Target Attainment and Clinical Efficacy for Vancomycin in Neonates: Systematic Review
Abstract
1. Introduction
2. Results
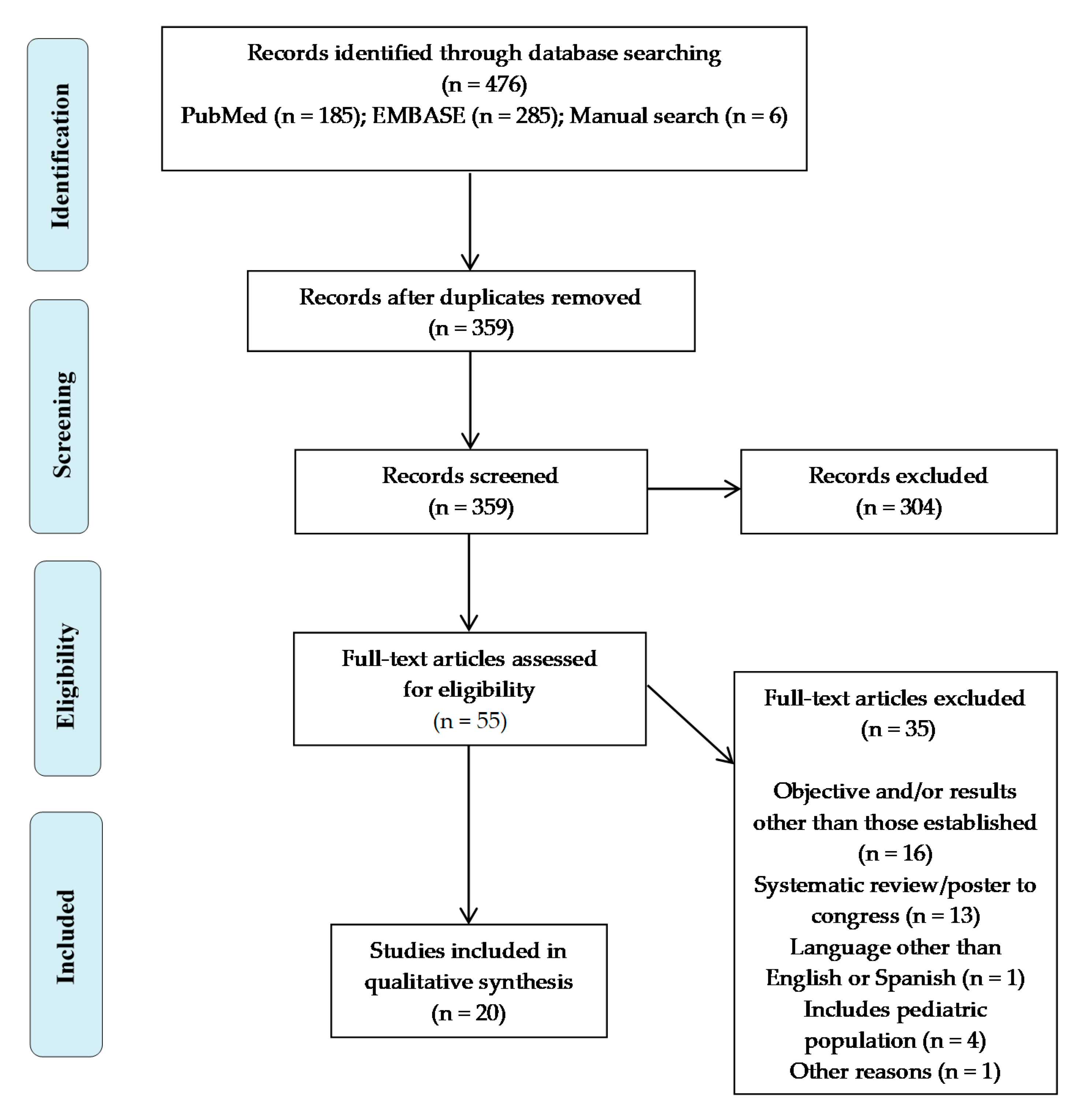
2.1. Bibliographic Search
2.2. Quality of the Included Studies
2.3. Characteristic of the Included Studies
2.4. Serum Concentrations and Dosage Form
2.5. Dosage Regimen Used
2.6. Main Findings
2.6.1. Efficacy
2.6.2. Safety
3. Discussion
Strengths and Limitations of the Study
4. Materials and Methods
4.1. Selection Criteria
- Population: neonatal and young–infant patients (from birth to three months old = 12 weeks) receiving empiric or directed vancomycin therapy.
- Intervention: monitoring of vancomycin serum concentrations.
