Occurrence and Survival of Livestock-Associated MRSA in Pig Manure and on Agriculture Fields
Abstract
1. Introduction
2. Results
2.1. Survival of LA-MRSA in Natural Positive Manure
2.2. Survival of LA-MRSA in Spiked Manure
2.3. Screening of LA-MRSA in Manure Storage Tank Samples from Farms
2.4. LA-MRSA in Boot Sock Samples from Fields before and after Fertilization
3. Discussion
4. Materials and Methods
4.1. LA-MRSA Survival in Liquid Pig Manure
4.1.1. Study Design
4.1.2. LA-MRSA Strains
4.1.3. CFU Counts and Estimation of Decimation Rates
4.1.4. Spiking Dosages
4.2. LA-MRSA in Liquid Manure Storage Tank Samples and Agriculture Fields
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasschaert, G.; Van Elst, D.; Colson, L.; Herman, L.; Cardoso de Carvalho Ferreira, H.; Dewulf, J.; Decrop, J.; Meirlaen, J.; Heyndrickx, M.; Daeseleire, E. Antibiotic residues and antibiotic-resistant bacteria in pig slurry used to fertilize agricultural fields. Antibiotics 2020, 9, 34. [Google Scholar] [CrossRef]
- Hölzel, C.S.; Schwaiger, K.; Harms, K.; Küchenhof, H.; Kunz, A.; Meyer, K.; Müller, C.; Bauer, J. Sewage sludge and liquid pig manure as possible sources of antibiotic resistant bacteria. Environ. Res. 2010, 110, 318–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, S.G.; Jang, H.M.; Kim, Y.B.; Lee, Y.; Kim, Y.M. Characterization of antibiotic resistance genes in representative organic solid wastes: Food waste-recycling wastewater, manure, and sewage sludge. Sci. Total Environ. 2017, 579, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Agency (EFSA). Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008—Part A: MRSA prevalence estimates. EFSA J. 2009, 7, 1376. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Reflection Paper on MRSA in Food Producing and Companion Animals in the European Union: Epidemiology and Control Options for Human and Animal Health. 2009. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-mrsa-food-producing-companion-animals-european-union-epidemiology-control-options_en.pdf (accessed on 15 April 2021).
- Lekkerkerk, W.S.N.; van de Sande-Bruinsma, N.; van der Sande, M.A.B.; Tjon-A-Tsien, A.; Groenheide, A.; Haenen, A.; Timen, A.; van den Broek, P.J.; van Wamel, W.J.B.; de Neeling, A.J.; et al. Emergence of MRSA of unknown origin in the Netherlands. Clin. Microbiol. Infect. 2012, 18, 656–661. [Google Scholar] [CrossRef]
- DANMAP-2016. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; Copenhagen, Denmark, 2017; ISSN 1600-2032. [Google Scholar]
- Danish Veterinary and Food Administration. Resultaterne af Screening for Husdyr-MRSA i Svin i 2016. 2017. Available online: https://www.foedevarestyrelsen.dk/Nyheder/Aktuelt/Documents/MRSA%20ekspertgruppe%20-%20resultatene%20forekomst%20af%20husdyr-MRSA%20i%20svin%202016.pdf (accessed on 15 April 2021).
