Quality and Utility of Information Captured by Surveillance Systems Relevant to Antimicrobial Resistance (AMR): A Systematic Review
Abstract
1. Introduction
- What is the effectiveness of AMR-relevant surveillance systems in providing information that can be used to inform healthcare professionals?
- What is the acceptability of these systems to users?
2. Materials and Methods
- Prospective observational studies (controlled and uncontrolled before and after studies).
- Retrospective observational evaluations, including case-control studies, retrospective cohort studies, and audits. Data sources included primary data collected for research and secondary data (for example, health insurance claim data).
- Interventions using an experimental design.
- Bacteria whose antibiotic susceptibility status was recorded by the surveillance system.
- Bacteria relevant to AMR. A list was collated from the key AMR threats that have been identified by the WHO [4], the CDC [5], European Centre for Disease Control (ECDC) [6], European Food Safety Authority (EFSA) [7], and the key drug-bug combinations identified by Public Health England in the UK AMR Strategy [8].
- The following types of evaluation study were excluded from the review:
- Evaluations of public surveillance systems that monitor non-bacterial microorganisms (for example, viruses or fungi).
- Evaluations of surveillance systems that monitor bacterial microorganisms that are not on any of the priority lists described in the inclusion criteria above.
- Screening systems that are limited to a single or group of hospitals, and where the information is not shared outside the hospital system.
- Studies published prior to 1988, when the first CDC guidelines for evaluating Public Health Surveillance systems were published.
- Articles published in languages other than English.
2.1. Outcomes
2.2. Quality Assessment of Studies
2.3. Analysis
3. Results
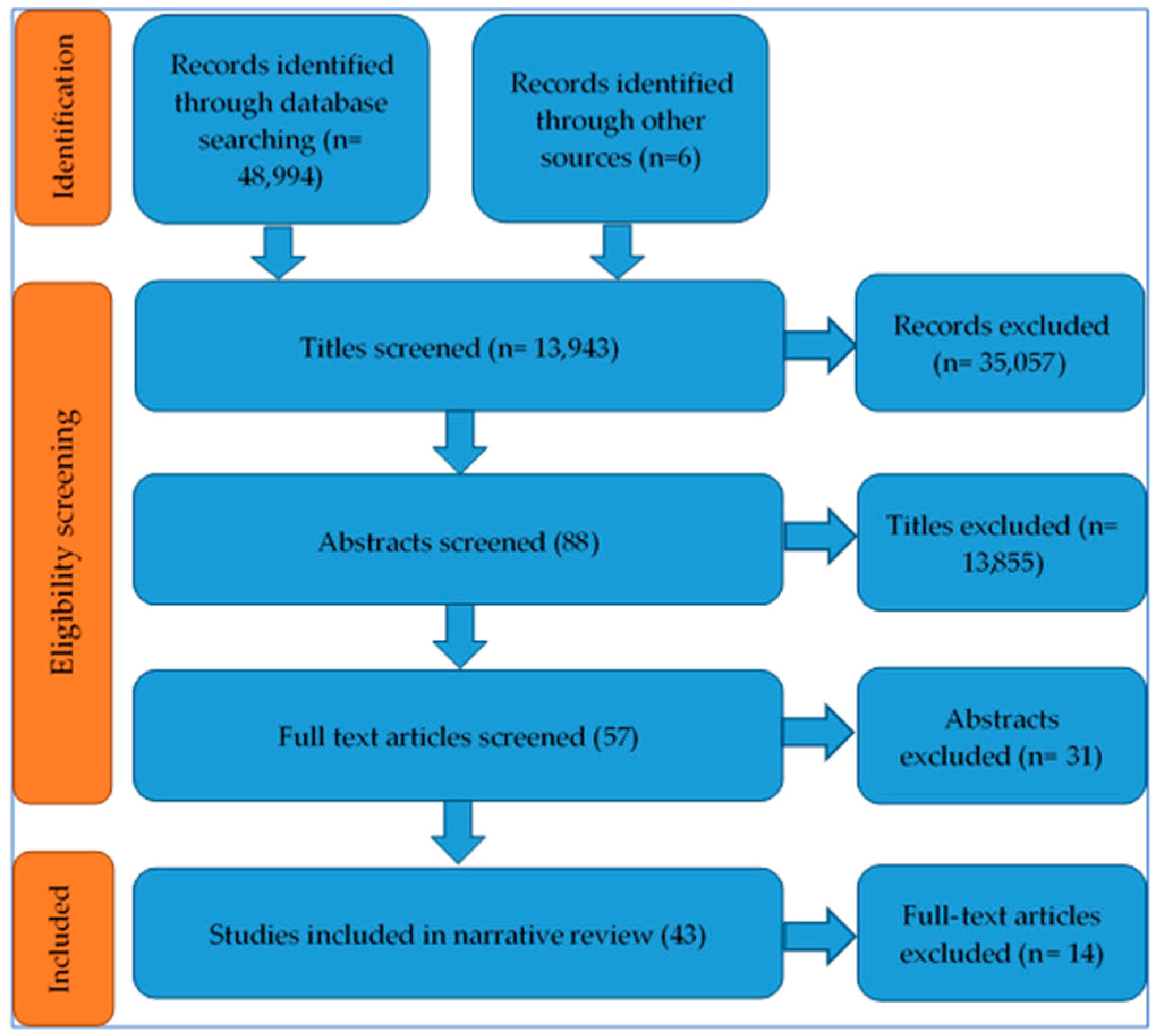
3.1. Outcome of Study Identification Process
3.2. Characteristics of Included Studies
3.2.1. Study Design
- Use of another surveillance system (such as the CDC Emerging Infections Programme) as a high-quality reference standard against which to compare (for example, Nguyen et al. [12])
- Comparison between different methods of data collection and reporting, including comparing electronic reporting against other forms of reporting, for example, Saeed et al. [13].
3.2.2. Quality of Studies
3.2.3. Setting
3.2.4. Surveillance Systems’ Attributes Evaluated
3.2.5. Health Conditions and Microorganisms Monitored by Systems
3.3. Performance of Systems in Relation to Attributes Assessed
3.3.1. Specificity
3.3.2. Usefulness
3.3.3. Completeness
3.3.4. Concordance
3.3.5. Timeliness
3.3.6. Positive Predictive Value (PPV)
3.3.7. Representativeness
3.3.8. Acceptability
3.3.9. Flexibility
3.3.10. Simplicity
