A Highly Active Chimeric Lysin with a Calcium-Enhanced Bactericidal Activity against Staphylococcus aureus In Vitro and In Vivo
Abstract
1. Introduction
2. Results
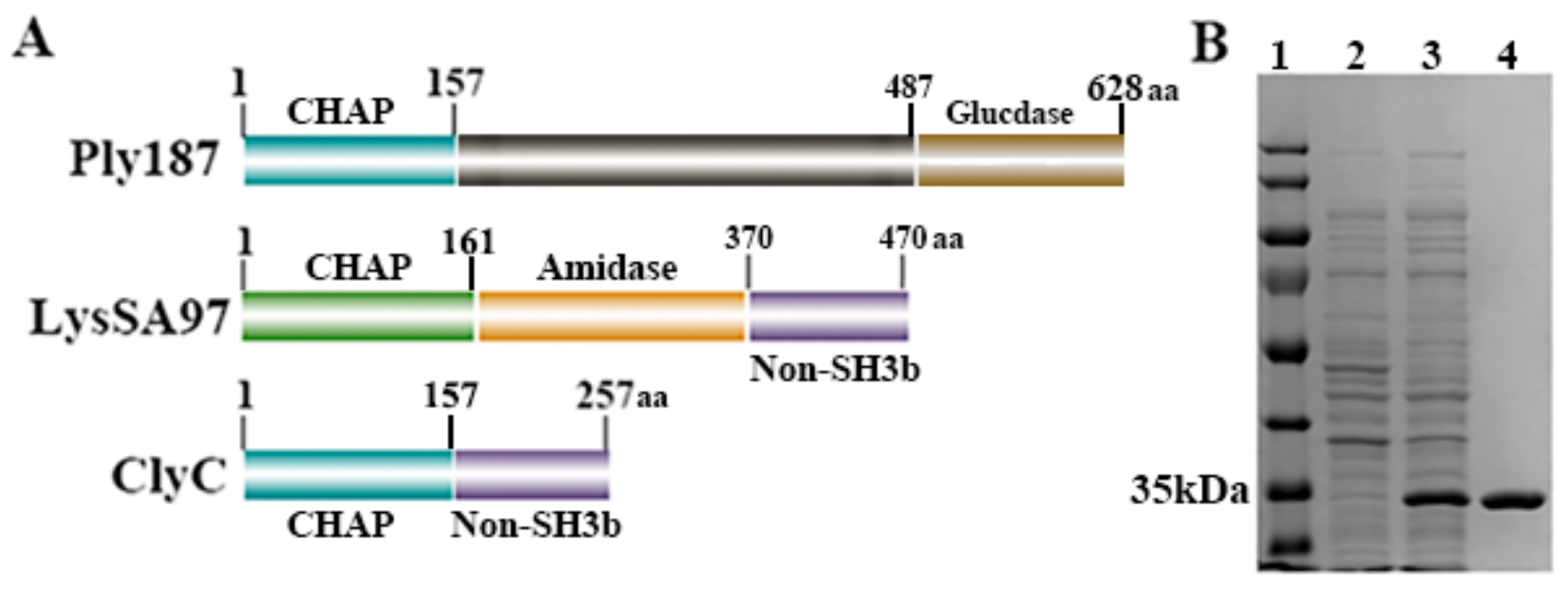
2.1. Construction and Purification of ClyC
2.2. Bactericidal Activity of ClyC
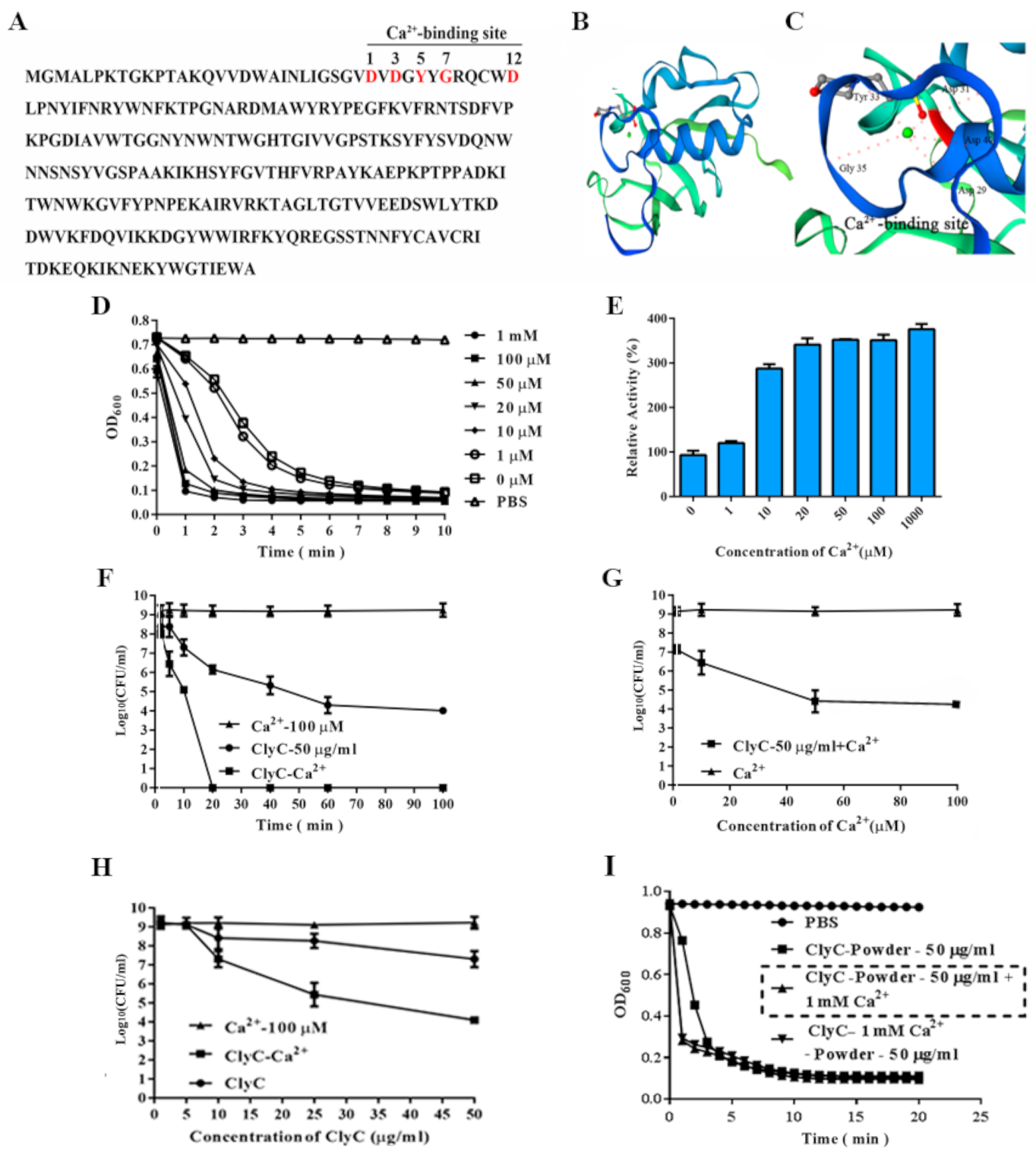
2.3. Characteristics of ClyC
2.4. Effects of Calcium on the Enzymatic Activity of ClyC
2.5. Effects of ClyC Alone against Planktonic and Sessile S. aureus or in Combination with Penicillin G
2.6. ClyC Cytotoxicity In Vitro
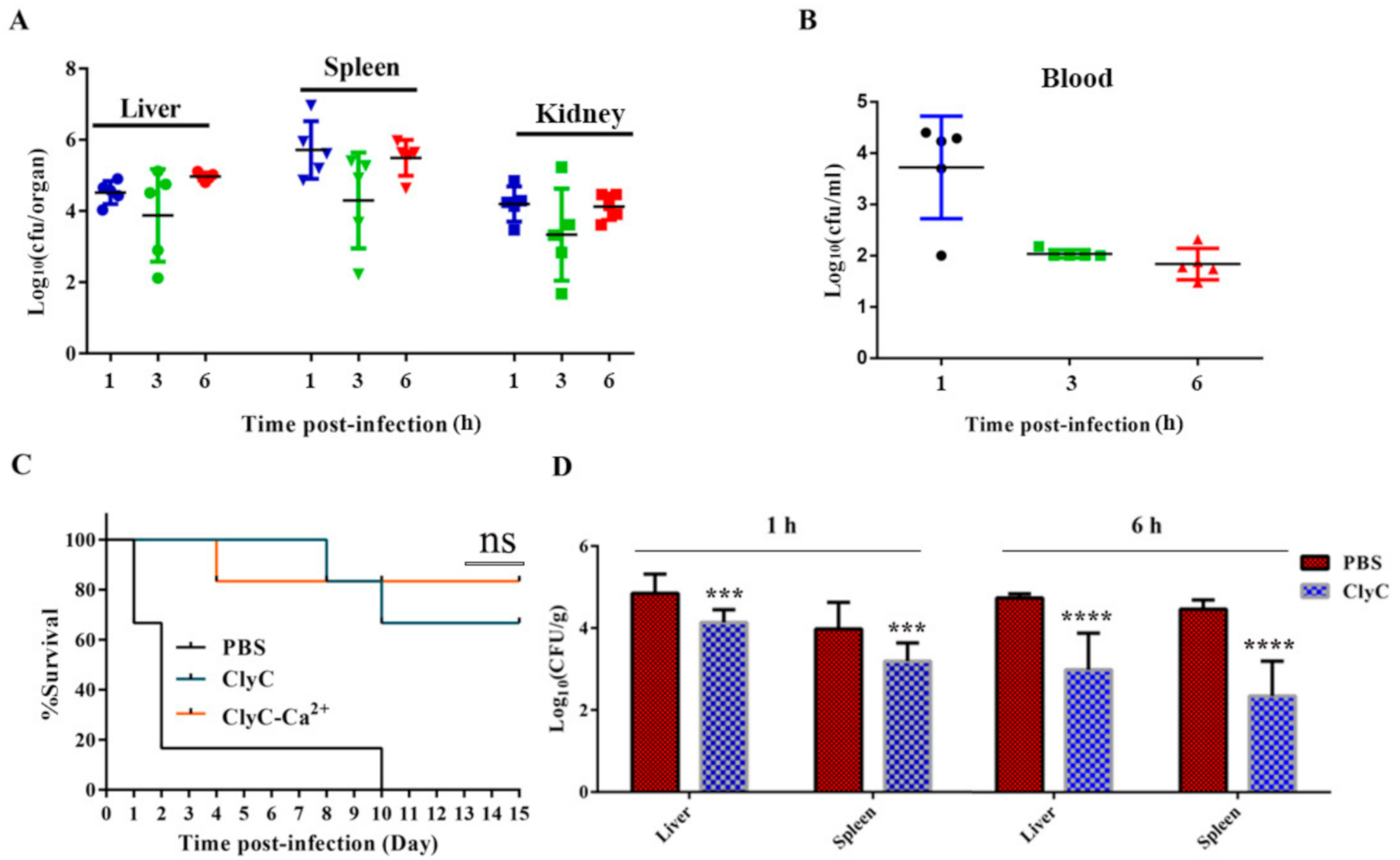
2.7. ClyC Protects Mice from Lethal S. aureus Infection
3. Discussion
4. Materials and Methods
4.1. Ethical Consideration
4.2. Bacterial Strains
4.3. Construction of Expression Plasmids and Protein Purification
4.4. Lytic Activity Assay
4.5. Lyophilization
4.6. MIC of ClyC Alone and in Combination with Penicillin G
4.7. Effects of Different Factors on ClyC Activity
4.8. Construction of 3D Models of ClyC
4.9. ClyC Cytotoxicity Assay
4.9.1. Biofilm Removal Efficacy of ClyC
4.9.2. Mouse Infection Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favero, M.S. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections. Infect. Control Hosp. Epidemiol. 2003, 24, 787. [Google Scholar]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Mohamed, N.; Scully, I.L.; Cooper, D.; Begier, E.; Eiden, J.; Jansen, K.U.; Gurtman, A.; Anderson, A.S. Staphylococcus aureus: The current state of disease, pathophysiology and strategies for prevention. Expert Rev. Vaccines 2016, 15, 1373–1392. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C. The evolution of a resistant pathogen–the case of MRSA. Curr. Opin. Pharmacol. 2003, 3, 474–479. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Szweda, P.; Schielmann, M.; Kotlowski, R.; Gorczyca, G.; Zalewska, M.; Milewski, S. Peptidoglycan hydrolases-potential weapons against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2012, 96, 1157–1174. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Are Phage Lytic Proteins the Secret Weapon To Kill Staphylococcus aureus. MBio 2018, 9, e01923-17. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef]
- Jun, S.Y.; Jang, I.J.; Yoon, S.; Jang, K.; Yu, K.-S.; Cho, J.Y.; Seong, M.-W.; Jung, G.M.; Yoon, S.J.; Kang, S.H. Pharmacokinetics and Tolerance of the Phage Endolysin-Based Candidate Drug SAL200 after a Single Intravenous Administration among Healthy Volunteers. Antimicrob. Agents Chemother. 2017, 61, e02629-16. [Google Scholar] [CrossRef]
- Oh, J.T.; Cassino, C.; Schuch, R. Postantibiotic and Sub-MIC Effects of Exebacase (Lysin CF-301) Enhance Antimicrobial Activity against Staphylococcus aureus. Antimicrob. Agents Chemother. 2019, 63, e02616-18. [Google Scholar] [CrossRef]
- Totté, J.E.E.; van Doorn, M.B.; Pasmans, S.G.M.A. Successful Treatment of Chronic Staphylococcus aureus-Related Dermatoses with the Topical Endolysin Staphefekt SA.100: A Report of 3 Cases. Case Rep. Dermatol. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Yang, H.; Gong, Y.; Zhang, H.; Etobayeva, I.; Miernikiewicz, P.; Luo, D.; Li, X.; Zhang, X.; Dąbrowska, K.; Nelson, D.C.; et al. ClyJ Is a Novel Pneumococcal Chimeric Lysin with a Cysteine- and Histidine-Dependent Amidohydrolase/Peptidase Catalytic Domain. Antimicrob. Agents Chemother. 2019, 63, e02043-18. [Google Scholar] [CrossRef]
- Mao, J.; Schmelcher, M.; Harty, W.J.; Foster-Frey, J.; Donovan, D.M. Chimeric Ply187 endolysin kills Staphylococcus aureus more effectively than the parental enzyme. FEMS Microbiol. Lett. 2013, 342, 30–36. [Google Scholar] [CrossRef]