- Comparison: with a comparator (age range, regimen, etc.) or without a comparator.
- Outcomes: clinical efficacy and/or target attainment, the latter defined as reaching target serum concentrations. Safety of vancomycin treatment through obtaining serum concentrations in those studies that are available.
- Study design: clinical trials and observational studies.
4.2. Data Sources
4.3. Study Selection
4.4. Quality Assessment
4.5. Data Extraction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, W.-H.; Brown, N.; Kelsall, A.W.; McClure, R.J. Dose regimen for vancomycin not needing serum peak levels? Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, 214–216. [Google Scholar] [CrossRef]
- Gwee, A.; Cranswick, N.; Donath, S.M.; Hunt, R.; Curtis, N. Protocol for a randomised controlled trial of continuous infusions of vancomycin to improve the attainment of target vancomycin levels in young infants: The VANC trial. BMJ Open 2018, 8, e022603. [Google Scholar] [CrossRef] [PubMed]
- Germovsek, E.; Osborne, L.; Gunaratnam, F.; A Lounis, S.; Busquets, F.B.; Standing, J.F.; Sinha, A.K. Development and external evaluation of a population pharmacokinetic model for continuous and intermittent administration of vancomycin in neonates and infants using prospectively collected data. J. Antimicrob. Chemother. 2019, 74, 1003–1011. [Google Scholar] [CrossRef]
- Murphy, J.E. Clinical Pharmacokinetics, 6th ed.; American Society of Health–System Pharmacists: Bethesda, MD, USA, 2016. [Google Scholar]
- Kearns, G.L.; Abdel–Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin–Resistant Staphylococcus aureus Infections in Adults and Children: Executive Summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; A Mueller, B.; Pai, M.P.; Wong–Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin–resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health–System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Pharm. 2020, 77, 835–864. [Google Scholar]
- Girand, H.L. Continuous Infusion Vancomycin in Pediatric Patients: A Critical Review of the Evidence. J. Pediatr. Pharmacol. Ther. 2020, 25, 198–214. [Google Scholar] [CrossRef]
- Moise–Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of Vancomycin and Other Antimicrobials in Patients with Staphylococcus aureus Lower Respiratory Tract Infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef]
- Young, T.E.; Mangum, B. Neofax, 22nd ed.; Montvale, N.J., Ed.; Thomson Reuters: New York, NY, USA, 2009; p. 86. [Google Scholar]
- Tseng, S.H.; Lim, C.P.; Chen, Q.; Tang, C.C.; Kong, S.T.; Ho, P.C. Evaluating the Relationship between Vancomycin Trough Concentration and 24–Hour Area under the Concentration–Time Curve in Neonates. Antimicrob. Agents Chemother. 2018, 62, e01647-17. [Google Scholar] [CrossRef]
- Aguilar, M.J.; Ferriols Lisart, R.; Tosca Segura, R.; Alós Almiñana, M. Diseño y validación de un esquema de dosificación de vancomicina en neonatos prematuros. An. Pediatr. 2008, 68, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ringenberg, T.; Robinson, C.; Meyers, R.; Degnan, L.; Shah, P.; Siu, A.; Sturgill, M. Achievement of Therapeutic Vancomycin Trough Serum Concentrations With Empiric Dosing in Neonatal Intensive Care Unit Patients. Pediatr. Infect. Dis. J. 2015, 34, 742–747. [Google Scholar] [CrossRef]
- Dersch–Mills, D.; Bengry, T.; Akierman, A.; Alshaikh, A.; Yusuf, K. Assessment of initial vancomycin dosing in neonates. Paeditr. Child. Health 2014, 19, 30–34. [Google Scholar] [CrossRef]
- Leroux, S.; Jacqz–Aigrain, E.; Biran, V.; Lopez, E.; Madeleneau, D.; Wallon, C. Clinical Utility and Safety of a Model–Based Patient–Tailored Dose of Vancomycin in Neonates. Antimicrob. Agents Chemother. 2016, 60, 2039–2042. [Google Scholar] [CrossRef]
