In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris
Abstract
1. Introduction
2. Results
2.1. Checkerboard Assays and Analysis
2.2. Time-Kill Procedures
3. Discussion
4. Materials and Methods
4.1. Fungal Strains
4.2. Antifungal Agents
4.3. Checkerboard Assay
4.4. Data Analysis
4.4.1. FICI
4.4.2. Greco Model
4.4.3. Bliss Independence Model
4.5. Time-Kill Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidaud, A.L.; Chowdhary, A.; Dannaoui, E. Candida auris: An emerging drug resistant yeast—A mini-review. J. Mycol. Med. 2018, 28, 568–573. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [CrossRef]
- WHO. First Meeting of the WHO Antifungal Expert Group on Identifying Priority Fungal Pathogens: Meeting Report. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789240006355 (accessed on 8 February 2021).
- Kenters, N.; Kiernan, M.; Chowdhary, A.; Denning, D.W.; Peman, J.; Saris, K.; Schelenz, S.; Tartari, E.; Widmer, A.; Meis, J.F.; et al. Control of Candida auris in healthcare institutions: Outcome of an International Society for Antimicrobial Chemotherapy expert meeting. Int. J. Antimicrob. Agents 2019, 54, 400–406. [Google Scholar] [CrossRef]
- Biagi, M.J.; Wiederhold, N.P.; Gibas, C.; Wickes, B.L.; Lozano, V.; Bleasdale, S.C.; Danziger, L. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum. Infect. Dis. 2019, 6, ofz262. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Chowdhary, A.; Prakash, A.; Vaezi, A.; Dannaoui, E.; Meis, J.F.; Badali, H. In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.L.; Botterel, F.; Chowdhary, A.; Dannaoui, E. In vitro antifungal combination of flucytosine with amphotericin B, voriconazole, or micafungin against Candida auris shows no antagonism. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef]