- Yang, H.; Linden, S.B.; Wang, J.; Yu, J.; Nelson, D.C.; Wei, H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015, 5, 17257. [Google Scholar] [CrossRef]
- Swift, S.M.; Seal, B.S.; Garrish, J.K.; Oakley, B.B.; Hiett, K.; Yeh, H.-Y.; Woolsey, R.; Schegg, K.M.; Line, J.E.; Donovan, D.M. A Thermophilic Phage Endolysin Fusion to a Clostridium perfringens-Specific Cell Wall Binding Domain Creates an Anti-Clostridium Antimicrobial with Improved Thermostability. Viruses 2015, 7, 3019–3034. [Google Scholar] [CrossRef]
- Gilmer, D.B.; Schmitz, J.E.; Euler, C.W.; Fischetti, V.A. Novel Bacteriophage Lysin with Broad Lytic Activity Protects against Mixed Infection by Streptococcus pyogenes and Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 2743–2750. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.-P.; et al. Engineered Endolysin-Based “Artilysins” To Combat Multidrug-Resistant Gram-Negative Pathogens. MBio 2014, 5, e01379-14. [Google Scholar] [CrossRef]
- De Maesschalck, V.; Gutiérrez, D.; Paeshuyse, J.; Lavigne, R.; Briers, Y. Advanced engineering of third-generation lysins and formulation strategies for clinical applications. Crit. Rev. Microbiol. 2020, 46, 548–564. [Google Scholar] [CrossRef]
- Indiani, C.; Sauve, K.; Raz, A.; Abdelhady, W.; Xiong, Y.Q.; Cassino, C.; Bayer, A.S.; Schuch, R. The Antistaphylococcal Lysin, CF-301, Activates Key Host Factors in Human Blood To Potentiate Methicillin-Resistant Staphylococcus aureus Bacteriolysis. Antimicrob. Agents Chemother. 2019, 63, e02291-18. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; Christiansen, T.F.; Komarmy, L.; Siggaard-Andersen, O. Measurement of free calcium ion in capillary blood and serum. Clin. Chem. 1978, 24, 1545–1552. [Google Scholar] [CrossRef]