- Hansen, J.E.; Larsen, A.R.; Skov, R.L.; Chriél, M.; Larsen, G.; Angen, Ø.; Larsen, J.; Lassen, D.C.K.; Pedersen, K. Livestock-associated methicillin-resistant Staphylococcus aureus is widespread in farmed mink (Neovison vison). Vet. Microbiol. 2017, 207, 44–49. [Google Scholar] [CrossRef]
- Hansen, J.E.; Ronco, T.; Stegger, M.; Sieber, R.; Fertner, M.E.; Martin, H.L.; Farre, M.; Toft, N.; Larsen, A.R.; Pedersen, K. MRSA CC398 in dairy cattle and veal calf farms indicates spillover from pig production. Front. Microbiol. 2019, 10, 2733. [Google Scholar] [CrossRef]
- Bisdorff, B.; Scholhölter, J.L.; Claussen, K.; Pulz, M.; Novak, D.; Radon, K. MRSA-ST398 in livestock farmers and neighbouring residents in a rural area in Germany. Epidemiol. Infect. 2012, 140, 1800–1808. [Google Scholar] [CrossRef]
- Cuny, C.; Wieler, L.; Witte, W. Livestock-associated MRSA: The impact on humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Mulders, M.N.; Haenen, A.P.J.; Geenen, P.L.; Vesseur, P.C.; Poldervaart, E.S.; Bosch, T.; Huijsdens, X.W.; Hengevelt, P.D.; Dam-Deisz, W.D.C.; Graat, E.A.M.; et al. Prevalence of livestock-associated MRSA in broiler flocks and risk factors for slaughterhouse personnel in The Netherlands. Epidemiol. Infect. 2010, 138, 743–755. [Google Scholar] [CrossRef]
- Dahms, C.; Hübner, N.-O.; Cuny, C.; Kramer, A. Occurrence of methicillin-resistant Staphylococcus aureus in farm workers and the livestock environment in Mecklenburg-Western Pomerania, Germany. Acta Vet. Scand. 2014, 56, 53. [Google Scholar] [CrossRef]
- Danish Veterinary and Food Administration. MRSA Risiko og Håndtering. Rapport ved MRSA-Ekspertgruppen. 2017. Available online: https://www.foedevarestyrelsen.dk/SiteCollectionDocuments/Dyresundhed/Dyresygdomme/15082017%20MRSA%20rapport.pdf (accessed on 15 April 2021).
- Toft, N.; Larsen, A.R.; Pedersen, K.; Koch, A. OHLAM-Projektet, en One-Health Forskningsindsats om Husdyr-MRSA Hos Dyr Og Mennesker; Technical University of Denmark and Statens Serum Institut: Copenhagen, Denmark, 2019; Available online: https://diagnostik.dtu.dk/-/media/Andre_Universitetsenheder/Diagnostik/Nyheder/Nyheder-i-2019/OHLAM-rapport-version-2.ashx?la=da&hash=CD4980F362C4B2DFD202C06ABBF335FB310BC2C0 (accessed on 15 April 2021).
- Stelder, J.J.; Kjær, L.J.; Jensen, L.B.; Boklund, A.E.; Denwood, M.; Carlsen, M.; Bødker, R. Livestock-associated MRSA survival on house flies (Musca domestica) and stable flies (Stomoxys calcitrans) after removal from a Danish pig farm. Nat. Sci. Rep. 2021, 11, 3527. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.A.; Curriero, F.C.; Cosgrove, S.E.; Nachman, K.E.; Schwartz, B.S. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern. Med. 2013, 173, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Börjesson, S.; Mernelius, S.; Matussek, A.; Lindgren, P.-E. A seasonal study of the mecA gene and Staphylococcus aureus including methicillin-resistant S. aureus in a municipal wastewater treatment plant. Water Res. 2009, 43, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Munch, B.; Errebo Larsen, H.; Aalbӕk, B. Experimental studies on the survival of pathogenic and indicator bacteria in aerated and non-aerated cattle and pig slurry. Biol. Wastes 1987, 22, 49–65. [Google Scholar] [CrossRef]
