclofenac, ibuprofen and 17α-ethinylestradiol differentially affect the activity of acetogens and methanogens in anaerobic communities. Ecotoxicology 2020, 29, 866–875. [Google Scholar] [CrossRef]
- Mai, D.T.; Stuckey, D.C.; Oh, S. Effect of ciprofloxacin on methane production and anaerobic microbial community. Bioresour. Technol. 2018, 261, 240–248. [Google Scholar] [CrossRef]
- Zhi, S.; Zhang, K. Antibiotic residues may stimulate or suppress methane yield and microbial activity during high-solid an-aerobic digestion. Chem. Eng. J. 2019, 359, 1303–1315. [Google Scholar] [CrossRef]
- Gurmessa, B.; Pedretti, E.F.; Cocco, S.; Cardelli, V.; Corti, G. Manure anaerobic digestion effects and the role of pre- and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. Sci. Total Environ. 2020, 721, 137532. [Google Scholar] [CrossRef]
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017. EMA/294674/2019. 2019. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2017_en.pdf (accessed on 10 January 2021).
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Casas, M.E.; Ottosen, L.D.M.; Møller, H.B.; Bester, K. Removal of antibiotics during the anaerobic digestion of pig manure. Sci. Total Environ. 2017, 603, 219–225. [Google Scholar] [CrossRef]
- Figueroa-Diva, R.A.; Vasudevan, D.; Mackay, A.A. Trends in soil sorption coefficients within common antimicrobial families. Chemosphere 2010, 79, 786–793. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Escario, M.; Miguel, E.; Pérez, R.A. Persistence and availability of veterinary antibiotics in soil and soil-manure systems. Sci. Total Environ. 2018, 643, 1562–1570. [Google Scholar] [CrossRef]