- O’Brien, B.; Chaturvedi, S.; Chaturvedi, V. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Li, X.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimicrob. Agents 2018, 52, 754–761. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin interacts synergistically with echinocandins against Candida auris. Int. J. Antimicrob. Agents 2020, 55, 105901. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Totten, M.; Memon, W.; Ying, C.; Zhang, S.X. In vitro antifungal susceptibility of the emerging multidrug-resistant pathogen Candida auris to miltefosine alone and in combination with amphotericin B. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Schwarz, P.; Bidaud, A.L.; Dannaoui, E. In vitro synergy of isavuconazole in combination with colistin against Candida auris. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Warn, P.A.; Sharp, A.; Parmar, A.; Majithiya, J.; Denning, D.W.; Hope, W.W. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: Mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob. Agents Chemother. 2009, 53, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.S.; Deshpande, L.M.; Rhomberg, P.R.; Utt, E.A.; Castanheira, M. Evaluation of synergistic activity of isavuconazole or voriconazole plus anidulafungin and the occurrence and genetic characterisation of Candida auris detected in a surveillance program. Antimicrob. Agents Chemother. 2021, in press. [Google Scholar] [CrossRef]
- Katragkou, A.; McCarthy, M.; Meletiadis, J.; Hussain, K.; Moradi, P.W.; Strauss, G.E.; Myint, K.L.; Zaw, M.H.; Kovanda, L.L.; Petraitiene, R.; et al. In vitro combination therapy with isavuconazole against Candida spp. Med. Mycol. 2017, 55, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Dudiuk, C.; Berrio, I.; Leonardelli, F.; Morales-Lopez, S.; Theill, L.; Macedo, D.; Yesid-Rodriguez, J.; Salcedo, S.; Marin, A.; Gamarra, S.; et al. Antifungal activity and killing kinetics of anidulafungin, caspofungin and amphotericin B against Candida auris. J. Antimicrob. Chemother. 