Population Pharmacokinetics of Piperacillin in Non-Critically Ill Patients with Bacteremia Caused by Enterobacteriaceae
Abstract
1. Introduction
2. Results
2.1. Patients
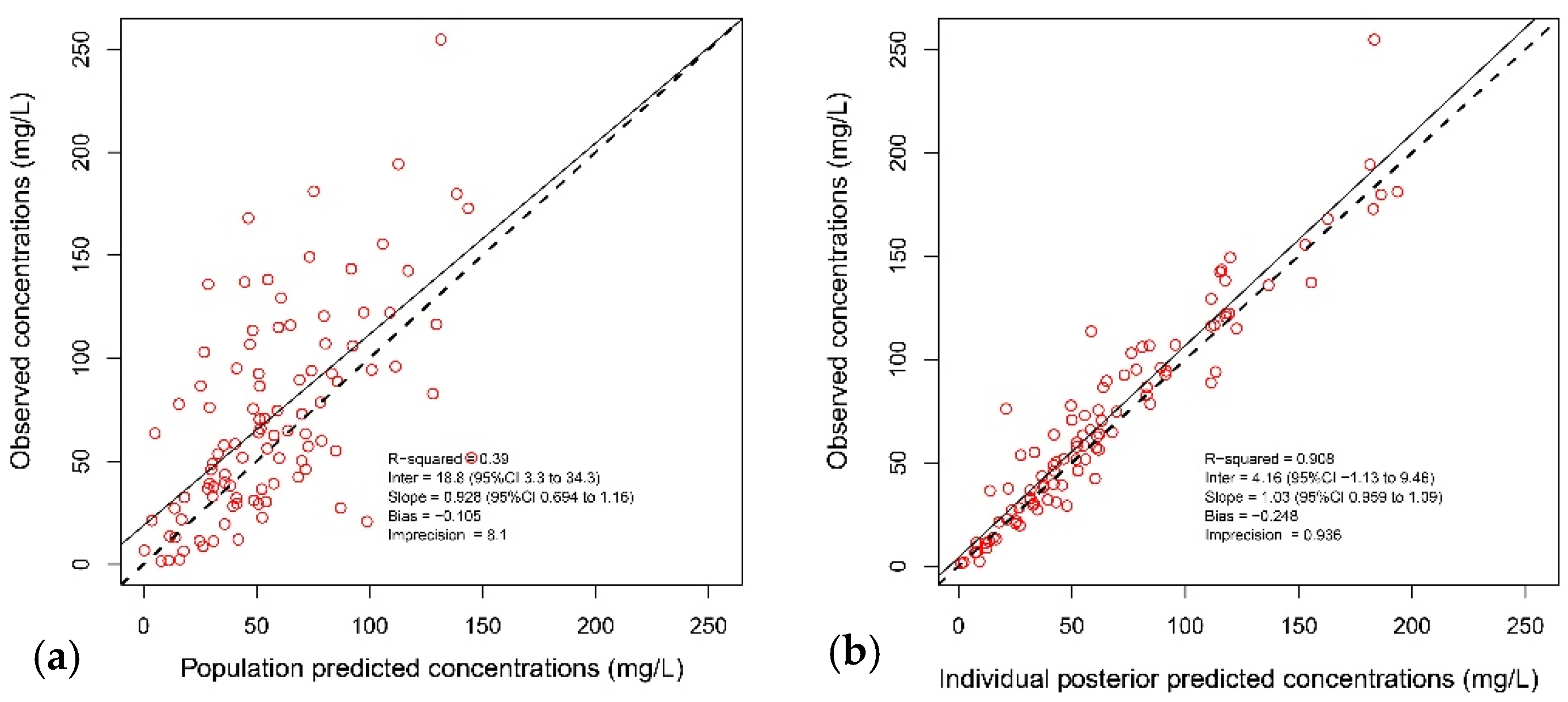
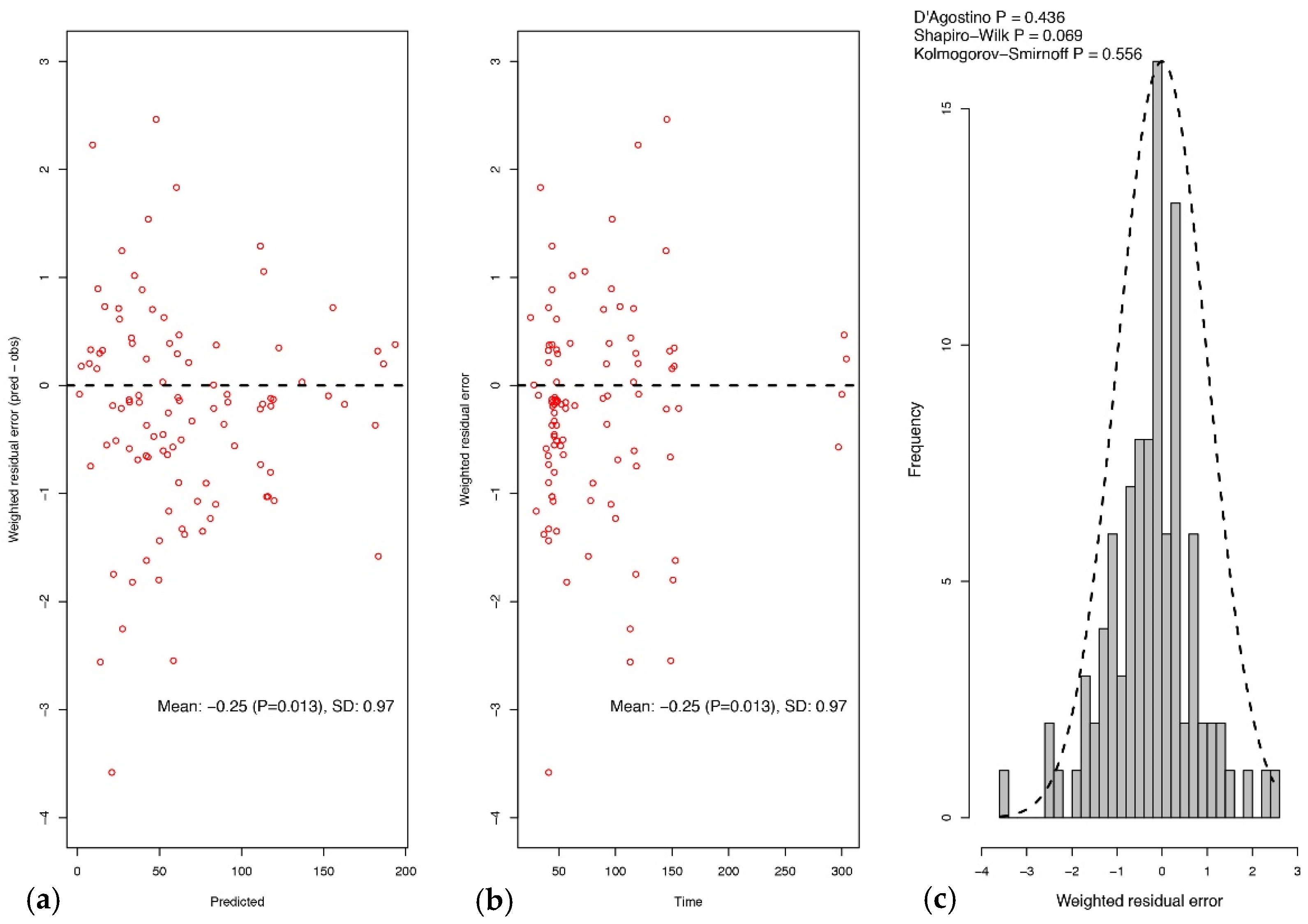
2.2. Pharmacokinetic Model
2.3. Probability of Target Attainment
2.4. Piperacillin Neurotoxicity and Nephrotoxicity
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Antibiotic Therapy
4.3. Piperacillin Serum Concentration Assay
4.4. Pharmacokinetic Analyses
4.5. Simulations and Probability of Target Attainment
4.6. Toxicodynamic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Baño, J.; Picón, E.; Gijón, P.; Hernández, J.R.; Ruíz, M.; Peña, C.; Almela, M.; Almirante, B.; Grill, F.; Colomina, J.; et al. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: Risk factors and prognosis. Clin. Infect. Dis. 2010, 50, 40–48. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Pascual, A. Clinical significance of extended-spectrum β-lactamases. Expert Rev. Anti. Infect. Ther. 2008, 6, 671–683. [Google Scholar] [CrossRef]
- Rottier, W.C.; Ammerlaan, H.S.M.; Bonten, M.J.M. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum -lactamase-producing Enterobacteriaceae and patient outcome: A meta-analysis. J. Antimicrob. Chemother. 2012, 67, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Retamar, P.; Portillo, M.M.; López-Prieto, M.D.; Rodríguez-López, F.; de Cueto, M.; García, M.V.; Gómez, M.J.; Del Arco, A.; Muñoz, A.; Sánchez-Porto, A.; et al. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: A propensity score-based analysis. Antimicrob. Agents Chemother. 2012, 56, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Gin, A.; Dilay, L.; Karlowsky, J.A.; Walkty, A.; Rubinstein, E.; Zhanel, G.G. Piperacillin-tazobactam: A beta-lactam/beta-lactamase inhibitor combination. Expert Rev. Anti. Infect. Ther. 2007, 5, 365–383. [Google Scholar] [CrossRef]
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef]