- Trouchon, T.; Lefebvre, S. A review of enrofloxacin for veterinary use. Open J. Vet. Med. 2016, 6, 40–58. [Google Scholar] [CrossRef]
- Ma, R.; Huang, L.; Wei, W.; Wang, Y.; Zou, X.; Zhou, J.; Li, X.; Fang, W. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in Pacific white shrimp Litopenaeus vannamei after multiple-dose oral administration. Fish. Sci. 2018, 84, 869–876. [Google Scholar] [CrossRef]
- Idowu, O.R.; Peggins, J.O.; Cullison, R.; von Bredow, J. Comparative pharmacokinetics of enrofloxacin and ciprofloxacin in lactating dairy cows and beef steers following intravenous administration of enrofloxacin. Res. Vet. Sci. 2010, 89, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Siddique, M.B.A.; Kamal, A.; Coyne, M.S. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, B.; Zou, R.; Dai, Y.; Xie, S.; Yuan, B. Biodegradation of antibiotic ciprofloxacin: Pathways, influential factors, and bacterial community structure. Environ. Sci. Pollut. Res. 2016, 23, 7911–7918. [Google Scholar] [CrossRef]
- Davis, J.L.; Foster, D.M.; Papich, M.G. Pharmacokinetics and tissue distribution of enrofloxacin and its active metabolite ciprofloxacin in calves. J. Vet. Pharmacol. Ther. 2007, 30, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Monno, R.; D’Addabbo, P.; Pesole, G.; Dionisi, A.; Scrascia, M.; Chiara, M.; Horner, D.; Manzari, C.; Luzzi, I.; et al. A novel group of IncQ1 plasmids conferring multidrug resistance. Plasmid 2017, 89, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Das, K.R.; Naik, M.M. Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat. Chemosphere 2019, 215, 846–857. [Google Scholar] [CrossRef]
- Duggett, N.; AbuOun, M.; Randall, L.; Horton, R.; Lemma, F.; Rogers, J.; Crook, D.; Teale, C.; Anjum, M.F. The importance of using whole genome sequencing and extended spectrum beta-lactamase selective media when monitoring antimicrobial resistance. Sci. Rep. 2020, 10, 19880. [Google Scholar] [CrossRef]
- Ma, J.; Gu, J.; Wang, X.; Peng, H.; Wang, Q.; Zhang, R.; Hu, T.; Bao, J. Effects of nano-zerovalent iron on antibiotic resistance genes during the anaerobic digestion of cattle manure. Bioresour. Technol. 2019, 289, 121688. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qian, X.; Gu, J.; Wang, X.; Duan, M. Mechanism and effect of temperature on variations in antibiotic resistance genes during anaerobic digestion of dairy manure. Nat. Publ. Gr. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Sun, W.; Gu, J.; Wang, X.; Qian, X.; Peng, H. Solid-state anaerobic digestion facilitates the removal of antibiotic resistance genes and mobile genetic elements from cattle manure. Bioresour. Technol. 2019, 274, 287–295. [Google Scholar] [CrossRef]
- Ezzariai, A.; Ha, M.; Khadra, A.; Aemig, Q.; El, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef]