- Levin-Edens, E.; Bonilla, N.; Scott Meschke, J.; Roberts, M.C. Survival of environmental and clinical strains of methicillin-resistant Staphylococcus aureus [MRSA] in marine and fresh waters. Water Res. 2011, 45, 5681–5686. [Google Scholar] [CrossRef]
- Masmoudi, S.; Denis, M.; Maalej, S. Inactivation of the gene katA or sodA affects the transient entry into the viable but non-culturable response of Staphylococcus aureus in natural seawater at low temperature. Mar. Pollut. Bull. 2010, 60, 2209–2214. [Google Scholar] [CrossRef]
- Feld, L.; Bay, H.; Angen, Ø.; Larsen, A.R.; Madsen, A.M. Survival of LA-MRSA in dust from swine farms. Ann. Work Expo. Health 2018, 62, 147–156. [Google Scholar] [CrossRef]
- Feingold, B.J.; Silbergeld, E.K.; Curriero, F.C.; van Cleef, B.A.G.L.; Heck, M.E.O.C.; Kluytmans, J.A.J.W. Livestock density as risk factor for livestock-associated methicillin-resistant Staphylococcus aureus, the Netherlands. Emerg. Infect. Dis. 2012, 18, 1841–1849. [Google Scholar] [CrossRef]
- Graveland, H.; Wagenaar, J.A.; Bergs, K.; Heesterbeek, H.; Heederik, D. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS ONE 2011, 6, e16830. [Google Scholar] [CrossRef] [PubMed]
- Van Cleef, B.A.G.L.; Graveland, H.; Haenen, A.P.J.; van de Giessen, A.W.; Heederik, D.; Wagenaar, J.A.; Kluytmans, J.A.J.W. Persistence of livestock-associated methicillin-resistant Staphylococcus aureus in field workers after short-term occupational exposure to pigs and veal calves. J. Clin. Microbiol. 2011, 49, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.E.H.; Verstappen, K.M.; van Cleef, B.A.G.L.; Dohmen, W.; Dorado-Garcia, A.; Graveland, H.; Duim, B.; Wagenaar, J.A.; Kluytmans, J.A.J.W.; Heederik, D.J.J. Transmission through air as a possible route of exposure for MRSA. J. Expo. Sci. Environ. Epidemiol. 2016, 2685, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Friese, A.; Schulz, J.; Hoehle, L.; Fetsch, A.; Tenhagen, B.A.; Hartung, J.; Roesler, U. Occurrence of MRSA in air and housing environment of pig barns. Vet. Microbiol. 2012, 158, 129–135. [Google Scholar] [CrossRef]
- Friese, A.; Schulz, J.; Zimmermann, K.; Tenhagen, B.-A.; Fetsch, A.; Hartung, J.; Rösler, U. Occurrence of livestock-associated methicillin-resistant Staphylococcus aureus in turkey and broiler barns and contamination of air and soil surfaces in their vicinity. Appl. Environ. Microbiol. 2013, 79, 2759–2766. [Google Scholar] [CrossRef]
- Schulz, J.; Friese, A.; Klees, S.; Tenhagen, B.A.; Fetsch, A.; Rösler, U.; Hartung, J. Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 5666–5671. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.G.; Green, C.F.; Tarwater, P.M.; Mota, L.C.; Mena, K.D.; Scarpino, P.V. Isolation of antibiotic-resistant bacteria from the air plume downwind of a swine confined or concentrated animal feeding operation. Environ. Health Perspect. 2006, 114, 1032–1037. [Google Scholar] [CrossRef]
- Hansen, J.E. Methicillin-Resistant Staphylococcus Aureus in Danish Production Animals. Ph.D. Thesis, DTU National Veterinary Institute, Kongens Lyngby, Denmark, 2018. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/149112156/PhD_thesis_Julie_Elvekj_r_Hansen.pdf (accessed on 15 April 2021).
- Bӕkbo, P.; Sommer, H.M.; Pedersen, K.; Nielsen, M.W.; Fertner, M.E.; Espinosa-Gongora, C. Forsøg med Nedsӕttelse af Forekomsten af MRSA i Grise og i Staldmiljø. SEGES Meddelelse nr. 1185, 2019. Available online: https://svineproduktion.dk/publikationer/kilder/lu_medd/2019/1185 (accessed on 15 April 2021).