3.3.11. Stability
4. Discussion
Strengths and Limitations of the Research
5. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Definitions of Attributes Identified as Important for Determining the Usefulness of a Surveillance System
Appendix B. Search Terms
Appendix C. Attributes Used in Evaluations
| Author | Acceptability | Completeness (Proportion of Cases) | Completeness (Variables Collected for Each Case) | Concordance | Flexibility | Positive Predictive Value (PPV) | Representativeness | Simplicity | Specificity | Stability | Timeliness | Usefulness |
| Heunis | √ | |||||||||||
| Reijn | √ | |||||||||||
| Devine | √ | |||||||||||
| Gimenez-Duran | √ | |||||||||||
| Auld | √ | √ | ||||||||||
| Podewils | √ | √ | √ | |||||||||
| Trei | √ | √ | ||||||||||
| Lo | √ | √ | ||||||||||
| Nguyen | √ | |||||||||||
| Cojocaru | √ | |||||||||||
| San Gabriel | √ | |||||||||||
| Lirio | √ | |||||||||||
| Santos | √ | |||||||||||
| Takahashi | √ | |||||||||||
| Grills | √ | √ | √ | √ | √ | |||||||
| Khue | √ | |||||||||||
| Guerrin-Tran | √ | √ | ||||||||||
| da Silva | √ | √ | √ | √ | ||||||||
| Mancuso | √ | √ | ||||||||||
| Teo | √ | |||||||||||
| Saeed | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Alkhalawi | √ | √ | √ | |||||||||
| Jansson | √ | |||||||||||
| Marks | √ | |||||||||||
| Jansson | √ | |||||||||||
| Severi | √ | √ | ||||||||||
| Birkhead | √ | |||||||||||
| Dominguez | √ | |||||||||||
| Kirk | √ | |||||||||||
| Altmann | √ | |||||||||||
| Kang | √ | √ | ||||||||||
| Migliori | √ | √ | ||||||||||
| Trepka | √ | |||||||||||
| Nicolay | √ | √ | ||||||||||
| Curtis | √ | |||||||||||
| Driver | √ | √ | ||||||||||
| Tanihara | √ | |||||||||||
| Samaan | √ | √ | √ | √ | √ | |||||||
| Stenhem | √ | √ | ||||||||||
| Van Leth | √ | |||||||||||
| Jones | √ | |||||||||||
| Olowokure | √ | |||||||||||
| Sprinson | √ | √ | √ |
Appendix D. Attributes Examined by Health Condition or Microorganism
| Condition/Microorganism | Acceptability | Completeness (Proportion of Cases) | Completeness (Variables Collected for Each Case) | Concordance | Flexibility | Positive Predictive Value (PPV) | Representativeness | Simplicity | Specificity | Stability | Timeliness | Usefulness |
| TB | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Salmonella/salmonellosis | √ | √ | √ | √ | √ | |||||||
| Infections with penicillin-resistant pneumococci | √ | √ | ||||||||||
| MRSA | √ | √ | √ | √ | ||||||||
| Neisseria gonorrhoeae/Gonoccoal infections | √ | √ | √ | √ | √ | √ | ||||||
| Shiga-toxin producing or enter-haemorrhagic Escherichia Coli | √ | |||||||||||
| Shigellosis | √ | |||||||||||
| Campylobacter | √ | √ | √ | √ | √ | |||||||
| Haemophilus influenzae | √ |
References
- World Health Organisation. Antimicrobial Resistance. Available online: https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 April 2021).