2019, 74, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Faessel, H.; Levasseur, L. The search for cytotoxic synergy between anticancer agents: A case of Dorothy and the ruby slippers? J. Natl. Cancer Inst. 1996, 88, 699–700. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Meletiadis, J.; Verweij, P.E.; TeDorsthorst, D.T.; Meis, J.F.; Mouton, J.W. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: Comparison of different drug interaction models. Med. Mycol. 2005, 43, 133–152. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar]
- de Miranda Silva, C.; Hajihosseini, A.; Myrick, J.; Nole, J.; Louie, A.; Schmidt, S.; Drusano, G.L. Effect of linezolid plus bedaquiline against Mycobacterium tuberculosis in log phase, acid phase, and nonreplicating-persister phase in an in vitro assay. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Otto, R.G.; van Gorp, E.; Kloezen, W.; Meletiadis, J.; van den Berg, S.; Mouton, J.W. An alternative strategy for combination therapy: Interactions between polymyxin B and non-antibiotics. Int. J. Antimicrob. Agents 2019, 53, 34–39. [Google Scholar] [CrossRef]
- Brill, M.J.E.; Kristoffersson, A.N.; Zhao, C.; Nielsen, E.I.; Friberg, L.E. Semi-Mechanistic pharmacokinetic-pharmacodynamic modelling of antibiotic drug combinations. Clin. Microbiol. Infect. 2018, 24, 697–706. [Google Scholar] [CrossRef]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- de la Pena, A.; Grabe, A.; Rand, K.H.; Rehak, E.; Gross, J.; Thyroff-Friesinger, U.; Muller, M.; Derendorf, H. PK-PD modelling of the effect of cefaclor on four different bacterial strains. Int. J. Antimicrob. Agents 2004, 23, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitan, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-Lopez, A.I.; Martinez-Morel, H.; Calabuig, E.; Salavert-Lleti, M.; Ramirez, P.; Lopez-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. The European Committee for Antimicrobial Susceptibility Testing. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. EUCAST Definitive Document E.def 7.3.2. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 8 February 2021).
- D’Argenio, D.Z.; Schumitzky, A.; Wang, X. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software; Biomedical Simulations Resource: Los Angeles, CA, USA, 2009. [Google Scholar]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef]
- Gil-Alonso, S.; Jauregizar, N.; Canton, E.; Eraso, E.; Quindos, G. In vitro fungicidal activities of anidulafungin, caspofungin, and micafungin against Candida glabrata, Candida bracarensis, and Candida nivariensis evaluated by time-kill studies. Antimicrob. Agents Chemother. 2015, 59, 3615–3618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef] [PubMed]
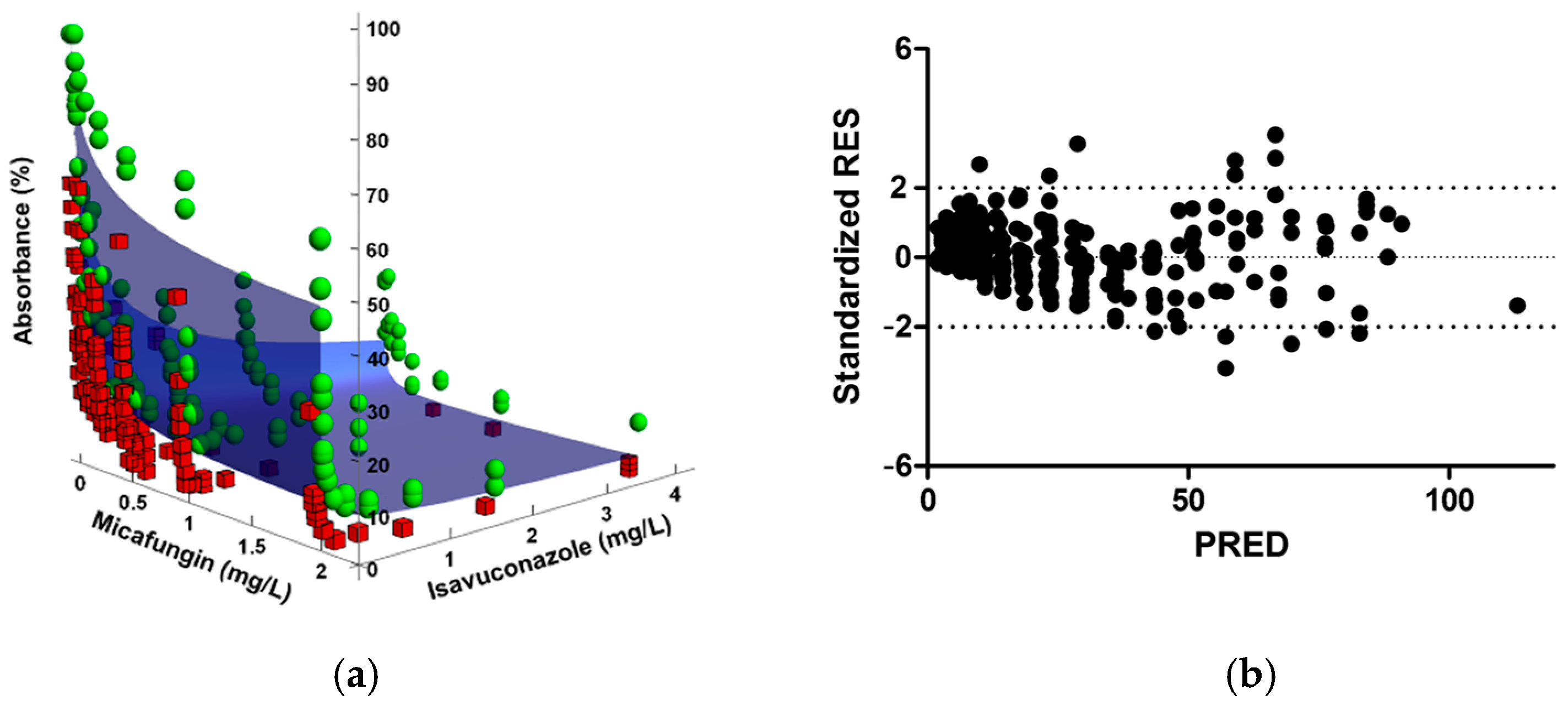
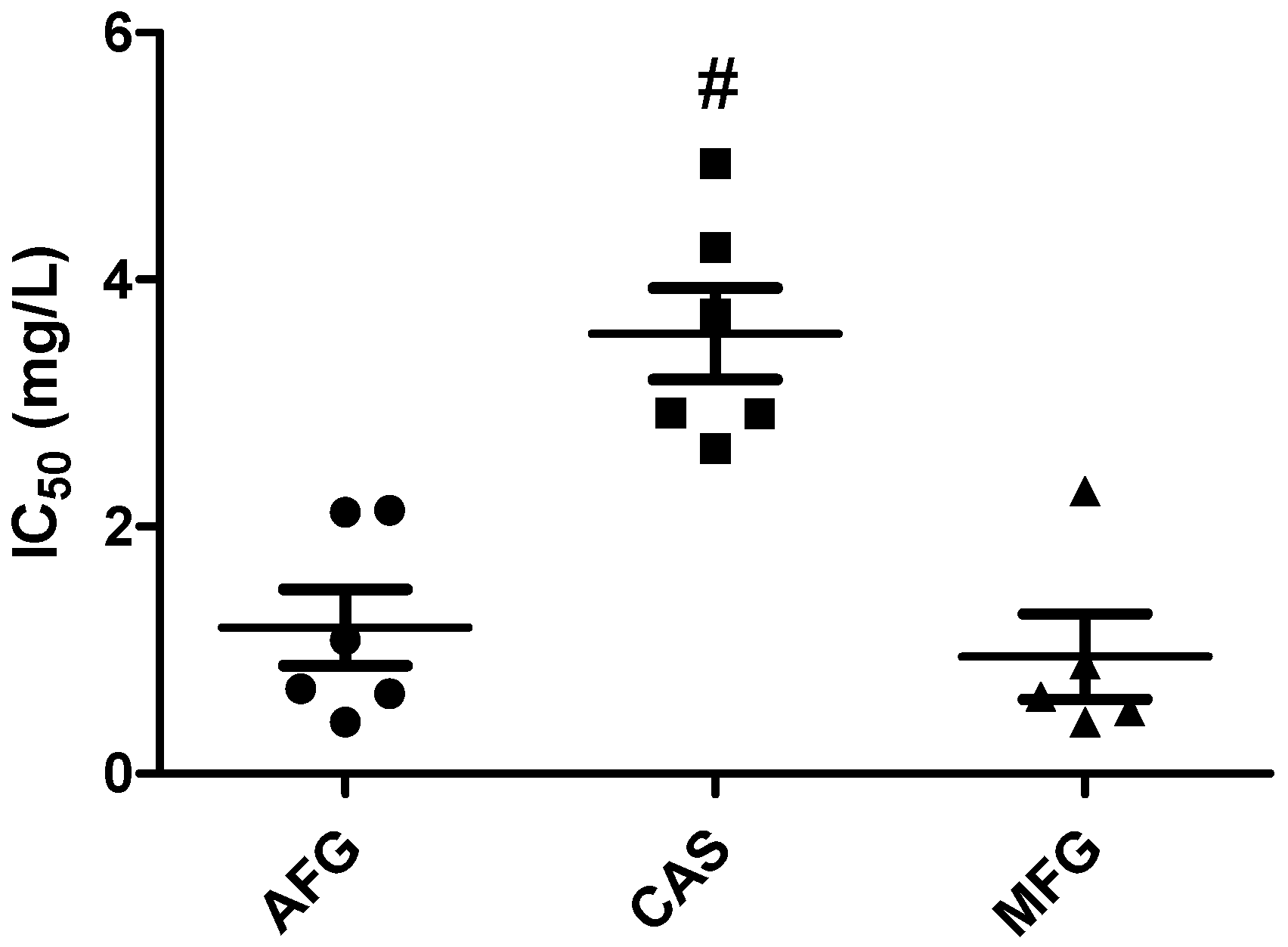

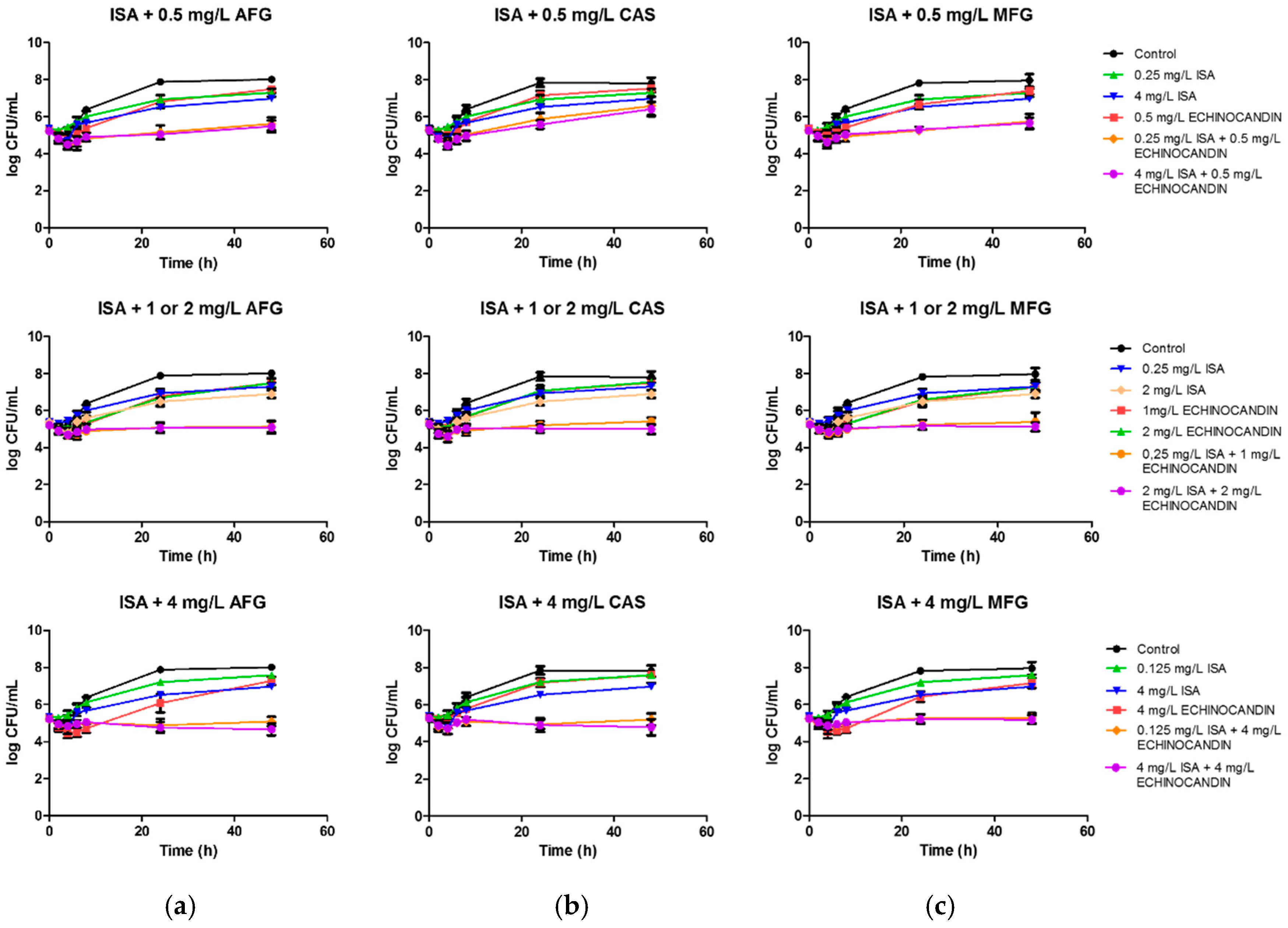
| C. auris isolate | ISA + AFG b | ISA + CAS b | ISA + MFG b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FICI | Greco | Bliss | FICI | Greco | Bliss | FICI | Greco | Bliss | |
| Median (Range) | α (95% CI) | ΣSYN_ANT (ΣSYN; ΣANT) | Median (Range) | α (95% CI) | ΣSYN_ANT (ΣSYN; ΣANT) | Median (Range) | α (95% CI) | ΣSYN_ANT (ΣSYN; ΣANT) | |
| CJ94 | 0.27 (0.24–0.30) | 151 (16.53–285.5) | 86.91 (87.22; −0.31) | 0.26 (0.25–0.52) | 38.59 (−8.106–85.28) | 36.56 (41.22; −4.66) | 0.24 (0.15–0.38) | 112.8 (22.70–202.9) | 66.01 (66.79; −0.78) |
| CJ97 | 0.36 (0.25–0.49) | 21.70 (5.105–38.38) | 29.24 (30.27; −1.03) | 0.37 (0.36–1.25) | 22.23 (1.016–43.44) | 11.44 (20.75; −8.56) | 0.15 (0.13–0.25) | 216.7 (11.37–422.0) | 57.59 (59.49; −1.90) |