- Pawlotsky, F.; Thomas, A.; Kergueris, M.F.; Debillon, T.; Roze, J.C. Constant rate infusion of vancomycin in premature neonates: A new dosage schedule. Br. J. Clin. Pharmacol. 1998, 46, 163–167. [Google Scholar] [CrossRef]
- Tauzin, M.; Cohen, R.; Durrmeyer, X.; Dassieu, G.; Barre, J.; Caeymaex, L. Continuous–Infusion Vancomycin in Neonates: Assessment of a Dosing Regimen and Therapeutic Proposal. Front Pediatr. 2019, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Lee, S.E.; Abraham, T.; Saad, N.N.; Gad, A. Evaluation of Vancomycin Target Trough Attainment With Published Dosing Regimens in the Neonatal Intensive Care Unit Population. J. Neonatal. Perinatal Med. 2019, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Radu, L.; Bengry, T.; Akierman, A.; Alshaikh, B.; Yusuf, K.; Dersch–Mills, D. Evolution of Empiric Vancomycin Dosing in a Neonatal Population. J. Perinatol. 2018, 38, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.; O’Brien, C.; Bhushan, S.; Tonna, A. Neonatal vancomycin trough level audit using British National Formulary for Children dosing. Arch. Dis. Child. Fetal Neonatal. 2015, 100, 278–279. [Google Scholar] [CrossRef]
- Reilly, A.M.; Ding, M.X.; Rower, J.E.; Kiser, T.H. The Effectiveness of a Vancomycin Dosing Guideline in the Neonatal Intensive Care Unit for Achieving Goal Therapeutic Trough Concentrations. J. Clin. Pharmacol. 2019, 59, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lopez, E.; Biran, V.; Durrmeyer, X.; Fakhoury, M.; Jacqz–Aigrain, E. Vancomycin continuous infusion in neonates: Dosing optimisation and therapeutic drug monitoring. Arch. Dis. Child. 2013, 98, 449–453. [Google Scholar] [CrossRef]
- De Hoog, M.; Schoemaker, R.C.; Mouton, J.W.; van den Anker, J.N. Vancomycin Population Pharmacokinetics in Neonates. Clin. Pharmacol. Ther. 2000, 67, 360–367. [Google Scholar] [CrossRef]
- Sinkeler, F.S.; de Haan, T.R.; Hodiamont, C.J.; Bijleveld, Y.A.; Pajkrt, D.; AMathôt, R.A. Inadequate Vancomycin Therapy in Term and Preterm Neonates: A Retrospective Analysis of Trough Serum Concentrations in Relation to Minimal Inhibitory Concentrations. BMC Pediatr. 2014, 28, 193. [Google Scholar] [CrossRef] [PubMed]
- Adigan, T.; Teng, C.B.; Koshaish, J.; Johnson, K.R.; Graner, K.K.; Banerjee, R. Optimization of Vancomycin Dosing in Very Low–Birth–Weight Preterm Neonates. Am. J. Perinatol. 2015, 32, 83–86. [Google Scholar]
- Badran, E.F.; Shamayleh, A.; Irshaid, Y.M. Pharmacokinetics of vancomycin in neonates admitted to the neonatology unit at the Jordan University Hospital. Int. J. Clin. Pharmacol. Ther. 2011, 49, 252–257. [Google Scholar] [CrossRef] [PubMed]
- McDougal, A.; Ling, E.W.; Levine, M. Vancomycin Pharmacokinetics and Dosing in Premature Neonates. Ther. Drug Monit. 1995, 17, 319–326. [Google Scholar] [CrossRef]
- Patel, A.D.; Anand, D.; Lucas, C.; Thompson, A.H. Continuous infusion of vancomycin in neonates. Arch. Dis Child. 2013, 98, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Plan, O.; Cambonie, G.; Barbotte, E.; Meyer, P.; Devine, C.; Milesi, C.; Pidoux, O.; Badr, M.; Picaud, J.C. Continuous–infusion vancomycin therapy for preterm neonates with suspected or documented Gram–positive infections: A new dosage schedule. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F418–F421. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Imamoglu, E.; Gursoy, T.; Demirel, U.; Topcuoglu, S.; Karatekin, G.; Demirel, U. Comparison of intermittent versus continuous vancomycin infusion for the treatment of late–onset sepsis in preterm infants. J. Neonatal Perinat. Med. 2015, 8, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gwee, A.; Cranswick, N.; McMullan, B.; Perkins, E.; Bolisetty, S.; Gardiner, K.; Daley, A.; Ward, M.; Chiletti, R.; Donath, S.; et al. Continuous Versus Intermittent Vancomycin Infusions in Infants: A Randomized Controlled Trial. Pediatrics 2019, 143, e20182179. [Google Scholar] [CrossRef] [PubMed]