- Qian, X.; Sun, W.; Gu, J.; Wang, X.J.; Sun, J.J.; Yin, Y.N.; Duan, M.L. Variable effects of oxytetracycline on antibiotic resistance gene abundance and the bacterial community during aerobic composting of cow manure. J. Hazard. Mater. 2016, 315, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Amarakoon, I.D.; Zaheer, R.; Smith, A.; Sura, S.; Wang, G.; Reuter, T.; Zvomuya, F.; Cessna, A.J.; Larney, F.J.; et al. Dissipation of antimicrobial resistance genes in compost originating from cattle manure after direct oral administration or post-excretion fortification of antimicrobials. J. Environ. Sci. Health Part A 2017, 4529, 1–12. [Google Scholar] [CrossRef]
- Xie, W.; Yang, X.; Li, Q.; Wu, L.; Shen, Q.; Zhao, F.J. Changes in antibiotic concentrations and antibiotic resistome during com-mercial composting of animal manures. Environ. Pollut. 2016, 219, 182–190. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 2015, 245, 89–97. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Sun, W.; Wang, X.-J.; Su, J.-Q.; Stedfeld, R. Diversity, abundance, and persistence of antibiotic resistance genes in various types of animal manure following industrial composting. J. Hazard. Mater. 2018, 344, 716–722. [Google Scholar] [CrossRef]
- Massé, D.I.; Saady, N.M.C.; Gilbert, Y. Potential of biological processes to eliminate antibiotics in livestock manure: An overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, H.; Xia, D.; Du, J.; Xie, H.; Chen, J. Determination of 21 antibiotics in sea cucumber using accelerated solvent extraction with in-cell clean-up coupled to ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 258, 87–94. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Alder, A.C.; Giger, W.; Theiß, N.; Löffler, D.; Ternes, T.A. Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge. J. Chromatogr. A 2005, 1085, 179–189. [Google Scholar] [CrossRef]