- ISO 4833-2:2013. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Skov, M.N.; Carstensen, B.; Tornøe, N.; Madsen, M. Evaluation of sampling methods for the detection of Salmonella in broiler flocks. J. Appl. Microbiol. 1999, 86, 695–700. [Google Scholar] [CrossRef]
- Maes, N.; Magdalena, J.; Rottiers, S.; De Gheldre, Y.; Struelens, M.J. Evaluation of a triplex PCR assay to discriminate Staphylococcus aureus from coagulase-negative staphylococci and determine methicillin resistance from blood cultures. J. Clin. Microbiol. 2002, 40, 1514–1517. [Google Scholar] [CrossRef]
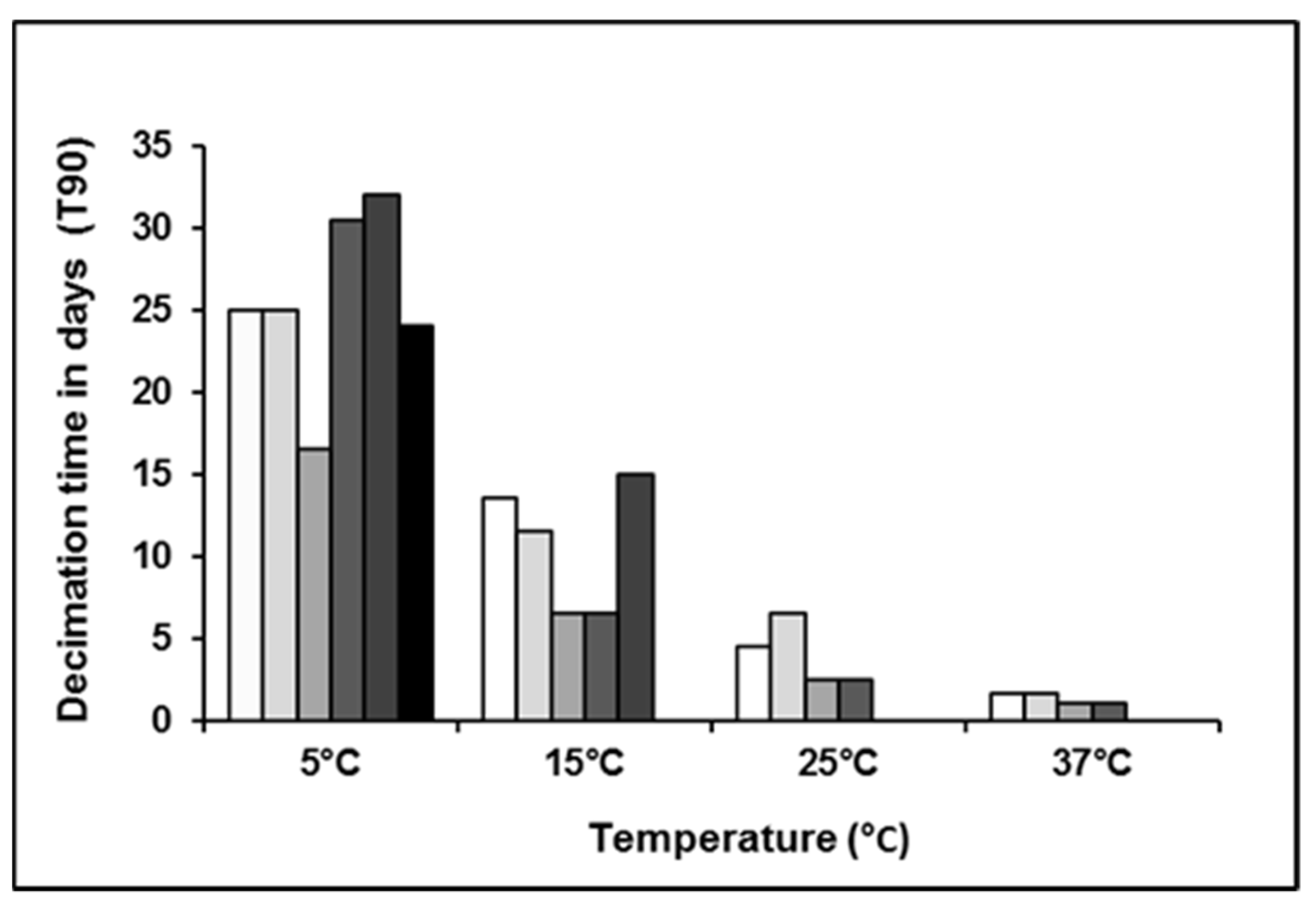

| Sample ID | Temperature (°C) | Initial Load CFU/mL | T90 (Days) |
|---|---|---|---|
| t011 low load | 5 | 7.40 × 102 | 25 |
| t034 low load | 5 | 9.10 × 102 | 25 |
| t011 high load | 5 | 1.72 × 107 | 16.5 |
| t034 high load | 5 | 2.83 × 107 | 30.5 |
| Natural positive | 5 | 2.00 × 101 | 24 |
| Natural positive | 5 | 1.05 × 102 | 32 |
| t011 low load | 15 | 6.75 × 102 | 13.5 |
| t034 low load | 15 | 7.55 × 102 | 11.5 |
| t011 high load | 15 | 1.67 × 107 | 6.5 |
| t034 high load | 15 | 2.87 × 107 | 6.5 |
| Natural positive | 15 | 1.15 × 102 | 15 |
| t011 low load | 25 | 7.30 × 102 | 4.5 |
| t034 low load | 25 | 8.55 × 102 | 6.5 |
| t011 high load | 25 | 2.13 × 107 | 2.5 |
| t034 high load | 25 | 2.62 × 107 | 2.5 |
| t011 low load | 37 | 5.85 × 102 | 1.6 |
| t034 low load | 37 | 8.45 × 102 | 1.6 |
| t011 high load | 37 | 1.82 × 107 | 1.1 |
| t034 high load | 37 | 2.76 × 107 | 1.1 |
| Farm ID n = 19 | Manure Status * | Sock Sample before Fertilization | Sock Sample after Fertilization | ||
|---|---|---|---|---|---|