- Tollner, U. Early diagnosis of septicemia in the newborn. Pediatr. Infect. Dis. J. 1982, 138, 331–337. [Google Scholar]
- Gwee, A.; Cranswick, N.; Metz, D.; Coghlan, B.; Daley, A.J.; Bryant, P.A. Neonatal Vancomycin Continuous Infusion Still a Confusion? Pediatric Infect. Dis. J. 2014, 33, 600–605. [Google Scholar] [CrossRef]
- Yoo, R.N.; Kim, S.H.; Lee, J. Impact of initial vancomycin trough concentration on clinical and microbiological outcomes of methicillin–resistant Staphylococcus aureus bacteremia in children. J. Korean Med. Sci. 2017, 32, 22–28. [Google Scholar] [CrossRef]
- Allegaert, K.; Flint, R.; Smits, A. Pharmacokinetic modelling and Bayesian estimation–assisted decision tools to optimize vancomycin dosage in neonates: Only one piece of the puzzle. Expert Opin. Drug Metab. Toxicol. 2019, 15, 735–749. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, M.; Mouton, J.W.; van den Anker, J.N. Vancomycin Pharmacokinetics and Administration Regimens in Neonates. Clin. Pharm. 2004, 43, 417–440. [Google Scholar] [CrossRef] [PubMed]
- Zhe, T.; Jing, C.; Weiwei, S. Analysis of Influencing Factors on Vancomycin Trough Concentration in Neonates. Lat. Am. J. Pharm. 2018, 37, 2441–2447. [Google Scholar]
- Irikura, M.; Fujiyama, A.; Saita, F.; Fukushima, S.; Kitaoka, H.; Fukuda, T.; Kawase, A.; Kondo, Y.; Ishitsuka, Y.; Kondo, G.; et al. Evaluation of the vancomycin dosage regimen based on serum creatinine used in the neonatal intensive care unit. Pediatr. Int. 2011, 53, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Bhongsatiern, J.; Stockmann, C.; Roberts, J.K.; Yu, T.; Korgenski, K.E.; Spigarelli, M.G. Evaluation of Vancomycin Use in Late–Onset Neonatal Sepsis Using the Area Under the Concentration–Time Curve to the Minimum Inhibitory Concentration ≥400 Target. Ther. Drug Monit. 2015, 37, 756–765. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta–analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS–I: A tool for assessing risk of bias in non–randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
| Study | Risk of Bias Due to | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tool | Confounding | Selection of Participants | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result | Overall Bias | |
| [12] | ROBINS–I | M | L | L | L | L | M | L | M |
| [13] | S | L | M | S | L | M | M | S | |
| [14] | S | L | L | L | L | M | M | S | |
| [15] | M | L | L | L | M | M | M | M | |
| [16] | L | L | L | L | L | M | M | M | |
| [17] | M | L | L | L | L | M | M | M | |
| [18] | M | L | L | L | L | M | M | M | |
| [19] | S | L | L | L | L | M | M | S | |
| [20] | S | L | L | L | L | M | M | S | |
| [21] | M | L | L | L | L | M | S | S | |
| [22] | M | L | L | L | M | M | M | M | |
| [23] | M | L | L | L | L | M | M | M | |
| [24] | M | L | L | L | M | L | L | M | |
| [25] | M | L | M | L | M | M | M | M | |
| [26] | M | L | L | L | L | M | M | M | |
| [27] | M | L | L | L | L | M | M | M | |
| [28] | M | L | M | L | L | M | M | M | |
| [29] | M | L | M | L | L | M | M | M | |
| [30] | M | L | M | L | M | M | M | M | |
| Study | Tool | Randomization Process | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result | Overall Bias | ||
| [31] | ROB–2 | L | SC | L | L | SC | SC | ||
| Articles | Main Objective | Design | Main Variable | Target Serum Concentrations and Method of Administration | Target Population | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics and No. Subjects | Sex | Age: GE (w) PNA (d) PMA (w) | Current Weight (kg) | Basal Cr (mcmol/L) | Isolates | |||||