- Spataro, F.; Ademollo, N.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Antibiotic residues and endocrine disrupting compounds in municipal wastewater treatment plants in Rome, Italy. Microchem. J. 2019, 148, 634–642. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Gioia, R.; Aceña, J.; Pérez, S.; Casamayor, E.O.; Dachs, J. Degradation of sulfonamides as a microbial resistance mechanism. Water Res. 2017, 115, 309–317. [Google Scholar] [CrossRef]
- Kerrn, M.B.; Klemmensen, T.; Frimodt-Møller, N.; Espersen, F. Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. J. Antimicrob. Chemother. 2002, 50, 513–516. [Google Scholar] [CrossRef]
- Byrne-Bailey, K.G.; Gaze, W.H.; Zhang, L.; Kay, P.; Boxall, A.; Hawkey, P.M.; Wellington, E.M.H. Integron prevalence and diversity in manured soil. Appl. Environ. Microbiol. 2010, 77, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Mckinney, C.W.; Dungan, R.S.; Moore, A.; Leytem, A.B. Occurrence and abundance of antibiotic resistance genes in agricul-tural soil receiving dairy manure. FEMS Microbiol. Ecol. 2018, 94, 29360961. [Google Scholar] [CrossRef]
- Marti, E.; Balcázar, J.L. Real-time PCR assays for quantification of qnr genes in environmental water samples and chicken feces. Appl. Environ. Microbiol. 2013, 79, 1743–1745. [Google Scholar] [CrossRef]
