Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo
Abstract
1. Introduction
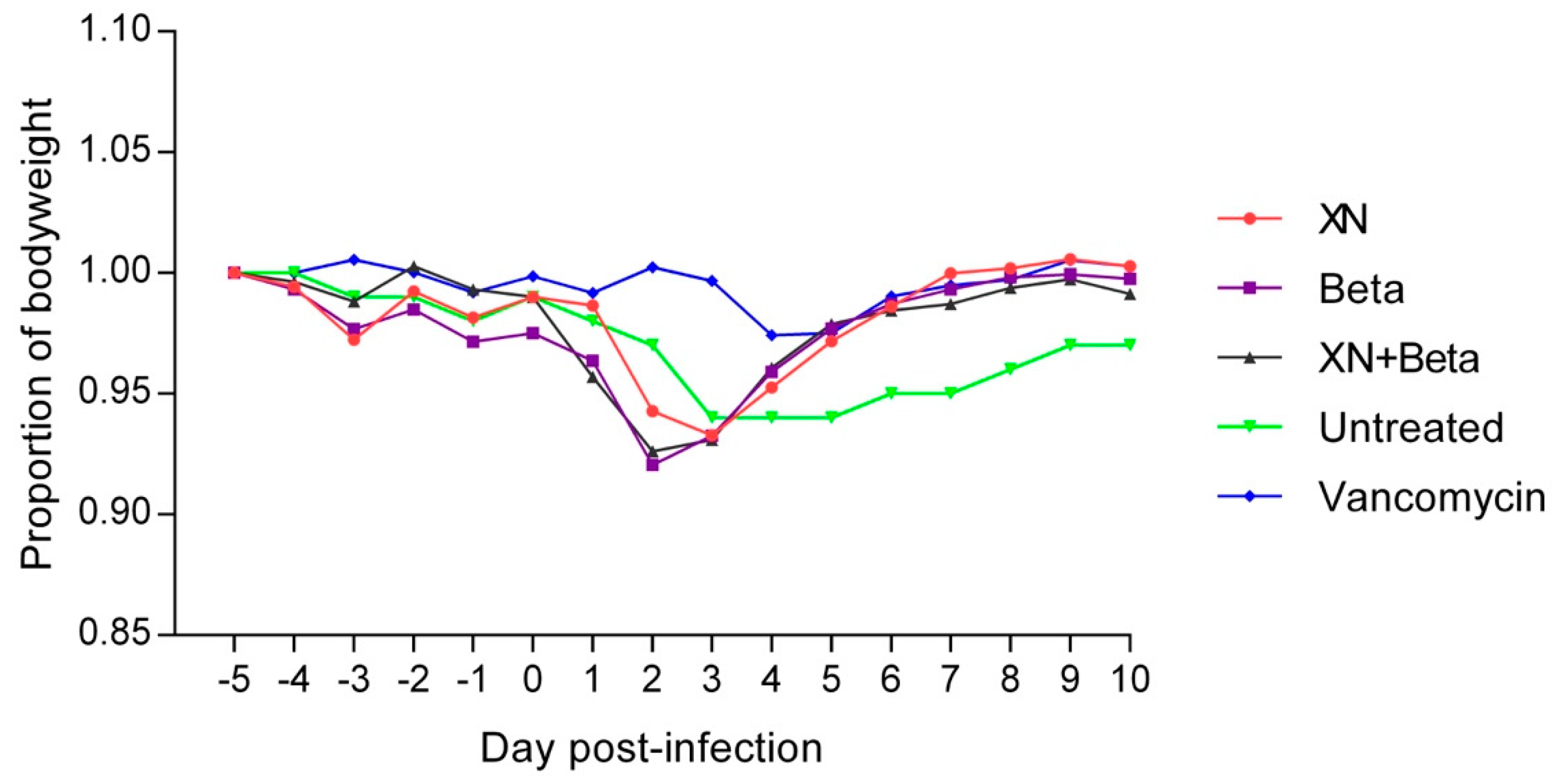
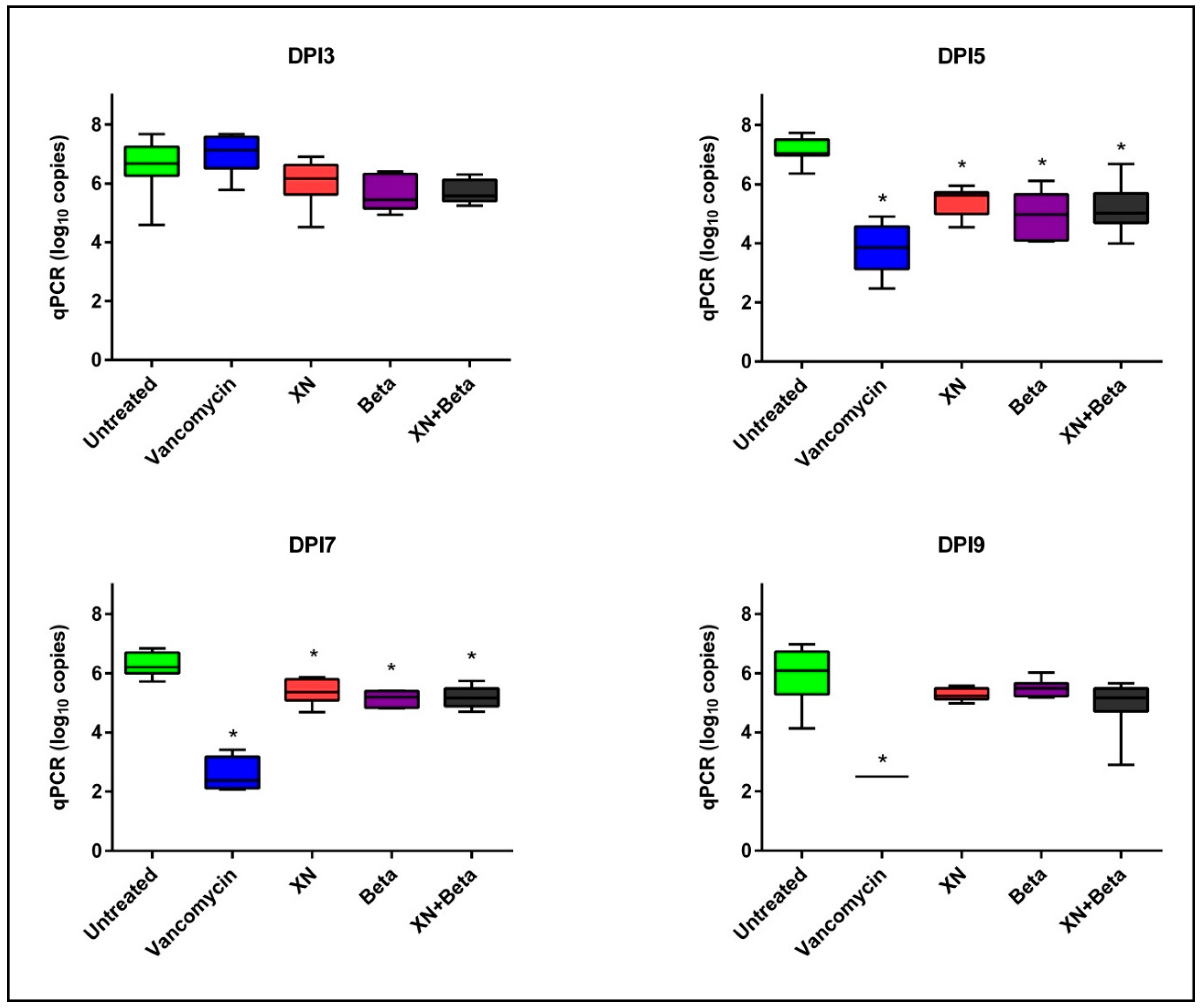
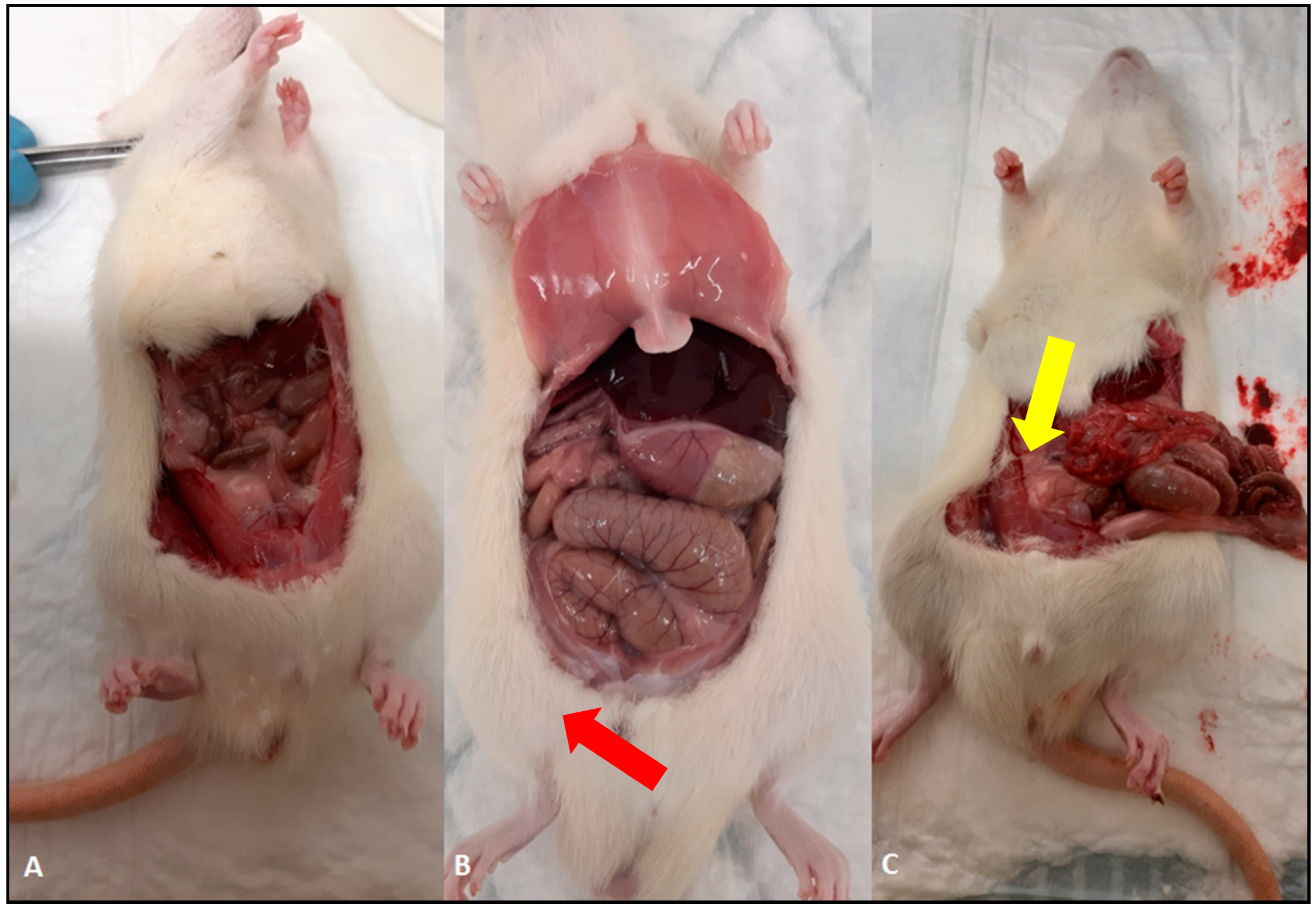
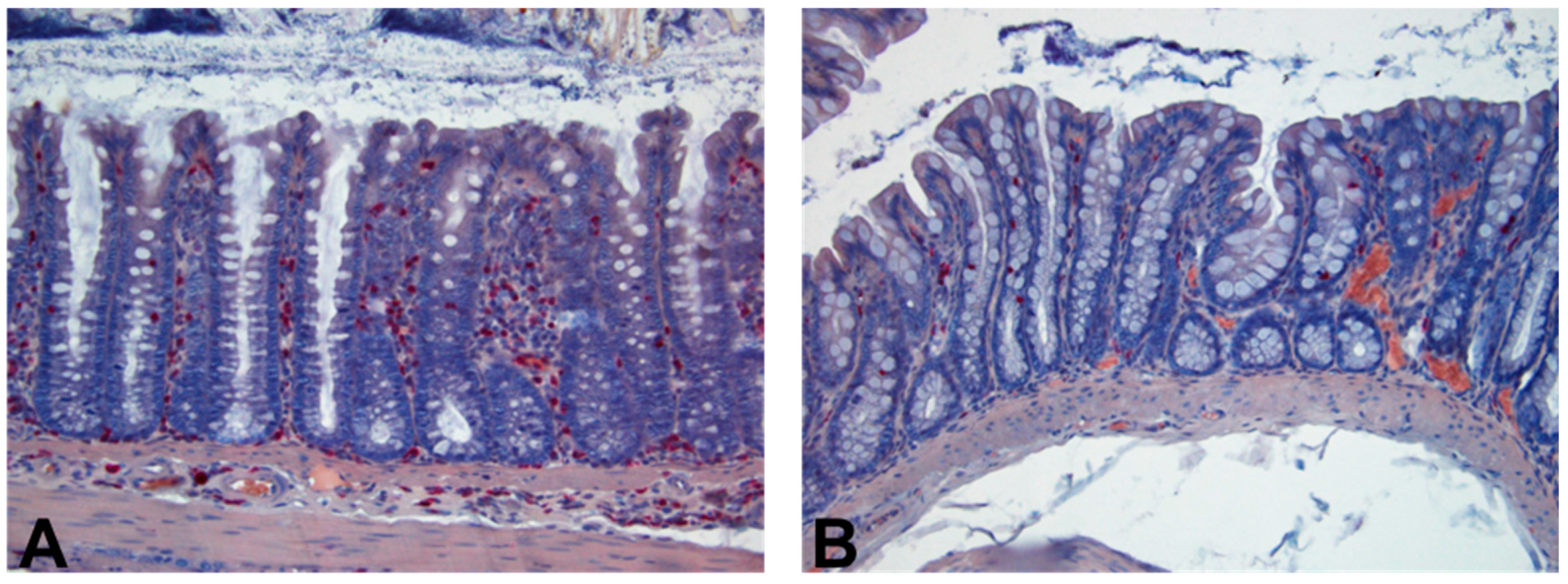
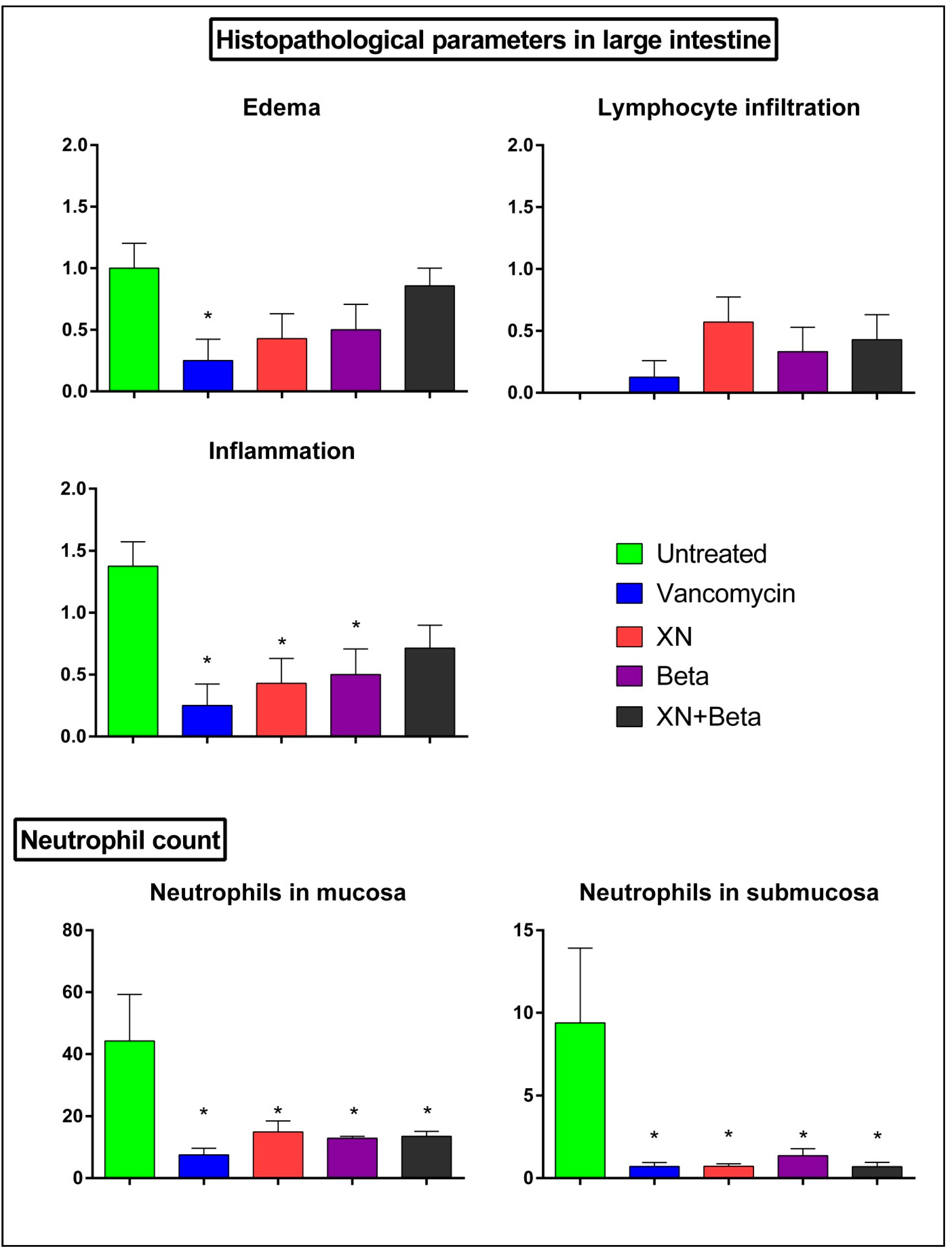
2. Results
3. Discussion
4. Materials and Methods
4.1. Hops Compounds
4.2. Bacterial Strain and Culture Conditions
4.3. Animals and Housing
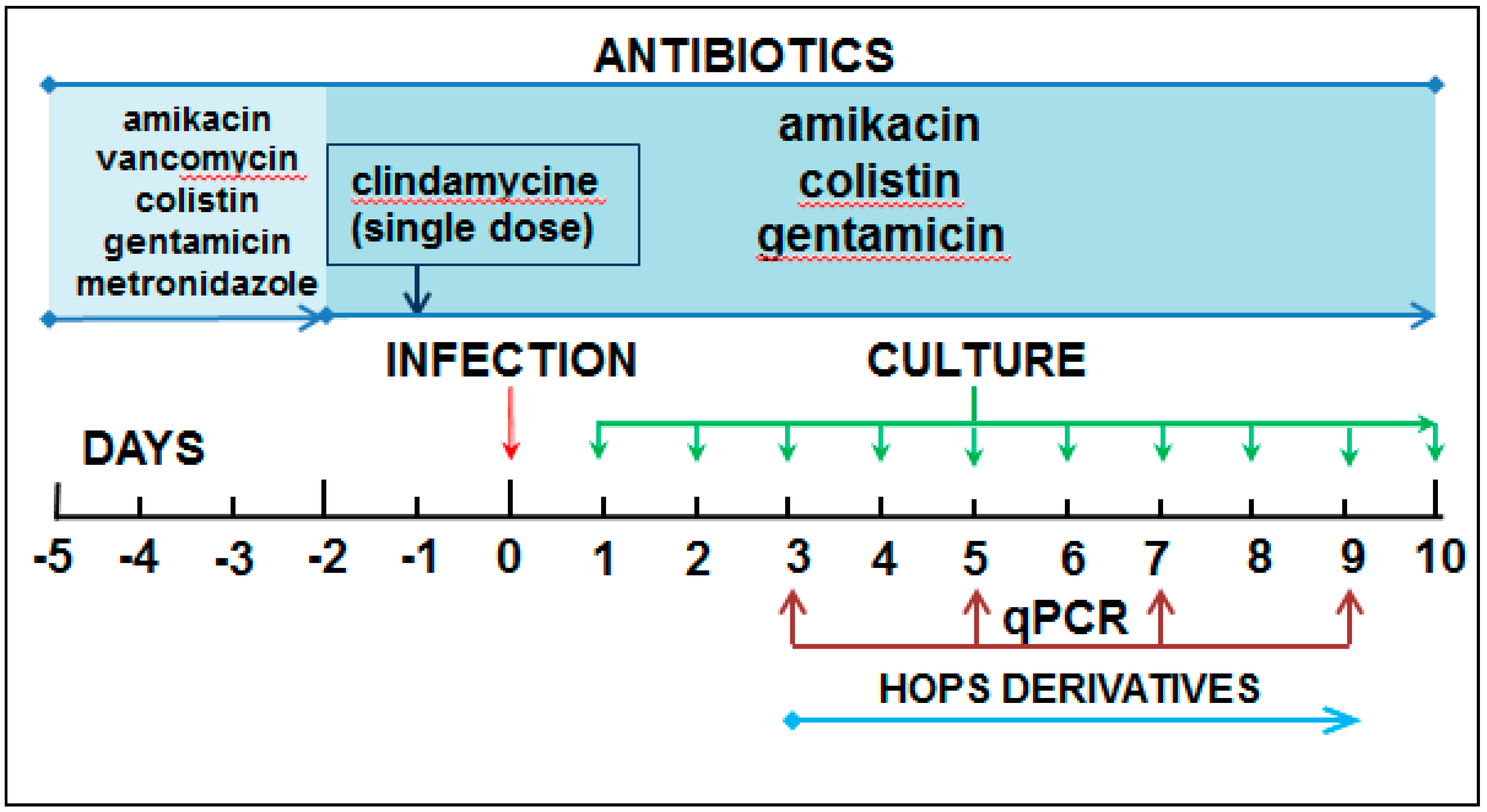
4.4. Experimental Model
4.5. DNA Isolation and Real-Time PCR
4.6. Histopathology Examination
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oka, K.; Osaki, T.; Hanawa, T.; Kurata, S.; Sugiyama, E.; Takahashi, M.; Tanaka, M.; Taguchi, H.; Kamiya, S. Establishment of an Endogenous Clostridium difficile Rat Infection Model and Evaluation of the Effects of Clostridium butyricum MIYAIRI 588 Probiotic Strain. Front. Microbiol. 2018, 9, 1264. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Dang, M.D.; Hasbun, R.; Koo, H.L.; Jiang, Z.D.; DuPont, H.L.; Garey, K.W. Clostridium difficile infection: Update on emerging antibiotic treatment options and antibiotic resistance. Expert Rev. Anti-Infect. Ther. 2010, 8, 555–564. [Google Scholar] [CrossRef]
- Zhu, D.; Sorg, J.A.; Sun, X. Clostridioides difficile Biology: Sporulation, Germination, and Corresponding Therapies for C. difficile Infection. Front. Cell. Infect. Microbiol. 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Janoir, C. Virulence factors of Clostridium difficile and their role during infection. Anaerobe 2016, 37, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Koenigsknecht, M.J.; Theriot, C.M.; Bergin, I.L.; Schumacher, C.A.; Schloss, P.D.; Young, V.B. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect. Immun. 2015, 83, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Dharbhamulla, N.; Abdelhady, A.; Domadia, M.; Patel, S.; Gaughan, J.; Roy, S. Risk Factors Associated With Recurrent Clostridium difficile Infection. J. Clin. Med. Res. 2019, 11, 1–6. [Google Scholar] [CrossRef]
- Dieterle, M.G.; Rao, K.; Young, V.B. Novel therapies and preventative strategies for primary and recurrent Clostridium difficile infections. Ann. N. Y. Acad. Sci. 2019, 1435, 110–138. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; van Beurden, Y.H.; Terveer, E.M.; Goorhuis, A.; Bauer, M.P.; Keller, J.J.; Mulder, C.J.J.; Kuijper, E.J. Update of treatment algorithms for Clostridium difficile infection. Clin. Microbiol. Infect. 2018, 24, 452–462. [Google Scholar] [CrossRef]
- Singh, T.; Bedi, P.; Bumrah, K.; Singh, J.; Rai, M.; Seelam, S. Updates in Treatment of Recurrent Clostridium difficile Infection. J. Clin. Med. Res. 2019, 11, 465–471. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Bartmanska, A.; Walecka-Zacharska, E.; Tronina, T.; Poplonski, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef]
- Bogdanova, K.; Roderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS 2017, 125, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Jeliazkova, E.; Zheljazkov, V.D.; Kacaniova, M.; Astatkie, T.; Tekwani, B.L. Sequential Elution of Essential Oil Constituents