- World Health Organisation. WHO Global Strategy for Containment of Antimicrobial Resistance; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Centers for Disease Control and Prevention. Updated Guidelines for Evaluating Public Health Surveillance Systems: Recommendations from the Guidelines Working Group. MMWR 2001, 50, 1–35. [Google Scholar]
- World Health Organisation. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Centers for Disease Control and Prevention. Biggest Threats; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Cormican, M.; Hopkins, S.; Jarlier, V.; Reilly, J.; Simonsen, G.S.; Strauss, R.; Vandenberg, O.; Zabicka, D.; Zarb, P.; Catchpole, M.; et al. ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food-producing animals. EFSA J. 2017, 15, 5017. [Google Scholar]
- EFSA. Scientific Report of EFSA. Technical specification on the harmonised monitoring and reporting of antimicrobial resistance in Salmonella, Campylobacter and indicator Escherichia coli and Enterococcus spp. Bacteria transmitted through food. EFSA J. 2012, 10, 2742. [Google Scholar]
- Department of Health and Department for Environment and Rural Affairs, UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. 2013. Available online: https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018 (accessed on 1 April 2021).
- Critical Appraisal Skills Programme. CASP Checklist: 10 Questions to Help You Make Sense of A Qualitative Research; Critical Appraisal Skills Programme: Oxford, UK, 2018. [Google Scholar]
- Evans, N.; Lasen, M.; Tsey, K. Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies, in A Systematic Review of Rural Development Research; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Popay, J.; Petticrew, H.R.A.S.M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme. Version 1. ESRC. 2006. Available online: https://www.researchgate.net/publication/233866356_Guidance_on_the_conduct_of_narrative_synthesis_in_systematic_reviews_A_product_from_the_ESRC_Methods_Programme (accessed on 1 April 2021).