- Yamane, K.; Wachino, J.-I.; Suzuki, S.; Arakawa, Y. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob. Agents Chemother. 2008, 52, 1564–1566. [Google Scholar] [CrossRef]
- Ruiz, E.; Ocampo-Sosa, A.A.; Arlet, G.; Torres, C. Changes in ciprofloxacin resistance levels in Enterobacter aerogenes isolates associated with variable expression of the aac(6’)-Ib-cr gene. Antimicrob. Agents Chemother. 2012, 56, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pu, C.; Yu, X.; Sun, Y.; Chen, J. Removal of tetracyclines, sulfonamides, and quinolones by industrial-scale composting and anaerobic digestion processes. Environ. Sci. Pollut. Res. 2018, 25, 35835–35844. [Google Scholar] [CrossRef]
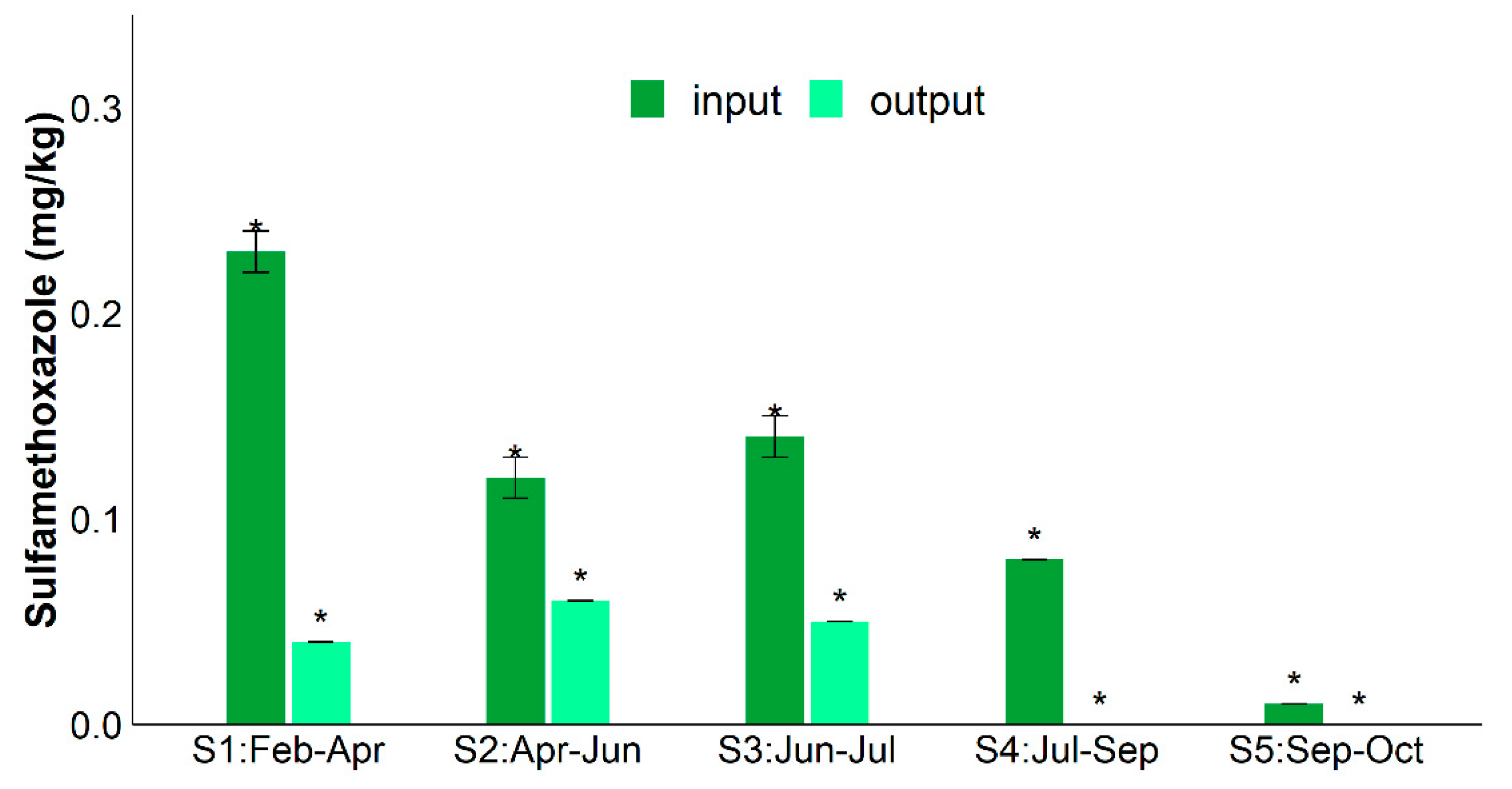
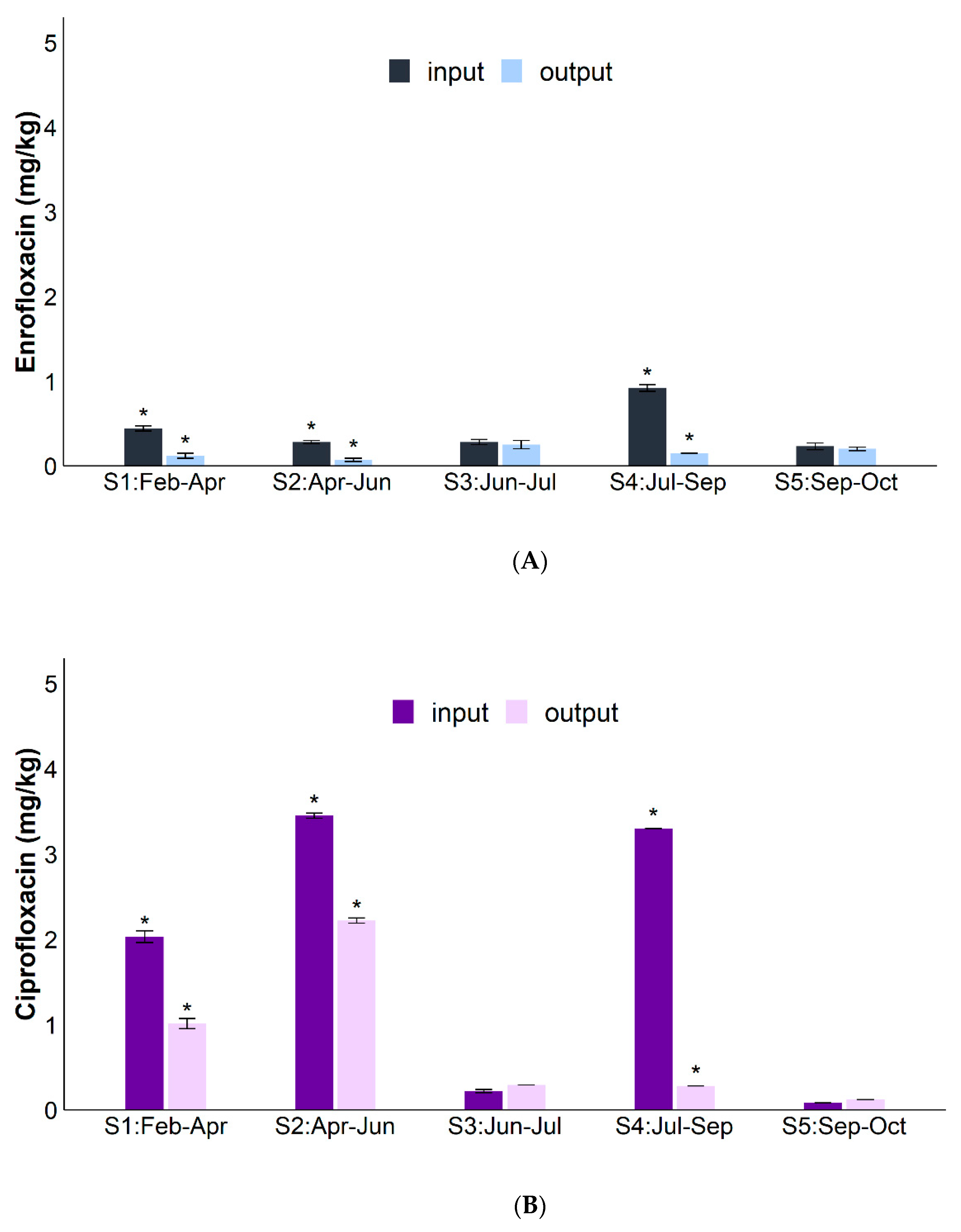
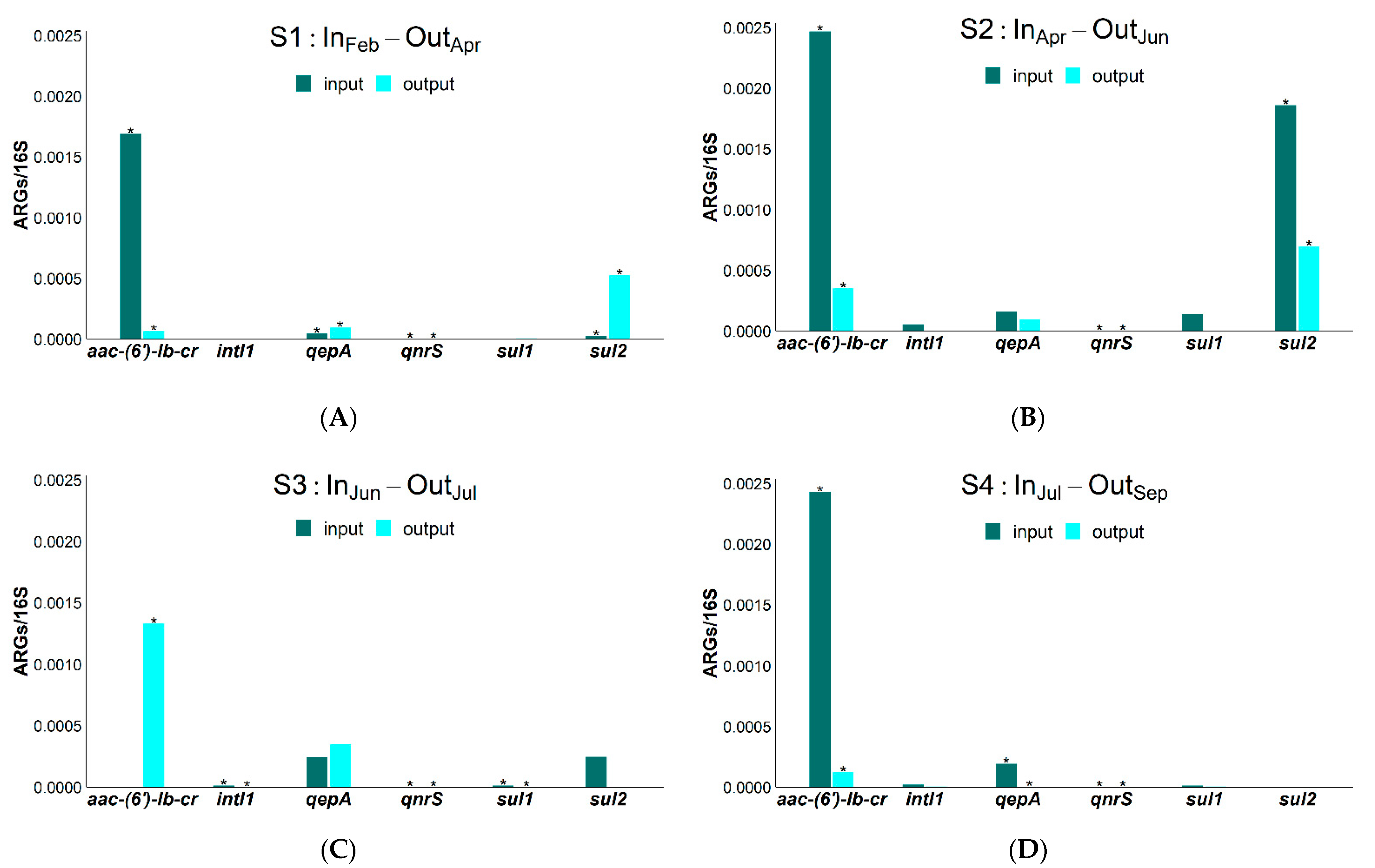
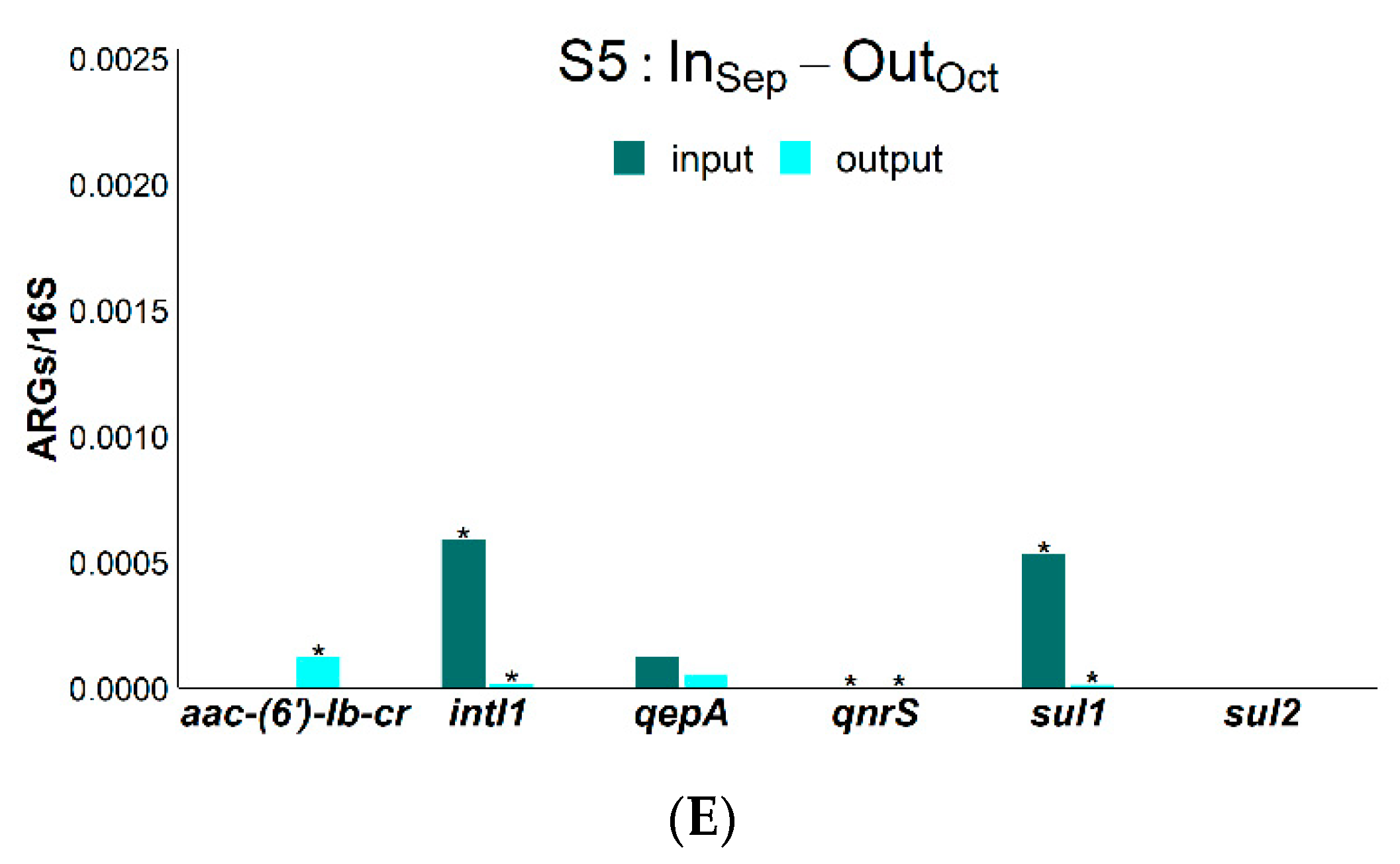
| RSMX (%) | RENR (%) | RCIP (%) | Rsul1 (%) | Raac-(6′)-Ib-cr (%) | RintI1 (%) | |
|---|---|---|---|---|---|---|
| S1 | 82.9 | 71.9 | 50.3 | - | 96.2 | - |
| S2 | 50.3 | 75.8 | 35.6 | 97.2 | 85.8 | 95.8 |
| S3 | 63.9 | 10.9 | - | 98.3 | 72.3 | 98.8 |
| S4 | 94.3 | 83.9 | 91.6 | 50.6 | 94.9 | 73.9 |
| S5 | 100.0 | 8.9 | - | 97.6 | 99.9 | 97.0 |
| Average | 78.3 ± 8.0% | 50.3 ± 16.0% | 37.0% ± 25.0% | 64.0 ± 24.0% | 90.0 ± 5.0% | 69.0 ± 22.0% |
| Farm Type | No. of Animals | Cattle Breed | Feeding of Cattle | Manure Storage | Digestate Treatment |
|---|---|---|---|---|---|