- Kim, M.K.; Xuan, D.; Quintiliani, R.; Nightingale, C.H.; Nicolau, D.P. Pharmacokinetic and pharmacodynamic profile of high dose extended interval piperacillin-tazobactam. J. Antimicrob. Chemother. 2001, 48, 259–267. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef]
- Falagas, M.E.; Tansarli, G.S.; Ikawa, K.; Vardakas, K.Z. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: A systematic review and meta-analysis. Clin. Infect. Dis. 2013, 56, 272–282. [Google Scholar]
- Tabah, A.; De Waele, J.; Lipman, J.; Zahar, J.R.; Cotta, M.O.; Barton, G.; Timsit, J.-F.; Roberts, J.A. The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef]
- Udy, A.A.; Lipman, J.; Jarrett, P.; Klein, K.; Wallis, S.C.; Patel, K.; Kirkpatrick, C.M.J.; Kruger, P.S.; Paterson, D.L.; Roberts, M.S.; et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care 2015, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Kirkpatrick, C.M.J.; Roberts, M.S.; Dalley, A.J.; Lipman, J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int. J. Antimicrob. Agents 2010, 35, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Alobaid, A.S.; Wallis, S.C.; Jarrett, P.; Starr, T.; Stuart, J.; Lassig-Smith, M.; Mejia, J.L.O.; Roberts, M.S.; Roger, C.; Udy, A.A.; et al. Population Pharmacokinetics of Piperacillin in Nonobese, Obese, and Morbidly Obese Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e01276-16. [Google Scholar] [CrossRef]
- Kuye, O.; Teal, J.; DeVries, V.G.; Morrow, C.A.; Tally, F.P. Safety profile of piperacillin/tazobactam in phase I and III clinical studies. J. Antimicrob. Chemother. 1993, 31 (Suppl. A), 113–124. [Google Scholar] [CrossRef]
- Quinton, M.-C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.-S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of β-lactam concentration–toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.; Drusano, G.L. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 2007, 44, 357–363. [Google Scholar] [CrossRef]
