The Governance and Implementation of the National Action Plan on Antimicrobial Resistance in Tanzania: A Qualitative Study
Abstract
1. Introduction
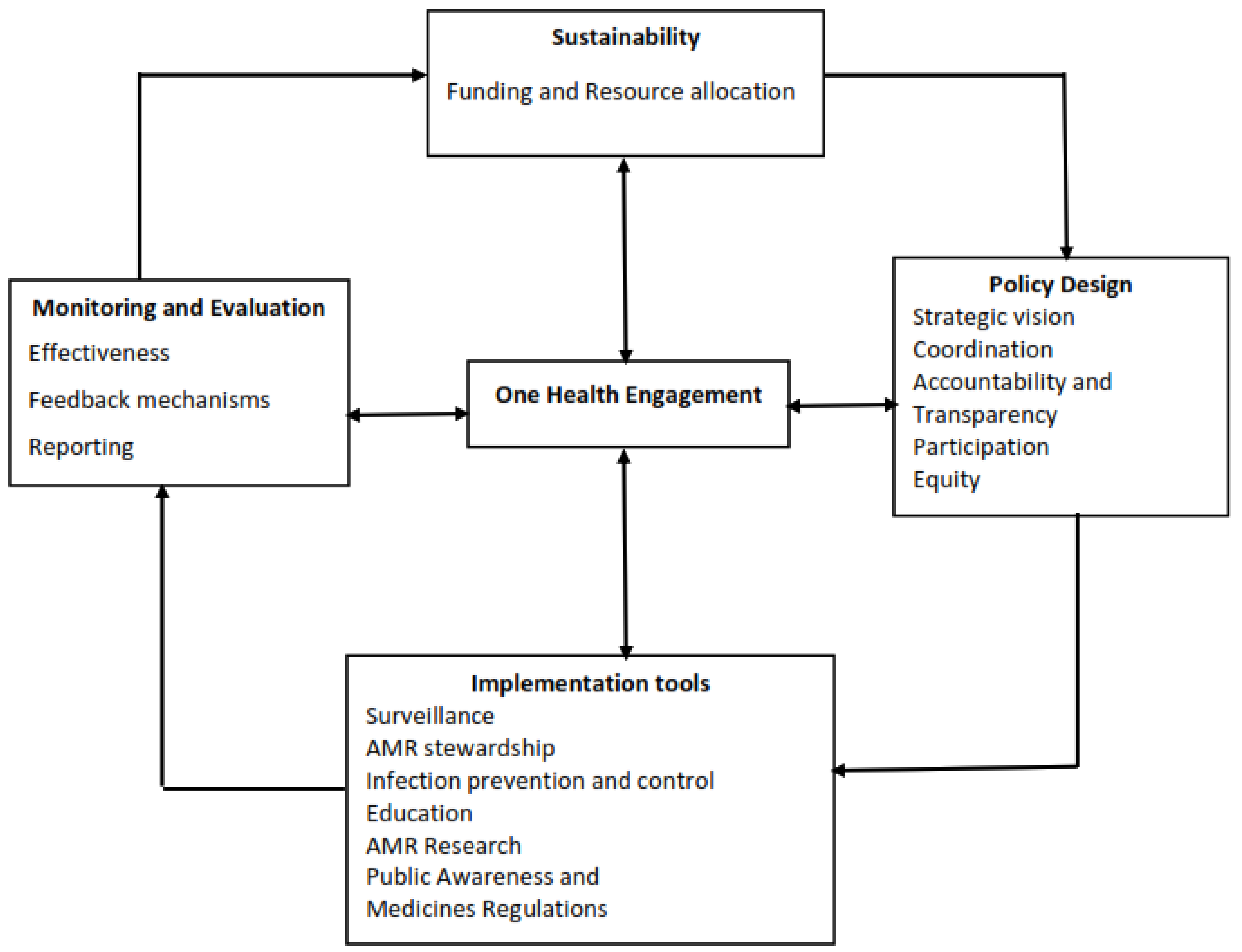
Conceptual Framework of Analysis
2. Results
2.1. Policy Design
2.1.1. Strategic Vision
2.1.2. Coordination
2.1.3. Participation
“I think all sectors were influential because each sector is directly involved and each sector has advantages in the action plan because we have seen that the issue of bacteria resistance doesn’t concern one sector, it affects all sectors”.(Key Informant (KI): scientist from National Laboratory).