| Dairy farm | 700 | Dairy Friesian | Corn shredded, triticale, soya, cotton seeds, corn flour | Open air pool | Solid/liquid separation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visca, A.; Barra Caracciolo, A.; Grenni, P.; Patrolecco, L.; Rauseo, J.; Massini, G.; Mazzurco Miritana, V.; Spataro, F. Anaerobic Digestion and Removal of Sulfamethoxazole, Enrofloxacin, Ciprofloxacin and Their Antibiotic Resistance Genes in a Full-Scale Biogas Plant. Antibiotics 2021, 10, 502. https://doi.org/10.3390/antibiotics10050502
Visca A, Barra Caracciolo A, Grenni P, Patrolecco L, Rauseo J, Massini G, Mazzurco Miritana V, Spataro F. Anaerobic Digestion and Removal of Sulfamethoxazole, Enrofloxacin, Ciprofloxacin and Their Antibiotic Resistance Genes in a Full-Scale Biogas Plant. Antibiotics. 2021; 10(5):502. https://doi.org/10.3390/antibiotics10050502
Chicago/Turabian StyleVisca, Andrea, Anna Barra Caracciolo, Paola Grenni, Luisa Patrolecco, Jasmin Rauseo, Giulia Massini, Valentina Mazzurco Miritana, and Francesca Spataro. 2021. "Anaerobic Digestion and Removal of Sulfamethoxazole, Enrofloxacin, Ciprofloxacin and Their Antibiotic Resistance Genes in a Full-Scale Biogas Plant" Antibiotics 10, no. 5: 502. https://doi.org/10.3390/antibiotics10050502
APA StyleVisca, A., Barra Caracciolo, A., Grenni, P., Patrolecco, L., Rauseo, J., Massini, G., Mazzurco Miritana, V., & Spataro, F. (2021). Anaerobic Digestion and Removal of Sulfamethoxazole, Enrofloxacin, Ciprofloxacin and Their Antibiotic Resistance Genes in a Full-Scale Biogas Plant. Antibiotics, 10(5), 502. https://doi.org/10.3390/antibiotics10050502