| CJ98 | 0.19 (0.015–0.19) | 102.1 (4.056–200.1) | 57.38 (57.73; −0.35) | 0.25 (0.08–0.5) | 42.64 (−14.72–100.00) | 40.67 (45.46; −4.79) | 0.37 (0.15–0.49) | 57.88 (0.85–114.9) | 50.88 (53.70; −2.82) |
| CJ99 | 0.18 (0.09–0.18) | 186.9 (−3.962–377.8) | 73.23 (73.32; −0.09) | 0.25 (0.18–0.37) | 114.4 (−1.911–230.6) | 75.71 (75.89; −0.18) | 0.16 (0.08–0.38) | 674.4 (−138.3–1487) | 111.56 (112.09; −0.53) |
| CJ100 | 0.18 (0.15–0.37) | 48.71 (−15.71–113.1) | 72.80 (75.17; −2.37) | 0.38 (0.25–0.49) | 37.12 (2.344–71.90) | 60.31 (60.64; −0.33) | 0.14 (0.12–0.15) | 175.1 (14.32–335.8) | 80.14 (80.37; −0.23) |
| CJ102 | 0.25 (0.14–0.36) | 204.1 (−5.106–413.9) | 69.61 (69.63; −0.02) | 0.36 (0.25–0.38) | 41.85 (−1.016–84.71) | 46.37 (48.08; −1.71) | 0.25 (0.13–0.49) | 95.66 (24.84–166.5) | 71.35 (71.72; −0.37) |
| Median | 0.22 | – | 71.205 | 0.31 | – | 43.52 | 0.20 | – | 68.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, U.; Kim, S.; Eraso, E.; Quindós, G.; Vozmediano, V.; Schmidt, S.; Jauregizar, N. In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics 2021, 10, 355. https://doi.org/10.3390/antibiotics10040355
Caballero U, Kim S, Eraso E, Quindós G, Vozmediano V, Schmidt S, Jauregizar N. In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics. 2021; 10(4):355. https://doi.org/10.3390/antibiotics10040355
Chicago/Turabian StyleCaballero, Unai, Sarah Kim, Elena Eraso, Guillermo Quindós, Valvanera Vozmediano, Stephan Schmidt, and Nerea Jauregizar. 2021. "In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris" Antibiotics 10, no. 4: 355. https://doi.org/10.3390/antibiotics10040355
APA StyleCaballero, U., Kim, S., Eraso, E., Quindós, G., Vozmediano, V., Schmidt, S., & Jauregizar, N. (2021). In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics, 10(4), 355. https://doi.org/10.3390/antibiotics10040355