- Filatova, L.; Donovan, D.; Swift, S.; Pugachev, V.; Emelianov, G.; Chubar, T.; Klaychko, N. Kinetics of inactivation of staphylolytic enzymes: Qualitative and quantitative description. Biochimie 2019, 162, 77–87. [Google Scholar] [CrossRef]
- Gu, J.; Feng, Y.; Feng, X.; Sun, C.; Lei, L.; Ding, W.; Niu, F.; Jiao, L.; Yang, M.; Li, Y.; et al. Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin. PLoS Pathog. 2014, 10, e1004109. [Google Scholar] [CrossRef]
- Loessner, M.J.; Gaeng, S.; Scherer, S. Evidence for a Holin-Like Protein Gene Fully Embedded Out of Frame in the Endolysin Gene of Staphylococcus aureus Bacteriophage 187. J. Bacteriol. 1999, 181, 4452–4460. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, J.; Yang, H.; Wei, C.; Yu, J.; Zhang, Y.; Huang, Y.; Zhang, X.-E.; Wei, H. Construction of a chimeric lysin Ply187N-V12C with extended lytic activity against staphylococci and streptococci. Microb. Biotechnol. 2015, 8, 210–220. [Google Scholar] [CrossRef]
- Chang, Y.; Shin, H.; Lee, J.-H.; Park, C.J.; Paik, S.-Y.; Ryu, S. Isolation and Genome Characterization of the Virulent Staphylococcus aureus Bacteriophage SA97. Viruses 2015, 7, 5225–5242. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S. Characterization of a novel cell wall binding domain-containing Staphylococcus aureus endolysin LysSA97. Appl. Microbiol. Biotechnol. 2017, 101, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Tack, B.F.; McCray, P.B.J.; Welsh, M.J. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L799–L805. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Wang, J.; Yu, J.; Wei, H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 40182. [Google Scholar] [CrossRef]
- Schmelcher, M.; Tchang, V.S.; Loessner, M.J. Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity. Microb. Biotechnol. 2011, 4, 651–662. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Yu, J.; Huang, Y.; Zhang, X.-E.; Wei, H. Novel Chimeric Lysin with High-Level Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus In Vitro. Antimicrob. Agents Chemother. 2014, 58, 536–542. [Google Scholar] [CrossRef]