- Nguyen, D.B.; See, I.; Gualandi, N.; Shugart, A.; Lines, C.; Bamberg, W.; Dumyati, G.; Harrison, L.H.; Lesher, L.; Nadle, J.; et al. Completeness of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection Reporting from Outpatient Hemodialysis Facilities to the National Healthcare Safety Network, 2013. Infect. Control. Hosp. Epidemiol. 2015, 37, 205. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Bano, R.; Asghar, R. Evaluation of national tuberculosis surveillance system in Afghanistan. East. Mediterr. Health J. 2013, 19, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, J.D.; Tobler, S.K.; Eick, A.A.; Olsen, C.H. An evaluation of the completeness and accuracy of active tuberculosis reporting in the United States military. Int. J. Tuberc. Lung Dis. 2010, 14, 1310–1315. [Google Scholar] [PubMed]
- Silva, G.D.M.D.; Bartholomay, P.; Cruz, O.G.; Garcia, L.P. Evaluation of data quality, timeliness and acceptability of the tuberculosis surveillance system in Brazil’s micro-regions. Cienc. Saude Coletiva 2017, 22, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Samaan, G.; Roche, P.W.; E Greig, J.; Tapsall, J.W.; Programme, A.G.S. Evaluation of the Australian Gonococcal Surveillance Programme. Commun. Dis. Intell. Q. Rep. 2005, 29, 143–149. [Google Scholar]
- Auld, S.C.; Kim, L.; Webb, E.K.; Podewils, L.J.; Uys, M. Completeness and concordance of TB and HIV surveillance systems for TB-HIV co-infected patients in South Africa. Int. J. Tuberc. Lung Dis. 2013, 17, 186–191. [Google Scholar] [CrossRef]
- Heunis, C.; Wouters, E.; Kigozi, G.; Engelbrecht, M.; Tsibolane, Y.; Van Der Merwe, S.; Motlhanke, S. Accuracy of Tuberculosis Routine Data and Nurses’ Views of the TB-HIV Information System in the Free State, South Africa. J. Assoc. Nurses AIDS Care 2011, 22, 67–73. [Google Scholar] [CrossRef]
- Effective Public Health Practice Project. Quality Assessment Tool for Quantitative Studies; Effective Public Health Practice Project: Hamilton, ON, USA, 1998. [Google Scholar]
- Alkhalawi, M.J.; McNabb, S.J.; Assiri, A.M.; Memish, Z.A. Evaluation of tuberculosis public health surveillance, Al-Madinah province, Kingdom of Saudi Arabia, 2012. J. Epidemiol. Glob. Health 2015, 6, 37. [Google Scholar] [CrossRef]
- Kang, H.-Y.; Yoo, H.; Park, W.; Go, U.; Jeong, E.; Jung, K.-S.; Son, H. Tuberculosis Notification Completeness and Timeliness in the Republic of Korea during 2012–2014. Osong Public Health Res. Perspect. 2016, 7, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Migliori, G.B.; Spanevello, A.; Ballardini, L.; Neri, M.; Gambarini, C.; Moro, M.L.; Trnka, L.; Raviglione, M. Validation of the surveillance system for new cases of tuberculosis in a province of Northern Italy. Eur. Respir. J. 1995, 8, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Trepka, M.J.; Beyer, T.O.; Proctor, M.E.; Davis, J.P. An evaluation of the completeness of tuberculosis case reporting using hospital billing and laboratory data; Wisconsin, 1995. Ann. Epidemiol. 1999, 9, 419–423. [Google Scholar] [CrossRef]
- Driver, C.R.; Braden, C.R.; Nieves, R.L.; Navarro, A.M.; Rullan, J.V.; Valway, S.E.; McCray, E. Completeness of tuberculosis case reporting, San Juan and Caguas Regions, Puerto Rico, 1992. Public Health Rep. 1996, 111, 157–161. [Google Scholar] [PubMed]
- Devine, M.J.; Aston, R. Assessing the completeness of tuberculosis notification in a health district. Commun. Dis. Rep. CDR Rev. 1995, 5, 137–140. [Google Scholar]
- Podewils, L.J.; Bantubani, N.; Bristow, C.; E Bronner, L.; Peters, A.; Pym, A.; Mametja, L.D. Completeness and Reliability of the Republic of South Africa National Tuberculosis (TB) Surveillance System. BMC Public Health 2015, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Trei, J.S.; Carvelli, K.M. Completeness and timeliness of Chlamydia trachomatis and Neisseria gonorrhoeae genital infection reporting in the U.S. Air Force. Mil. Med. 2008, 173, 313–317. [Google Scholar] [CrossRef][Green Version]