“Of course, when you talk about these issues involving more than one sector, the human health sector has often been taking a big part (okay)…, even in the plan it took a big part but at the end of the day it has to touch other sectors because they provide a response to human health. Human health is leading because it is a priority”.(KI: MoH official)
“There was no direct community involvement but the government prepared action plan. After the plan is ready the community will be informed and the community will be educated on what the plan is all about”.(KI: a scientist from the National Laboratory)
“I’m not sure about the community involvement. In the sessions which I attended I did not see a participant specifically from the community”.(KI: MoH official)
2.1.4. Accountability and Transparency
“The guidelines have helped to us observe drug resistance among patients, … we get information through weekly reports, daily reports and monthly reports”.(KI: health center respondent, Ilala)
2.1.5. Equity
“What I know is that every clinician must have guidelines that he/she uses to provide treatment to patients, guidelines for all the diseases. So, when the clinician finds that a patient has used a certain medicine for a long time without getting better, then he moves a patient to another medicine which is stronger”.(KI: health facility in-charge, Ilala)
“So, we came up with a strategy that a pharmacist should make sure we have all the required medicines. When there is shortage, we should take immediate actions to get alternative medicines for our patients”.(KI: health worker, Ilala)
2.2. Implementation Tools
2.2.1. Surveillance
2.2.2. Antimicrobial Stewardship
“We have now developed antimicrobial stewardship guidelines which will be released at any time”.(KI: TMDA official)
2.2.3. Infection Prevention and Control
“We already have a guideline and have begun to distribute it to various regions in preventing and controlling the infection. We are also conduct sensitization campaigns to create awareness on personal hygiene”.(KI: Ministry of Health official)
2.2.4. AMR Research
2.2.5. Education and Public Awareness
“Community health education has increased and it is not only from the Ministry but also from individual and other stakeholders. For example, there are Pharmacy student’s association collaborating with others people to educate the public about AMR”.(KI: TMDA official)
“There is a technical working group whose main task is to provide education on AMU and AMR to the general public and students organizes concerts and symposia and involves students who go around sensitizing the community on AMR”.(KI: MoH official)
“Government officials go direct to farmers and livestock keepers to provide education about antibiotics resistance”.(KI: Ministry of Livestock and Fisheries)
2.2.6. Medicine Regulation
“We expect that no person will be prescribed antibiotics before performing culture and sensitivity in order to be sure the medication you are prescribing is going to work. However, we failed because some hospitals are not equipped. I also think district hospitals do not have the capacity”.(KI: MoH official)
2.3. Monitoring and Evaluation
2.3.1. Reporting
2.3.2. Feedback Mechanisms
“The national level AMR focal persons do conduct supervision of the lower levels. This is because, currently, only the zonal levels are functioning. It will take time before the programme is rolled out nation-wide and to all levels”.(KI: MCC member)
2.3.3. Effectiveness of Monitoring and Evaluation
2.4. Sustainability
Funding and Resource Allocation
“Another challenge is insufficient funds compared to existing activities. And if I look clearly, I see that this AMR is more of donor funded project than the government so when donors leave, the situation will be difficult”.(KI: FAO representative to MCC)
2.5. One Health Engagement
“The preparation of this plan was coordinated by the Ministry of Health, as I explained it was the issue of one health so we were together with the Ministry of Livestock and Fisheries”.(KI: Ministry of Health official)
3. Discussion
4. Materials and Methods
4.1. Study Design and Aims
4.2. Study Settings
4.3. Selection and Recruitment of Study Participants
4.4. Data Collection, Management and Analysis
4.5. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMU | Antimicrobial use |
| AMR | Antimicrobial resistance |
| FAO | Food and Agriculture Organization |
| GLASS | The Global Antimicrobial Resistance and Use Surveillance System |
| IPC | Infection prevention and control |
| KI | Key informant |
| NAP-AMR | National Action Plan on Antimicrobial Resistance |
| MCC | Multi-Sectoral Coordinating Committee |
| TMDA | Tanzania Medicines and Medical Devices Authority |
| WASH | Water sanitation and hygiene |
References
- Spellberg, B.; Gilbert, D.N. The future of antibiotics and resistance: A tribute to a career of leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. S2), S71–S75. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Lipsitch, M.; Samore, M.H. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg. Infect. Dis. 2002, 8, 347–354. [Google Scholar] [CrossRef] [PubMed]
- McNulty, C.A.M.; Boyle, P.; Nichols, T.; Clappison, P.; Davey, P. The public’s attitudes to and compliance with antibiotics. J. Antimicrob. Chemother. 2007, 60 (Suppl. S1), 63–68. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Frumence, G.; Mboera, L.E.G.; Rweyemamu, M.; Mshana, S.E.; Matee, M.I.N. Assessment of drivers of antimicrobial use and resistance in poultry and domestic pig farming in the Msimbazi river basin in Tanzania. Antibiotics 2020, 9, 838. [Google Scholar] [CrossRef]
- Omolase, C.; Adeleke, O.; Afolabi, A.; Ofolabi, O. Self medication amongst general outpatients in a Nigerian community hospital. Ann. Ibadan Postgrad. Med. 2011, 5, 64–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future. World Bank Rep. 2016, 2, 1–132. Available online: www.worldbank.org (accessed on 13 July 2020).