| Aguilar MJ 2008 [12] | Design and validation of an empirical dosing regimen for vancomycin | Retrospective study (regimen design) and prospective validation | No. of neonates and serum concentrations that reach target levels | Intermittent inf: Cmin: 5–10 mcg/mL Cmax: 20–40 mcg/mL | Premature: 1) 53 neonates 2) 30 neonates | 1) Male: 43.4% (n = 23) 2) Male: 46.7% (n = 14) | 1) GE 30 ± 3 PNA 23 ± 13 2) GE 31 ± 2 PNA 13 ± 8 | 1) 1.3 ± 0.5 2) 1.6 ± 0.4 | No data | Empirical and targeted therapy |
| Ringenberg T 2015 [13] | To assess the percentage of neonates and young infants achieving a trough serum concentration | A multi–institutional retrospective chart review | Percentage of NICU patients achieving a trough serum concentration with initial vancomycin dosing | Intermittent inf: 10–20 mcg/mL | 141 patients (NICU patients) | Male: 46.1% Female: 53.9% | GE 28.2 ± 4.1 PNA 34.1 ± 34.6 PMA 33.1 ± 6.3 | 1.602 ± 1.015 | No data | Empirical: 58.6% Targeted: 41.4% |
| Dersch–Mills D 2014 [14] | To assess the performance of an empirical vancomycin dosing regimen in achieving target trough levels | Retrospective, observational study of vancomycin doses, levels and pharmacokinetics | Percentage of neonates with initial pre–vancomycin levels of <10 mcg/mL, 10–20 mcg/mL and >20 mcg/mL | Intermittent inf: 10–20 mcg/mL | 153 patients (NICU patients) | No data | 1) Preterm (n = 171): PNA: 12 (1–102) a) GE ≤29 (62%) b) GE 30–36 (28%) 2)Term (n = 20) PNA: 13 (1–70)*1 | No data | No data | No data |
| Leroux S 2016 [15] | To evaluate the clinical utility and safety of a model–based patient–tailored dose of vancomycin in neonates | Prospective study | Percentage of neonates with a first therapeutic drug monitoring vancomycin serum concentration achieving the target window | Continuous inf: 15–25 mcg/mL | 191 patients (NICU patients) | No data | GE: 31.1 ± 4.9 PNA: 16.7 ± 21.7 | 1.755 ± 0.873 | 48.6 ± 21.8 | Empirical and targeted therapy |
| Pawlotsky F 1998 [16] | To define a new dosage schedule in premature neonates | Prospective study (2 cohorts) | Mean vancomycin serum concentrations observed and percentage of patients attaining target concentrations at steady state in each group | Continuous inf target steady state: 12 mcg/mL Target range: 10–30 mcg/mL | 53 patients (NICU patients) | No data | 1) GE: 29.2 ± 2.9 PNA: 4.3 ± 3.1 PMA: 33.5 ± 3.7 2) GE: 30.5 ± 3.7 PNA: 3.4 ± 3.5 PMA: 33.9 ± 4.8 | 1) 1.5 ± 0.3 2) 1.8 ± 0.8 | No data | 75.5% (n = 40) Empirical 24.5% (n = 13) targeted therapy |
| Tauzin M 2019 [17] | To determine the proportion of neonates achieving an optimal therapeutic vancomycin level and which dosing regimen is the most suitable for neonates | Retrospective study | Proportion of neonates reaching the target vancomycin serum concentration | Continuous inf: 20–30 mcg/mL | 75 preterm neonates (n = 91 therapy episodes) | Male: 57.3% (n = 43) Female: 42.7% (n = 32) | GE: 27 (26–30.5) PNA: 15 (9–33) | 1.23 [0.94–1.79] | (n = 68) 52 [26.5–70] | 73.6% (n = 67) empirical 26.4% (n = 24) targeted therapy |
| Chung E 2018 [18] | To evaluate whether vancomycin dosing from published dosing algorithms correlate with the attainment of target trough concentrations | Retrospective study | Proportion of the first minimum levels within the target therapeutic range, as well as in the subtherapeutic range within therapeutic and subtherapeutic levels | Intermittent inf: 10–20 mcg/mL | 74 patients n = 97 levelsNICU patients | Male: 58.8% (n = 57) Female: 41.2% (n = 40) | Therapeutic (n = 34): GE: 27.6 ± 3.9 PNA: 22.1 ± 16.3 Subtherapeutic: GE 27.6 ± 3.1 PNA: 31.9±26.4 | Therapeutic (n = 34): 1.334±4.117 Subtherapeutic: (n = 63): 1.563 ± 0.736 | mg/dL: Therapeutic: 0.64 ± 0.25 Subtherapeutic: 0.45 ± 0.18 | 24.3% (n = 18) empirical 75.7% (n = 56) targeted therapy |