- San Gabriel, P.; Saiman, L.; Kaye, K.; Silin, M.; Onorato, I.; Schulte, J. Completeness of pediatric TB reporting in New York City. Public Health Rep. 2003, 118, 144–153. [Google Scholar] [CrossRef]
- Guerrin-Tran, E.; Thiolet, J.-M.; Rousseau, C.; Henry, S.; Poirier, C.; Che, D.; Vinas, J.-M.; Jarlier, V.; Robert, J. An evaluation of data quality in a network for surveillance of Mycobacterium tuberculosis resistance to antituberculosis drugs in Ile-de-France region-2001–2002. Eur. J. Epidemiol. 2006, 21, 783–785. [Google Scholar] [CrossRef]
- Olowokure, B.; Hawker, J.; Blair, I.; Spencer, N. Decrease in effectiveness of routine surveillance of Haemophilus influenzae disease after introduction of conjugate vaccine: Comparison of routine reporting with active surveillance system. BMJ 2000, 321, 731–732. [Google Scholar] [CrossRef]
- Lo, H.-Y.; Yang, S.-L.; Chou, P.; Chuang, J.-H.; Chiang, C.-Y. Completeness and timeliness of tuberculosis notification in Taiwan. BMC Public Health 2011, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Lírio, M.; Dos Santos, N.P.; Passos, L.A.R.; Kritski, A.; Galvão-Castro, B.; Grassi, M.F.R. Completeness of tuberculosis reporting forms for disease control in individuals with HIV/AIDS in priority cities of Bahia state. Ciência Saúde Coletiva 2015, 20, 1143–1148. [Google Scholar] [CrossRef][Green Version]
- Santos, N.P.D.; Lírio, M.; Passos, L.A.R.; Dias, J.P.; Kritski, A.L.; Galvão-Castro, B.; Grassi, M.F.R. Completeness of tuberculosis reporting forms in five Brazilian capitals with a high incidence of the disease. J. Bras. Pneumol. 2013, 39, 221–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicolay, N.; Garvey, P.; DeLappe, N.; Cormican, M.; McKeown, P. Completeness and timeliness of Salmonella notifications in Ireland in 2008: A cross sectional study. BMC Public Health 2010, 10, 568. [Google Scholar] [CrossRef]
- Stenhem, M.; Örtqvist, Å.; Ringberg, H.; Larsson, L.; Olsson-Liljequist, B.; Haeggman, S.; Kalin, M.; Ekdahl, K.; the Swedish Study Group on MRSA Epidemiology. Validity of routine surveillance data: A case study on Swedish notifications of methicillin-resistant Staphylococcus aureus. Eurosurveillance 2009, 14, 19281. [Google Scholar] [CrossRef]
- E Sprinson, J.; Lawton, E.S.; Porco, T.C.; Flood, J.M.; Westenhouse, J.L. Assessing the validity of tuberculosis surveillance data in California. BMC Public Health 2006, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Khuê, P.M.; Mallet, A.; Veziris, N.; Jarlier, V.; Robert, J. Evaluation of data quality in a laboratory-based surveillance of M. tuberculosis drug resistance and impact on the prevalence of resistance: France, 2004. Epidemiol. Infect. 2007, 136, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, S.; Suzuki, S. Estimation of the incidence of MRSA patients: Evaluation of a surveillance system using health insurance claim data. Epidemiol. Infect. 2016, 144, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Marks, G.B.; Stewart, G.J.; Simpson, S.E.; Sullivan, E.A. Specificity of notification for tuberculosis among screened refugees in NSW. Aust. N. Z. J. Public Health 1999, 23, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Reijn, E.; Swaan, C.M.; Kretzschmar, M.E.; Van Steenbergen, J.E. Analysis of timeliness of infectious disease reporting in the Netherlands. BMC Public Health 2011, 11, 409. [Google Scholar] [CrossRef]
- Takahashi, T. Evaluation of a public health Salmonella surveillance system in King County, Washington. Am. J. Infect. Control. 2004, 32, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Jansson, A.; Arneborn, M.; Skärlund, K.; Ekdahl, K. Timeliness of case reporting in the Swedish statutory surveillance of communicable diseases 1998–2002. Scand. J. Infect. Dis. 2004, 36, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Birkhead, G.; Chorba, T.L.; Root, S.; Klaucke, D.N.; Gibbs, N.J. Timeliness of national reporting of communicable diseases: The experience of the National Electronic Telecommunications System for Surveillance. Am. J. Public Health 1991, 81, 1313–1315. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Coll, J.J.; Fuentes, M.; Salleras, L. Timeliness of notification in infectious disease cases. Public Health Rep. 1992, 107, 474–476. [Google Scholar] [PubMed]