- Singh, P.K.; Donovan, D.M.; Kumar, A. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob. Agents Chemother. 2014, 58, 4621–4629. [Google Scholar] [CrossRef]
- Yoong, P.; Schuch, R.; Nelson, D.; Fischetti, V.A. Identification of a Broadly Active Phage Lytic Enzyme with Lethal Activity against Antibiotic-Resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 2004, 186, 4808–4812. [Google Scholar] [CrossRef]
- Celia, L.K.; Nelson, D.; Kerr, D.E. Characterization of a bacteriophage lysin (Ply700) from Streptococcus uberis. Vet. Microbiol. 2008, 130, 107–117. [Google Scholar] [CrossRef]
- Wu, M.; Lu, H.; Huang, Q. Expression and Antibacterial Activity of CHAP Catalytic Domain of Staphylococcus aureus Phage Lysin Ply187. Biotechnol. Bull. 2016, 32, 232–238. [Google Scholar]
- Thandar, M.; Lood, R.; Winer, B.Y.; Deutsch, D.R.; Euler, C.W.; Fischetti, V.A. Novel Engineered Peptides of a Phage Lysin as Effective Antimicrobials against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 2671–2679. [Google Scholar] [CrossRef]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Górski, A. Bacteriophages and Lysins in Biofilm Control. Virol. Sin. 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Manoharadas, S.; Witte, A.; Bläsi, U. Antimicrobial activity of a chimeric enzybiotic towards Staphylococcus aureus. J. Biotechnol. 2009, 139, 118–123. [Google Scholar] [CrossRef]