| Radu L 2018 [19] | To validate the empirical vancomycin dosage regimen in achieving target troughs | Multisite retrospective before–and–after cohort study | Proportion of neonates achieving target trough levels | Intermittent inf: 10–20 mcg/mL | 118 patients NICU Patients | No data | EG: 28.4 (26.3–34.3) PNA: 15 (8.0–37.5) PMA: 33.4 (29.1–38.5) | 1.814 (0.961) | No data | 80.51% (n = 95) empirical 19.49% (n = 23) targeted therapy |
| Petrie K 2015 [20] | To determine the initial trough level achievement of neonatal vancomycin given dosing according to the British National Formulary for Children | Retrospective study | Percentage of patients achieving a trough serum concentration with initial vancomycin dosing | Intermittent inf: 10–15 mcg/mL | 83 patients | No data | EG: 28 (23+1–41+3) PNA:12 (2–187) PMA: 30 (23–52) | 1.12 (0.56–4.7) | 42 (17–139) | No data |
| Reilly AM 2019 [21] | To evaluate the implementation of a new vancomycin dosing guideline in improving trough target attainment | Retrospective study | Percentage of neonates who achieve goal trough concentrations | Intermittent inf: 10–20 mcg/mL | Old guideline: 91 patients; New guideline: 121 patients NICU Patients | No data | Old: PNA: 28 ± 26 PMA: 32 ± 5 New: PNA: 18 ± 14 PMA: 29 ±4 | Old: 1.59 ± 0.93 New: 1.10 ± 0.58 | mg/dL: 0.56 ± 0.29 0.65 ± 0.34 | 1) 62.6% (n = 57) empirical 37.4% (n = 34) targeted therapy 2) 72.72% (n = 88) empirical 27.28% (n = 33) targeted therapy |
| Zhao W 2013 [22] | To evaluate the results of vancomycin TDM under three different dosing regimens and to optimize vancomycin therapy | Prospective study: dose optimization multicenter study (three hospitals (1,2,3)) and validation | Percentage ofneonates who achieve goal trough concentrations and concentration range | Continuous inf: 15–25 mcg/mL | a) Dose optimization: 207 samples 116 neonates b) Validation: 58 neonates | a) Male: 50.87% (n = 59) Female: 49.13% (n = 57) b) Male: 60.34% (n = 35) Female: 39.66% (n = 23) | a) PNA: 26 ± 25; 17(1,120) PMA: 33.8 ± 5.3; 32.7 (24.4, 49.4) b) PNA: 23 ± 33 11 (1–196) | a) Dose optimization: 1) 1.44 (0.46–5.68) 2) 1.64 (0.53–5.68) 3) 1.99 (0.620–4.50) b) Validation: 1.62 (0.66–3.89) | a) Dose optimization: 1) 46 (5–120) 2) 51 (8–228) 3) 48 (11–180) b) Validation: 45 (10–87) | No data |
| Matthijs de Hoog 1999 [23] | To incorporate new insights in an up–to–date dosing scheme for neonates of various gestational ages | Retrospective study with prospective validation | Number of patients presenting through and peak levels in the different established plasma ranges | Intermittent inf: Cmin: 5–10 mcg/mL Cmax: 20–40 mcg/mL | PNA < 29 days Retrospective: 108 newborns; Prospective: 22 neonates | No data | Retrospective: GE: 28.9 (24–410 PNA: 14 (3–27) PMA: 31 (26–42) Prospective: GE: 29 (25–42) PNA: 11 (7–21) PMA: 31 (27–43) | Retrospective group: 1.045 (0.51–4.41) Prospective group: 1.16 (0.73–3.42) | No data | Empirical and targeted therapy |
| Sinkeler FS 2014 [24] | To assess the percentage of therapeutic initial trough serum concentrations and to evaluate the adequacy of the therapeutic range in interrelationship with the observed MIC–values in neonates | Retrospective study | Total number (and %) of cases with trough concentrations below and above the therapeutic range (10–15 mg/L) | Intermittent inf: 10–15 mcg/mL | 112 neonates NICU patients | No data | GE: 28 (24–41) PNA: 14 (3–112) | 1.04(0.5– 4.31) | No data | Only patients with Gram–positive isolation were included |