- Altmann, M.; Wadl, M.; Altmann, D.; Benzler, J.; Eckmanns, T.; Krause, G.; Spode, A.; Der Heiden, M.A. Timeliness of Surveillance during Outbreak of Shiga Toxin–producingEscherichia coliInfection, Germany, 2011. Emerg. Infect. Dis. 2011, 17, 1906–1909. [Google Scholar] [CrossRef]
- Curtis, A.B.; McCray, E.; McKenna, M.; Onorato, I.M. Completeness and timeliness of tuberculosis case reporting. A multistate study. Am. J. Prev. Med. 2001, 20, 108–112. [Google Scholar] [CrossRef]
- Jones, G.; Le Hello, S.; Silva, N.J.-D.; Vaillant, V.; De Valk, H.; Weill, F.X.; Le Strat, Y. The French human Salmonella surveillance system: Evaluation of timeliness of laboratory reporting and factors associated with delays, 2007 to 2011. Eurosurveillance 2014, 19, 20664. [Google Scholar] [CrossRef]
- Teo, S.S.S.; Alfaham, M.; Evans, M.R.; Watson, J.M.; Riordan, A.; Sonnenberg, P.; Clark, J.; Hayward, A.; Sharland, M.; Moore-Gillon, J.; et al. An evaluation of the completeness of reporting of childhood tuberculosis. Eur. Respir. J. 2009, 34, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, C.; A Van Hest, N.; Mihaescu, T.; Davies, P.D. Completeness of notification of adult tuberculosis in Iasi County, Romania: A capture-recapture analysis. Int. J. Tuberc. Lung Dis. 2009, 13, 1094–1099. [Google Scholar] [PubMed]
- Van Leth, F.; Evenblij, K.; Wit, F.; Kiers, A.; Sprenger, H.; Verhagen, M.; Hillebregt, M.; Kalisvaart, N.; Schimmel, H.; Verbon, A. TB-HIV co-infection in the Netherlands: Estimating prevalence and under-reporting in national registration databases using a capture-recapture analysis. J. Epidemiol. Community Health 2016, 70, 556–560. [Google Scholar] [CrossRef]
- Hook, E.B.; Regal, R.R. Capture-Recapture Methods in Epidemiology: Methods and Limitations. Epidemiol. Rev. 1995, 17, 243–264. [Google Scholar] [CrossRef]
- Jansson, A.; Arneborn, M.; Ekdahl, K. Sensitivity of the Swedish statutory surveillance system for communicable diseases 1998–2002, assessed by the capture–recapture method. Epidemiol. Infect. 2005, 133, 401–407. [Google Scholar] [CrossRef]
- Gimenez-Duran, J.; Galmes-Truyols, A.; Luque-Fernández, M.Á.; Bonilla-Vargas, L.A.; Bosch-Isabel, C.; Nicolau-Riutort, A.; De Mateo-Ontañón, S. Assessment of tuberculosis surveillance by capture–recapture in the Balearic Islands, Spain, 2005–2007. Enferm. Infecc. Microbiol. Clín. 2015, 33, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Grills, N.J.; Rowe, S.L.; Gregory, J.E.; Lester, R.A.; Fielding, J.E. Evaluation of Campylobacter infection surveillance in Victoria. Commun. Dis. Intell. Q. Rep. 2010, 34, 110–115. [Google Scholar] [PubMed]
- Severi, E.; Dabrera, G.; Boxall, N.; Harvey-Vince, L.; Booth, L.; Balasegaram, S. Timeliness of Electronic Reporting and Acceptability of Public Health Follow-Up of Routine Nonparatyphoidal and Nontyphoidal Salmonella Infections, London and South East England, 2010 to 2011. J. Food Prot. 2014, 77, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Dalton, C.B.; Beers, M.Y.; Cameron, A.S.; Murray, C. Timeliness of Salmonella notifications in South Australia. Aust. N. Z. J. Public Health 1999, 23, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D. Antimicrobial resistance surveillance: Methods will depend on objectives. J. Antimicrob. Chemother. 2002, 49, 3–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organisation. Global Tuberculosis Report 2018; World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar]
- Johnson, A.P. Surveillance of antibiotic resistance. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140080. [Google Scholar] [CrossRef]
- Public Health England. Health Protection Report: Infection Report; Public Health England: London, UK, 2017.