- Tomson, G.; Vlad, I. The need to look at antibiotic resistance from a health systems perspective. Ups. J. Med. Sci. 2014, 119, 117–124. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Heal. 2019, 4, 4–6. [Google Scholar] [CrossRef]
- United Republic of Tanzania (URT). Tanzania National Antimicrobial Resistance Action Plan; Ministry of Health: Dar es Salaam, Tanzania, 2017; pp. 1–76.
- Sindato, C.; Mboera, L.E.G.; Katale, B.Z.; Frumence, G.; Kimera, S.; Clark, T.G.; Legido-Quigley, H.; Mshana, S.E.; Rweyemamu, M.M.; Matee, M. Knowledge, attitudes and practices regarding antimicrobial use and resistance among communities of Ilala, Kilosa and Kibaha districts of Tanzania. Antimicrob. Resist. Infect. Control 2020, 9, 1–17. [Google Scholar] [CrossRef]
- Manyahi, J.; Kibwana, U.; Mgimba, E.; Majigo, M. Multi-drug resistant bacteria predict mortality in bloodstream infection in a tertiary setting in Tanzania. PLoS ONE 2020, 15, e0220424. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, M.; Chatterjee, P.; Chauhan, A.S.; Grace, D.; Lindahl, J.; Beeche, A.; Jing, F.; Chotinan, S. Antimicrobial resistance in South East Asia: Time to ask the right questions. Glob. Health Action 2018, 11, 1483637. [Google Scholar] [CrossRef]
- The World Bank. Pulling Together to Beat Superbugs Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Chua, A.Q.; Verma, M.; Hsu, L.Y.; Legido-Quigley, H. An analysis of national action plans on antimicrobial resistance in Southeast Asia using a governance framework approach. Lancet Reg. Health West. Pac. 2021, 7, 100084. [Google Scholar] [CrossRef]
- United Republic of Tanzania. National Antimicrobial Resistance Surveillance Framework; COSTECH: Dar es Salaam, Tanzania, 2018.
- United Republic of Tanzania. Standard Treatment Guidelines and Essential Medicines List; COSTECH: Dar es Salaam, Tanzania, 2013.
- World Health Organization (WHO). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization (WHO). Surveillance and Monitoring for Antimicrobial Use and Resistance IACG Discussion Paper 1; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- United Republic of Tanzania. COSTECH Rolling Strategic Plan: 2016/17–2020/2021; COSTECH: Dar es Salaam, Tanzania, 2018.
- Horumpende, P.G.; Said, S.H.; Mazuguni, F.S.; Antony, M.L.; Kumburu, H.H.; Sonda, T.B.; Mwanziva, C.E.; Mshana, S.E.; Mmbaga, B.T.; Kajeguka, D.C.; et al. Prevalence, determinants and knowledge of antibacterial self-medication: A cross sectional study in North-eastern Tanzania. PLoS ONE 2018, 13, e020662. [Google Scholar] [CrossRef]
- Anderson, M.; Schulze, K.; Cassini, A.; Plachouras, D.; Mossialos, E. A governance framework for development and assessment of national action plans on antimicrobial resistance. Lancet Infect. Dis. 2019, 19, e371–e384. [Google Scholar] [CrossRef]
- WHO; FAO; OIE. Antimicrobial Resistance: A Manual for Developing National Action Plans; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Acharya, K.P.; Karki, S.; Shrestha, K.; Kaphle, K. One health approach in Nepal: Scope, opportunities and challenges. One Health 2019, 8, 100101. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Meeting the Challenge of Antimicrobial Resistance: From Communication to Collective Action; IACG Discussion Paper; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Kayunze, K.A.; Kiwara, A.; Lyamuya, E.; Kambarage, D.M.; Rushton, J.; Coker, R.; Kock, R. Practice of one health approaches: Bridges and barriers in Tanzania. Onderstepoort J. Vet. Res. 2014, 81, 1–8. [Google Scholar] [CrossRef]
- Mlozi, M.R.S.; Rumisha, S.F.; Mlacha, T.; Bwana, V.M.; Shayo, E.H.; Mayala, B.K.; Malima, R.C.; Mashoto, K.O.; Mboera, L.E.G. Challenges and opportunities for implementing an intersectoral approach in malaria control in Tanzania. Tanzan. J. Health Res. 2015, 17, 1–16. [Google Scholar]
- Sommanustweechai, A.; Tangcharoensathien, V.; Malathum, K.; Sumpradit, N.; Janejai, N.; Jaroenpoj, S. Implementing national strategies on antimicrobial resistance in Thailand: Potential challenges and solutions. Public Health 2018, 157, 142–146. [Google Scholar] [CrossRef]
- Ecumenical Pharmaceutical Network and ReAct. Moving Beyond Antimicrobial Resistance (AMR) National Action Plans Development to Implementation. In Proceedings of the Africa Annual Conference, Johannesburg, South Africa, 6 September 2017; Available online: https://www.reactgroup.org/wp-content/uploads/2017/10/RAN_Conference-2017-Report.pdf (accessed on 20 December 2020).