| Madigan T 2015 [25] | To compare vancomycin serum trough concentrations and 24–h area under the serum concentration–versus–time curve (AUC24) among very low–birthweight | Retrospective analysis: before and after implementation of a new vancomycin dosing protocol | Vancomycin trough concentrations and predicted AUC24 | Intermittent inf: 10–20 mcg/mL | 57 preterm < 1.5 kg (NICU patients) Control and intervention group | Control Male: 42.9% (n = 12) Female: 57.1% (n = 16) Intervention Male: 31% (n = 9) Female: 69% (n = 20) | Control: GE: 26 (24.0–30.1) PMA: 29.1(25–32.6) Intervention: GE: 25.9 (22.9–31.6) PMA: 28.1 (24–37.3) | Control: 0.94 (0.47–1.47) Intervention: 0.91 (0.49–1.49) | Control: 0.65 (0.2–1.4) Intervention: 0.50 (0.2–1.3) | Positive culture Control: 67.9% (n = 19) Intervention: 44.8% (n = 13) |
| Badran EF 2011 [26] | To evaluatethe pharmacokinetic parameters of vancomycinfrom data collected during regular monitoring of its serum concentrations | Prospective study | Percentage of patients reaching the target levels and pharmacokinetic variables in the different cohorts | Intermittent inf: Cmin: 5–10 mcg/mL Cmax: 20–40 mcg/mL | 151 neonates (NICU patients): divided into 3 groups | Male: 57% (n = 86) Female: 43% (n = 65) | 1) Group <28 weeks GE: 26.9 ± 0.4 PNA: 14.6 ± 11 2) Group 28–34 GE: 30.3 ± 1.7 PNA: 11.6 ± 7.9 3) Group 34 term GE: 36.7 ± 1.8 PNA: 9.8 ± 5.7 | No data | No data | No data |
| McDougal A 1995 [27] | To estimate the vancomycin pharmacokinetic parameters in a neonatal population and prospectively to evaluate these modified dosage guidelines | Prospective study | Clinical characteristics, pharmacokinetic variables, percentage that reach the target levels | Intermittent inf: Cmin: 5–10 mcg/mL Cmax: 25–35 mcg/mL | 44 patients (NICU patients) | No data | PMA: Range (27–44) PNA: Range (2–63) | Range (0.720–3.79) | No data | Empirical and targeted therapy |
| Patel AD 2013 [28] | Compare a dosing regimen with intermittent vs. continuous infusion | Prospective study: 2 groups | Proportion of patients reaching the target level with the first plasma level | Continuous inf: 15–25 mcg/mL Intermittent inf: 10–20 mcg/mL | 1) 60 courses + 60 courses 2) 17 patients: 20 courses | No data | 1) Continuous: GE: 29 (24–41) PMA: 36 (26–62) 2) Intermittent: GE: 30 (26–41) PMA: 39 (29–45) | Intermittent: 2.2 (1–4) Continuous: 2.22 (0.62–6.9) | Intermittent: 35 (11–79) Continuous: 33 (15–114) | 52.9% (n = 9) empirical 47.1% (n = 8) targeted therapy |
| Plan 2008 [29] | To evaluate a simplified dosage schedule for continuous–infusion vancomycin therapy | Prospective study: 2 groups | Percentage of patients reaching target levels and bacteriological data | Intermittent inf: 10–25 mcg/mL | 145 premature neonates (<34 weeks) | 1) Male: 44% (n = 32) 2) Male: 53% (n = 38) | 1) PNA: 11 (7–18) PMA: 28 (26–29) 2) PNA: 10 (8–15) PMA: 27.5 (26–29) | 1) 0.94 (0.795–1.14) 2) 0.87 (0.707–1.17) | 1) 70 (60–86) 2) 74 (55–104) | 43.45% (n = 63) Empirical 56.55% (n = 82) targeted: 80 with CoNS |
| Demirel 2015 [30] | To evaluate microbiological outcomes, clinical response and adverse events of vancomycin when administered via continuous intravenous infusion | Retrospective study (2 cohorts, intermittent or continuous intravenous) | Clinical response and microbiological outcomes; percentage of patients reaching target plasma levels | Intermittent inf: 5–10 mcg/mL; Continuous inf: 15–20 mcg/mL | 77 preterm NICU patients (<34 weeks) | 1) Male: 68.3% (n = 28) 2) Male: 52.8% (n = 19) | 1) GE: 29.3±2.9 PMA: 9 (4–29) 2) GE: 28.6±2.9 PMA: 11 (4–56) | No data | 1) –0.1 (–0.3/–0.05) 2) –0.15 (–0.4/–0.05) * | 1) Empirical: 53.7% (n = 22) 2) Empirical: 69.4% (n = 25) |