during Steam Distillation of Hops (Humulus lupulus L.) and Influence on Oil Yield and Antimicrobial Activity. J. Oleo Sci. 2018, 67, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Mody, D.; Athamneh, A.I.M.; Seleem, M.N. Curcumin: A natural derivative with antibacterial activity against Clostridium difficile. J. Glob. Antimicrob. Resist. 2020, 21, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Roehrer, S.; Behr, J.; Stork, V.; Ramires, M.; Medard, G.; Frank, O.; Kleigrewe, K.; Hofmann, T.; Minceva, M. Xanthohumol C, a minor bioactive hop compound: Production, purification strategies and antimicrobial test. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1095, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Best, E.L.; Freeman, J.; Wilcox, M.H. Models for the study of Clostridium difficile infection. Gut Microbes 2012, 3, 145–167. [Google Scholar] [CrossRef]
- De Wolfe, T.J.; Kates, A.E.; Barko, L.; Darien, B.J.; Safdar, N. Modified Mouse Model of Clostridioides difficile Infection as a Platform for Probiotic Efficacy Studies. Antimicrob. Agents Chemother. 2019, 63, e00111-19. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yang, S.; Zhang, Y.; Qian, K.; Zhang, Z.; Liu, Y.; Wang, Y.; Bai, Y.; Fan, H.; Zhao, X.; et al. Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front. Microbiol. 2018, 9, 2976. [Google Scholar] [CrossRef]
- Shelby, R.D.; Tengberg, N.; Conces, M.; Olson, J.K.; Navarro, J.B.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. Development of a Standardized Scoring System to Assess a Murine Model of Clostridium difficile Colitis. J. Investig. Surg. 2020, 33, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Li, T.; Liu, W.; Zhou, C.; Gao, F. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: An updated randomized controlled trial meta-analysis. PLoS ONE 2019, 14, e0210016. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic Compounds from Humulus lupulus as Natural Antimicrobial Products: New Weapons in the Fight against Methicillin Resistant Staphylococcus aureus, Leishmania mexicana and Trypanosoma brucei Strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Cheon, D.; Kim, J.; Jeon, D.; Shin, H.C.; Kim, Y. Target Proteins of Phloretin for Its Anti-Inflammatory and Antibacterial Activities Against Propionibacterium acnes-Induced Skin Infection. Molecules 2019, 24, 1319. [Google Scholar] [CrossRef]
- Krofta, K.; Liskova, H.; Vrabcova, S. Process for Preparing Pure Beta Acids of Hop. Patent Number CZ303017B6, 29 February 2012. [Google Scholar]
- Biendl, M. Isolation of Prenylflavovnoids from Hops. International Society for Horticultural Science. Available online: http://www.actahort.org/books/1010/1010_15.htm (accessed on 15 January 2021).
- Pejchal, J.; Novotny, J.; Marak, V.; Osterreicher, J.; Tichy, A.; Vavrova, J.; Sinkorova, Z.; Zarybnicka, L.; Novotna, E.; Chladek, J.; et al. Activation of p38 MAPK and expression of TGF-beta1 in rat colon enterocytes after whole body gamma-irradiation. Int. J. Radiat. Biol. 2012, 88, 348–358. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sleha, R.; Radochova, V.; Mikyska, A.; Houska, M.; Bolehovska, R.; Janovska, S.; Pejchal, J.; Muckova, L.; Cermak, P.; Bostik, P. Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo. Antibiotics 2021, 10, 392. https://doi.org/10.3390/antibiotics10040392
Sleha R, Radochova V, Mikyska A, Houska M, Bolehovska R, Janovska S, Pejchal J, Muckova L, Cermak P, Bostik P. Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo. Antibiotics. 2021; 10(4):392. https://doi.org/10.3390/antibiotics10040392
Chicago/Turabian StyleSleha, Radek, Vera Radochova, Alexander Mikyska, Milan Houska, Radka Bolehovska, Sylva Janovska, Jaroslav Pejchal, Lubica Muckova, Pavel Cermak, and Pavel Bostik. 2021. "Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo" Antibiotics 10, no. 4: 392. https://doi.org/10.3390/antibiotics10040392
APA StyleSleha, R., Radochova, V., Mikyska, A., Houska, M., Bolehovska, R., Janovska, S., Pejchal, J., Muckova, L., Cermak, P., & Bostik, P. (2021). Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo. Antibiotics, 10(4), 392. https://doi.org/10.3390/antibiotics10040392