- Craig, W.A. State-of-the-Art Clinical Article: Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Felton, T.W.; Hope, W.W.; Lomaestro, B.M.; Butterfield, J.M.; Kwa, A.L.; Drusano, G.L.; Lodise, T.P. Population pharmacokinetics of extended-infusion piperacillin-tazobactam in hospitalized patients with nosocomial infections. Antimicrob. Agents Chemother. 2012, 56, 4087–4094. [Google Scholar] [CrossRef]
- Langgartner, J.; Lehn, N.; Glück, T.; Herzig, H.; Kees, F. Comparison of the Pharmacokinetics of Piperacillin and Sulbactam during Intermittent and Continuous Intravenous Infusion. Chemotherapy 2007, 53, 370–377. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Optimal Doripenem Dosing Simulations in Critically Ill Nosocomial Pneumonia Patients With Obesity, Augmented Renal Clearance, and Decreased Bacterial Susceptibility. Crit. Care Med. 2013, 41, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.K.; Mercer, D.; Itani, K.M.; Nicolau, D.P.; Kuti, J.L.; Mansfield, D.; Dana, A. Randomized, Open-Label, Comparative Study of Piperacillin-Tazobactam Administered by Continuous Infusion versus Intermittent Infusion for Treatment of Hospitalized Patients with Complicated Intra-Abdominal Infection. Antimicrob. Agents Chemother. 2006, 50, 3556–3561. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 2013, 56, 236–244. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Lipman, J.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Dulhunty, J.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; et al. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother. 2016, 71, 196–207. [Google Scholar] [PubMed]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.-B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.J.; MacVane, S.H.; Kuti, J.L.; Scheetz, M.H. Impact of loading doses on the time to adequate predicted beta-lactam concentrations in prolonged and continuous infusion dosing schemes. Clin. Infect. Dis. 2014, 59, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Brinkman, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; El Helali, N.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11. 2021. Available online: http://www.eucast.org (accessed on 24 March 2021).