- Acharya, K.P.; Subramanya, S.H.; Lopes, B.S. Combatting antimicrobial resistance in Nepal: The need for precision surveillance programmes and multi-sectoral partnership. JAC Antimicrob. Resist. 2019, 1, 2–3. [Google Scholar] [CrossRef]
- Castro-Sánchez, E.; Iwami, M.; Ahmad, R.; Atun, R.; Holmes, A.H. Articulating citizen participation in national anti-microbial resistance plans: A comparison of European countries. Eur. J. Public Health 2018, 28, 928–934. [Google Scholar] [CrossRef]
- Munga, M.A.; Mæstad, O. Measuring inequalities in the distribution of health workers: The case of Tanzania. Hum. Resour. Health 2009, 7, 1–12. [Google Scholar] [CrossRef]
- Shemdoe, A.; Mbaruku, G.; Dillip, A.; Bradley, S.; William, J.J.; Wason, D.; Hildon, Z.J.L. Explaining retention of healthcare workers in Tanzania: Moving on, coming to “look, see and go”, or stay? Hum. Resour. Health 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Kariuki, S.; Dougan, G. Antibacterial resistance in sub-Saharan Africa: An underestimated emergency. Ann. N. Y. Acad. Sci. 2014, 1323, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Ndihokubwayo, J.B.; Yahaya, A.A.; Desta, A.T.; Ki-Zerbo, G.; Odei, E.A.; Keita, B.; Pana, A.P.; Nkhoma, W. Antimicrobial Resistance in the African Region: Issues, Challenges and Actions Proposed. Key Determinants for the African Region; WHO Office for Africa: Brazzaville, Republic of Congo, 2013; Volume 16, pp. 27–30. [Google Scholar]
- Elton, L.; Thomason, M.J.; Tembo, J.; Velavan, T.P.; Pallerla, S.R.; Arruda, L.B.; Vairo, F.; Montaldo, C.; Ntoumi, F.; Abdel Hamid, M.M.; et al. Antimicrobial resistance preparedness in sub-Saharan African countries. Antimicrob. Resist. Infect. Control 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Ministry of Health, Ghana. National Policy and Guidelines for Infection Prevention and Control in Health Care Settings; Ministry of Health: Accra, Ghana, 2015.