- Daniel, A.; Euler, C.; Collin, M.; Chahales, P.; Gorelick, K.J.; Fischetti, V.A. Synergism between a Novel Chimeric Lysin and Oxacillin Protects against Infection by Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 1603–1612. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chaitanyakumar, A.; Subramanian, P.; Mageswari, A.; Gomathi, A.; Aswini, V.; Sankar, A.M.; Ramya, M.; Gothandam, K.M. Recombinant engineered phage-derived enzybiotic in Pichia pastoris X-33 as whole cell biocatalyst for effective biocontrol of Vibrio parahaemolyticus in aquaculture. Int. J. Biol. Macromol. 2020, 154, 1576–1585. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Pikal, M.J.; Chang, B.S.; Randolph, T.W. Rational Design of Stable Lyophilized Protein Formulations: Some Practical Advice. Pharm. Res. 1997, 14, 969–975. [Google Scholar] [CrossRef]
- Kiefer, F.; Arnold, K.; Künzli, M.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009, 37, D387–D392. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, S.; Nyaruaba, R.; Liu, H.; Yang, H.; Wei, H. A Highly Active Chimeric Lysin with a Calcium-Enhanced Bactericidal Activity against Staphylococcus aureus In Vitro and In Vivo. Antibiotics 2021, 10, 461. https://doi.org/10.3390/antibiotics10040461
Li X, Wang S, Nyaruaba R, Liu H, Yang H, Wei H. A Highly Active Chimeric Lysin with a Calcium-Enhanced Bactericidal Activity against Staphylococcus aureus In Vitro and In Vivo. Antibiotics. 2021; 10(4):461. https://doi.org/10.3390/antibiotics10040461
Chicago/Turabian StyleLi, Xiaohong, Shujuan Wang, Raphael Nyaruaba, Huan Liu, Hang Yang, and Hongping Wei. 2021. "A Highly Active Chimeric Lysin with a Calcium-Enhanced Bactericidal Activity against Staphylococcus aureus In Vitro and In Vivo" Antibiotics 10, no. 4: 461. https://doi.org/10.3390/antibiotics10040461
APA StyleLi, X., Wang, S., Nyaruaba, R., Liu, H., Yang, H., & Wei, H. (2021). A Highly Active Chimeric Lysin with a Calcium-Enhanced Bactericidal Activity against Staphylococcus aureus In Vitro and In Vivo. Antibiotics, 10(4), 461. https://doi.org/10.3390/antibiotics10040461