- Spanish Agency of Medicines and Medical Devices (AEMPS). Piperacilina/Tazobactam SPC, Ficha técnica Piperacilina/Tazobactam Sandoz 4, g./.0.; 5 g polvo para solución para perfusión, E.F.G. Agencia Española Del Medicamento y Productos Sanitarios (AEMPS). Available online: https://cima.aemps.es/cima/dochtml/ft/71286/FT_71286.html (accessed on 24 March 2021).
- McWhinney, B.C.; Wallis, S.C.; Hillister, T.; Roberts, J.A.; Lipman, J.; Ungerer, J.P.J. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2010, 878, 2039–2043. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Service s Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM). Guidance for Industry, Bioanalytical Method Validation. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 24 March 2021).
- Neely, M.N.; van Guilder, M.G.; Yamada, W.M.; Schumitzky, A.; Jelliffe, R.W. Accurate Detection of Outliers and Subpopulations with Pmetrics, a Nonparametric and Parametric Pharmacometric Modeling and Simulation Package for R. Ther. Drug Monit. 2012, 34, 467–476. [Google Scholar] [CrossRef]
- Goutelle, S.; Bourguignon, L.; Maire, P.H.; Van Guilder, M.; Conte, J.E.; Jelliffe, R.W. Population Modeling and Monte Carlo Simulation Study of the Pharmacokinetics and Antituberculosis Pharmacodynamics of Rifampin in Lungs. Antimicrob. Agents Chemother. 2009, 53, 2974–2981. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of β-lactam antibiotics in critically ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-I.; Kim, S.-H.; Park, W.B.; Lee, K.-D.; Kim, H.-B.; Kim, E.-C.; Oh, M.-D.; Choe, K.-W. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: Risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 2005, 49, 760–766. [Google Scholar] [CrossRef]
- Peralta, G.; Lamelo, M.; Álvarez-García, P.; Velasco, M.; Delgado, A.; Horcajada, J.P.; Montero, M.; Roiz, M.P.; Fariñas, M.C.; Alonso, J.; et al. Impact of empirical treatment in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. bacteremia. A multicentric cohort study. BMC Infect. Dis. 2012, 12, 245. [Google Scholar] [CrossRef] [PubMed]

| Variable | No. of Cases (%) Unless Otherwise Stated |
|---|---|
| Male gender | 17 (62.96) |
| Age in years, median (range) | 76.5 (48–86) |
| Body mass index ≥ 25 | 19 (79.1) |
| CrCl in mL/min, median (range) | 50.7 (45.3–255.3) |
| Charlson score, median (range) | 2.5 (0–8) |
| Comorbidities: | |
| Diabetes mellitus | 13 (48.1) |
| Chronic pulmonary disease | 4 (14.8) |
| Cancer | 11 (40.7) |
| Liver cirrhosis | 1 (3.7) |
| Immunosuppressive therapy | 1 (3.7) |
| Source of bacteremia | |
| Urinary tract | 18 (66.67) |
| Biliary tract | 7 (25.9) |
| Other intraabdominal infection | 2 (7.4) |
| Community-acquired bacteremia | 11 (40.7) |
| Pitt score, median (range) | 2 (0–5) |
| SOFA score at diagnosis, median (range) | 3 (0–8) |
| Microorganism: | |
| Escherichia coli | 15 (55.5) |
| Klebsiella oxytoca | 6 (22.2) |
| Klebsiella pneumoniae | 3 (11.1) |
| Enterobacter aerogenes | 2 (7.4) |