- Yevutsey, S.K.; Buabeng, K.O.; Aikins, M.; Anto, B.P.; Biritwum, R.B.; Frimodt-Møller, N.; Gyansa-Lutterodt, M. Situational analysis of antibiotic use and resistance in Ghana: Policy and regulation. BMC Public Health 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.K.; Obeng-Nkrumah, N.; Bjerrum, S.; Aryee, N.A.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Physicians knowledge, attitudes, and perceptions concerning antibiotic resistance: A survey in a Ghanaian tertiary care hospital. BMC Health Serv. Res. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asare, A.; Enweronu-Laryea, C.C.; Newman, M.J. Hand hygiene practices in a neonatal intensive care unit in Ghana. J. Infect. Dev. Ctries. 2009, 3, 352–356. [Google Scholar] [PubMed]
- Basu, S.; Garg, S. Journal of Medical Ethics and History of Medicine Letter Antibiotic prescribing behavior among physicians: Ethical challenges in resource-poor settings. J. Med. Ethics Hist. Med. 2018, 11, 2–5. [Google Scholar]
- Opintan, J.A. Leveraging donor support to develop a national antimicrobial resistance policy and action plan: Ghana’s success story. Afr. J. Lab. Med. 2018, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Njukeng, P.A.; Ako-Arrey, D.E.; Amin, E.T.; Njumkeng, C.; Wirsiy, F.S. Antimicrobial Resistance in the Central African Region: A Review. J. Environ. Sci. Public Health 2019, 3, 358–378. [Google Scholar]
- Mathew, P.; Jaguga, C.; Mpundu, M.; Chandy, S.J. Building knowledge and evidence base on antimicrobial resistance in Africa, through ‘One Health’ based surveillance. Clin. Epidemiol. Glob. Health 2020, 8, 313–317. [Google Scholar] [CrossRef]
- Storr, J.; Twyman, A.; Zingg, W.; Damani, N.; Kilpatrick, C.; Reilly, J.; Price, L.; Egger, M.; Grayson, M.L.; Kelley, E.; et al. Core components for effective infection prevention and control programmes: New WHO evidence-based recommendations. Antimicrob. Resist. Infect. Control 2017, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.C.; Gordon, N.C.; Islam, J.; Peacock, S.J.; Scott, J.A.G. AMR surveillance in low and middle-income settings-A roadmap for participation in the Global Antimicrobial Surveillance System (GLASS). Wellcome Open Res. 2017, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tanzania National Health Research Ethics Committee. Guidelines of Ethics for Health Research in Tanzania; Tanzania National Health Research Ethics Committee: Dar es Salaam, Tanzania, 2009; Volume 2009. [Google Scholar]
- Fereday, J.; Muir-Cochrane, E. Demonstrating rigor using thematic analysis: A hybrid approach of inductive and deductive coding and theme development. Int. J. Qual. Methods 2006, 5, 80–92. [Google Scholar] [CrossRef]
| Participants’ Category | National Level | Ilala | Kibaha | Kilosa |
|---|---|---|---|---|
| Ministry of Health, Community Development, Gender, Elderly and Children | 7 | - | - | - |
| Ministry of Livestock and Fisheries | 1 | - | - | - |
| Implementing Partner (FAO) | 1 | - | - | - |
| Environmental Officer from Ilala Municipality | 1 | - | - | |
| Pharmacist and Pharmaceutical Assistants | 4 | 3 | 2 | |
| Public and Private Health Facility Laboratory Technologists/Technicians | 7 | 8 | 8 | |
| Livestock Field Officers | 3 | 4 | 5 | |
| Agro-vets | 4 | 5 | 6 | |
| In-charges of Health Facilities | 9 | 6 | 8 | |
| Dispensers | 8 | 6 | 5 | |
| Total | 9 | 36 | 32 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frumence, G.; Mboera, L.E.G.; Sindato, C.; Katale, B.Z.; Kimera, S.; Metta, E.; Durrance-Bagale, A.; Jung, A.-S.; Mshana, S.E.; Clark, T.G.; et al. The Governance and Implementation of the National Action Plan on Antimicrobial Resistance in Tanzania: A Qualitative Study. Antibiotics 2021, 10, 273. https://doi.org/10.3390/antibiotics10030273
Frumence G, Mboera LEG, Sindato C, Katale BZ, Kimera S, Metta E, Durrance-Bagale A, Jung A-S, Mshana SE, Clark TG, et al. The Governance and Implementation of the National Action Plan on Antimicrobial Resistance in Tanzania: A Qualitative Study. Antibiotics. 2021; 10(3):273. https://doi.org/10.3390/antibiotics10030273
Chicago/Turabian StyleFrumence, Gasto, Leonard E. G. Mboera, Calvin Sindato, Bugwesa Z. Katale, Sharadhuli Kimera, Emmy Metta, Anna Durrance-Bagale, Anne-Sophie Jung, Stephen E. Mshana, Taane G. Clark, and et al. 2021. "The Governance and Implementation of the National Action Plan on Antimicrobial Resistance in Tanzania: A Qualitative Study" Antibiotics 10, no. 3: 273. https://doi.org/10.3390/antibiotics10030273
APA StyleFrumence, G., Mboera, L. E. G., Sindato, C., Katale, B. Z., Kimera, S., Metta, E., Durrance-Bagale, A., Jung, A.-S., Mshana, S. E., Clark, T. G., Rweyemamu, M., Legido-Quigley, H., & Matee, M. I. N. (2021). The Governance and Implementation of the National Action Plan on Antimicrobial Resistance in Tanzania: A Qualitative Study. Antibiotics, 10(3), 273. https://doi.org/10.3390/antibiotics10030273