- Elliot, A.J.; Harcourt, S.E.; Hughes, H.E.; Loveridge, P.; Morbey, R.A.; Smith, S.; Soriano, A.; Bains, A.; Smith, G.E.; Edeghere, O.; et al. The COVID-19 pandemic: A new challenge for syndromic surveillance. Epidemiol. Infect. 2020, 148, e122. [Google Scholar] [CrossRef] [PubMed]
| Study Design and Analysis Approaches | Number of Studies |
|---|---|
| Observational: Retrospective analysis of routinely collected data | 30 |
| Mixed methods: Retrospective analysis of routinely collected data combined with semi-structured interviews | 5 |
| Observational: Retrospective analysis of routinely collected data combined with capture-recapture statistical methods | 5 |
| Observational: Retrospective analysis of routinely collected data combined with a questionnaire survey | 2 |
| Observational: Prospective analysis of routinely collected data | 1 |
| Total | 43 |
| Country of Study | Number of Studies |
|---|---|
| Afghanistan | 1 |
| Australia | 4 |
| Brazil | 3 |
| France | 3 |
| Germany | 1 |
| Ireland | 1 |
| Italy | 1 |
| Japan | 1 |
| Netherlands | 2 |
| Republic of Korea | 1 |
| Romania | 1 |
| Saudi Arabia | 1 |
| South Africa | 3 |
| Spain | 2 |
| Sweden | 3 |
| Taiwan | 1 |
| UK | 4 |
| USA | 10 |
| Total | 43 |
| Attribute Name. | Description of Attribute as Used by Evaluators |
|---|---|
| Acceptability | Awareness of, and adherence to, the surveillance system protocol by staff. |
| Completeness (also described as sensitivity, coverage, validity) | Either: The proportion of cases reported by the system (established by looking at other systems or by estimating using the capture-recapture method); also known as sensitivity or coverage. |
| Or: Extent (or proportion) of the fields that are completed in the forms. In some studies, critical categories to be completed were identified; also known as validity. | |
| Concordance (also known as reliability or consistency) | The level of agreement between the different systems on the data collected for each case. |
| Flexibility | The degree to which a system can adapt to changing information needs or operating conditions with little additional time, personnel, or allocated funds (CDC Definition) [3]. |
| Positive Predictive Value (also known as Predictive Value Positive) PPV | The proportion of reported cases that actually have the health-related event under surveillance (CDC Definition) [3]. |
| Representativeness | Geographic or population coverage of system. |
| Simplicity | Features that make a system easy to use (including the method of notification). |
| Specificity | Correctly identifying patients who are free of the condition. |
| Stability | Ability to collect, manage, and provide data properly without failure and ability to be operational when needed [3]. |
| Timeliness | Period between different time points in the notification process. |
| Usefulness | Ability of a system to provide information that can be (or is) acted on; also known as efficacy. |
| Health Condition/Microorganism | Number of Included Evaluations |
|---|---|
| TB (Pulmonary or extra-pulmonary) | 22 |
| Salmonella/salmonellosis | 8 |
| Infections with penicillin-resistant pneumococci | 2 |
| MRSA | 3 |
| Neisseria gonorrhoeae/Gonococcal infections | 2 |
| Shiga-toxin producing or enterhaemorrhagic Escherichia coli | 2 |
| Shigellosis | 1 |
| TB in HIV patients | 4 |
| Campylobacter | 1 |
| Haemophilus influenzae | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Haboubi, M.; Glover, R.E.; Eastmure, E.; Petticrew, M.; Black, N.; Mays, N. Quality and Utility of Information Captured by Surveillance Systems Relevant to Antimicrobial Resistance (AMR): A Systematic Review. Antibiotics 2021, 10, 431. https://doi.org/10.3390/antibiotics10040431
Al-Haboubi M, Glover RE, Eastmure E, Petticrew M, Black N, Mays N. Quality and Utility of Information Captured by Surveillance Systems Relevant to Antimicrobial Resistance (AMR): A Systematic Review. Antibiotics. 2021; 10(4):431. https://doi.org/10.3390/antibiotics10040431
Chicago/Turabian StyleAl-Haboubi, Mustafa, Rebecca E. Glover, Elizabeth Eastmure, Mark Petticrew, Nick Black, and Nicholas Mays. 2021. "Quality and Utility of Information Captured by Surveillance Systems Relevant to Antimicrobial Resistance (AMR): A Systematic Review" Antibiotics 10, no. 4: 431. https://doi.org/10.3390/antibiotics10040431
APA StyleAl-Haboubi, M., Glover, R. E., Eastmure, E., Petticrew, M., Black, N., & Mays, N. (2021). Quality and Utility of Information Captured by Surveillance Systems Relevant to Antimicrobial Resistance (AMR): A Systematic Review. Antibiotics, 10(4), 431. https://doi.org/10.3390/antibiotics10040431