| Enterobacter cloacae | 1 (3.7) |
| ESBL producer | 3 (11.1) |
| Hours from blood culture until first TZP dose, median (range) | 1.6 (0–11) |
| MIC | 25 (92.5) |
| 1 mg/L | 3 (12) |
| 2 mg/L | 13 (52) |
| 4 mg/L | 5 (20) 1 |
| 8 mg/L | 3 (12) 2 |
| 16 mg/L | 0 |
| >16 mg/L | 1 (3.7) |
| Outcome: | |
| Lack of improvement, day 2 | 5 (18.5) |
| Clinical failure, day 14 | 4 (14.8) |
| Mortality, day 30 | 1 (3.7) |
| Parameter | Mean | SD | Median |
|---|---|---|---|
| Drug Clearance, CL (L/h) CL = Intercept + slope × creatinine clearance (L/h) | |||
| Intercept (L/h) 1 | 4.556 | 5.035 | 3.503 |
| Slope | 1.353 | 1.032 | 1.39 |
| Volume of distribution, Vc (L) | 30.68 | 23.349 | 20.039 |
| Target fT > MIC = 50% | Normal (90–129 mL/min/1.73 m2) | Severely Decreased (15–29 mL/min/1.73 m2) Kidney Failure (<15 mL/min/1.73 m2) | ||||
|---|---|---|---|---|---|---|
| Dosage | Dosage | |||||
| MIC (mg/L) | 1 | 2 | 3 | 4 | 5 | 6 |
| 0.06 | 99.8 | 100 | 100 | 99.4 | 99.9 | 100 |
| 0.125 | 99.2 | 100 | 100 | 99.2 | 99.9 | 100 |
| 0.25 | 99 | 100 | 100 | 99.2 | 99.9 | 100 |
| 0.5 | 98.8 | 100 | 100 | 99.2 | 99.8 | 100 |
| 1 | 98.4 | 100 | 100 | 98.9 | 99.7 | 100 |
| 2 | 97.4 | 100 | 100 | 98.8 | 99.6 | 100 |
| 4 | 93.4 | 100 | 100 | 98.5 | 99.5 | 99.9 |
| 8 EUCAST (S) | 76.4 | 100 | 100 | 92.9 | 96.1 | 99.7 |
| 16 EUCAST (I) | 56.8 | 95.8 | 94.6 | 77.3 | 90.7 | 94.3 |
| 32 (EUCAST R) | 14.2 | 28 | 45.8 | 38.7 | 61.1 | 81.3 |
| 64 | 1.2 | 2 | 8.4 | 6.9 | 11.7 | 31.8 |
| 128 | 0 | 0 | 0.2 | 1.2 | 1.2 | 4.6 |
| 256 | 0 | 0 | 0 | 0.2 | 0.2 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino-Bohórquez, V.; Docobo-Pérez, F.; Valiente-Méndez, A.; Delgado-Valverde, M.; Cameán, M.; Hope, W.W.; Pascual, Á.; Rodríguez-Baño, J. Population Pharmacokinetics of Piperacillin in Non-Critically Ill Patients with Bacteremia Caused by Enterobacteriaceae. Antibiotics 2021, 10, 348. https://doi.org/10.3390/antibiotics10040348
Merino-Bohórquez V, Docobo-Pérez F, Valiente-Méndez A, Delgado-Valverde M, Cameán M, Hope WW, Pascual Á, Rodríguez-Baño J. Population Pharmacokinetics of Piperacillin in Non-Critically Ill Patients with Bacteremia Caused by Enterobacteriaceae. Antibiotics. 2021; 10(4):348. https://doi.org/10.3390/antibiotics10040348
Chicago/Turabian StyleMerino-Bohórquez, Vicente, Fernando Docobo-Pérez, Adoración Valiente-Méndez, Mercedes Delgado-Valverde, Manuel Cameán, William W. Hope, Álvaro Pascual, and Jesús Rodríguez-Baño. 2021. "Population Pharmacokinetics of Piperacillin in Non-Critically Ill Patients with Bacteremia Caused by Enterobacteriaceae" Antibiotics 10, no. 4: 348. https://doi.org/10.3390/antibiotics10040348
APA StyleMerino-Bohórquez, V., Docobo-Pérez, F., Valiente-Méndez, A., Delgado-Valverde, M., Cameán, M., Hope, W. W., Pascual, Á., & Rodríguez-Baño, J. (2021). Population Pharmacokinetics of Piperacillin in Non-Critically Ill Patients with Bacteremia Caused by Enterobacteriaceae. Antibiotics, 10(4), 348. https://doi.org/10.3390/antibiotics10040348