| Status | Positive/n Samples | Status | Positive/n Samples | ||
| 1 | − | + | 3/5 | − | 0/5 |
| 2 | − | − | 0/1 | − | 0/5 |
| 3 | − | + | 1/2 | + | 1/5 |
| 4 | − | − | 0/5 | + | 2/5 |
| 5 | + | + | 1/3 | − | 0/3 |
| 6 | − | − | 0/1 | − | 0/5 |
| 8 | − | − | 0/4 | − | 0/5 |
| 9 | − | − | 0/5 | − | 0/5 |
| 10 | − | − | 0/5 | − | 0/5 |
| 11 | − | − | 0/4 | − | 0/3 |
| 12 | − | + | 1/5 | − | 0/5 |
| 13 | + | − | 0/5 | − | 0/5 |
| 14 | + | − | 0/5 | − | 0/5 |
| 15 | + | − | 0/5 | NA ** | NA |
| 16 | + | − | 0/5 | − | 0/5 |
| 17 | + | − | 0/5 | − | 0/5 |
| 18 | + | − | 0/5 | − | 0/5 |
| 19 | − | + | 1/5 | + | 2/5 |
| 20 | − | − | 0/5 | − | 0/5 |
| Total pos | 7/19 | 5/19 | 7/80 | 3/18 | 5/86 |
| % pos | 37% | 26% | 9% | 17% | 6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astrup, L.B.; Hansen, J.E.; Pedersen, K. Occurrence and Survival of Livestock-Associated MRSA in Pig Manure and on Agriculture Fields. Antibiotics 2021, 10, 448. https://doi.org/10.3390/antibiotics10040448
Astrup LB, Hansen JE, Pedersen K. Occurrence and Survival of Livestock-Associated MRSA in Pig Manure and on Agriculture Fields. Antibiotics. 2021; 10(4):448. https://doi.org/10.3390/antibiotics10040448
Chicago/Turabian StyleAstrup, Lӕrke Boye, Julie Elvekjӕr Hansen, and Karl Pedersen. 2021. "Occurrence and Survival of Livestock-Associated MRSA in Pig Manure and on Agriculture Fields" Antibiotics 10, no. 4: 448. https://doi.org/10.3390/antibiotics10040448
APA StyleAstrup, L. B., Hansen, J. E., & Pedersen, K. (2021). Occurrence and Survival of Livestock-Associated MRSA in Pig Manure and on Agriculture Fields. Antibiotics, 10(4), 448. https://doi.org/10.3390/antibiotics10040448