| Gwee A 2019 [31] | To determine if CIV or intermittent infusions of vancomycin better achieves target vancomycin concentrations at the first steady–state level and to compare the frequency of drug–related adverse effects | Multicenter prospective randomized controlled trial: 2 groups | The difference in the proportion ofparticipants achieving target vancomycin levels at their first steady–state level | Continuous inf: 15–25 mcg/mL Intermittent inf: 10–20 mcg/mL | 104 patients Intermittent: 51 Continuous: 53 | Intermittent: Male: 53% (n = 27) Continuous: Male: 47.2% (n = 25) | Intermittent: GE: 34.4 ± 5.2 PNA: 23 ± 21 Continuous: GE: 34.0 ± 4.4 PNA: 23 ± 19 | Intermittent: 2.503 ± 1.137 Continuous: 2.595 ± 0.970 | No data | 77.88% (n = 81) empirical 22.12% (n = 23) targeted therapy |
| Dosage Regimen Used | Main Findings | |||||
|---|---|---|---|---|---|---|
| Articles | Variables Involved | Loading Dose | Maintenance dose | Clinics /Levels in Therapeutic Range | Infra /Supratherapeutic | Security |
| Aguilar MJ 2008 [12] | Weight and age (PNA) 1) < 1 kg + <15 d 2.1) < 1 kg + >15 d 2.2) > 1 kg + <15 d 3) > 1 kg + >15 d | N/A | 1) 10 mg/kg e/12 h 2.1) 15 mg/kg e/12 h 2.2) 15 mg/kg e/12 h 3) 13 mg/kg e/8 h | Validation (n = 30) 1) Cmin: 50% and Cmax: 55% 2) Cmin: 62% and Cmax: 75% 3) Cmin: 70% and Cmax: 80% Total: Cmin: 60%; Cmax: 73% | Validation (n = 30) 1) Cmin: 50% and Cmax: 45% 2) Cmin: 38% and Cmax: 25% 3) Cmin: 30% and Cmax: 20% | No data |
| Ringenberg T 2015 [13] | Age (PMA and PNA) PMA ≤ 29 w + PNA 0–14 d PMA ≤ 29 w + PNA >14 d PMA 30–36 w + PNA 0–14 d PMA 30–36 w + PNA >14 d PMA 37–44 w + PNA 0–7 d PMA 37–44 w + PNA >7 dPMA ≥ 45 w + PNA All | N/A | 10 mg/kg e/18 h 10 mg/kg e/12 h 10 mg/kg e/12 h 10 mg/kg e/8 h 10 mg/kg e/12 h 10 mg/kg e/8 h 10 mg/kg e/6 h | n = 171 10–20 mcg/mL: 25.1% (n = 43) | n = 171 < 10 mcg/mL: 71.9% > 20 mcg/mL: 2.9% | No nephrotoxicity 2 patients: reversible 50% increase in their creatinine. No other adverse drug reactions. |
| Dersch–Mills D 2014 [14] | Weight and age (PNA) < 1200 kg + 0–7 d 1200–2000 kg + 0–7 d 2000 kg + 0–7 d < 1200 kg + >7 d 1200–2000 kg + >7 d > 2000 kg + > 7 d | N/A | 15 mg/kg e/24 h 15 mg/kg e/18 h 15 mg/kg e/12 h 15 mg/kg e/24 h 15 mg/kg e/12 h 15 mg/kg e/8 h | 15% (n = 3) 17% (n = 1) 71% (n = 5) 15% (n = 8) 45% (n = 20) 75% (n = 15) Total: 34% (n = 52) | I: 85%; S: 0% I: 83%; S: 0% I: 29%; S: 0% I: 85%; S: 0% I: 52%; S: 3% I: 20%; S: 5% I: 65%; S: 1% | No data |
| Leroux S 2016 [15] | Birth weight (g), current weight (g), PNA (days), creatinine (mcmol/L) | Target [ ] × Vd Mean: 11.1 mg/kg | Target × CL × 24 h Mean: 28.3 mg/kg/d | n = 91 15–25 mcg/mL: 72% (n = 136) | n = 191 <10 mcg/mL: 3.1% > 30 mcg/mL: 6.3% | No nephrotoxicity |
| Pawlotsky F 1998 [16] | Age (PMA) Cohort 1: 25–30 w 31–34 w 35–38 w 39–40 w >41 w Cohort 2: 25–26 w 27–28 w 29–30 w 31–32 w 33–34 w 35–36 w 37–38 w 39–40 w 41–42 w 43–44 w >45 w | Cohort 1: N/A Cohort 2: 7 mg/kg | Cohort 1: 10 mg/kg/day 17 mg/kg/day 20 mg/kg/day 24 mg/kg/day 30 mg/kg/day Cohort 2: 10 mg/kg/day 12 mg/kg/day 15 mg/kg/day 18 mg/kg/day 20 mg/kg/day 23 mg/kg/day 26 mg/kg/day 29 mg/kg/day 31 mg/kg/day 34 mg/kg/day 40 mg/kg/day | Cohort 1: 10–30 mcg/mL: 56% (n = 13) Cohort 2: 10–30 mcg/mL: 88% (n = 26) | Cohort 1: <10 mcg/mL: 44% >30 mcg/mL: 0% Cohort 2: <10 mcg/mL: 8.6% >30 mcg/mL: 3.4% | No cases of hypotension, flushing, red man syndrome. One patient: reversible creatinine increase |
| Tauzin M 2019 [17] | N/A | 15 mg/kg | 30 mg/kg/d | n = 91 20–30 mcg/mL: 30.8% (n = 28): GA < 28 n = 17; GA ≥ 28 n = 12 PNA ≤ 14 d n = 17; >14 d n = 12 ≤1 kg n = 11; > 1 kg n = 18 | n = 91 <20 mg/L: 44% >30 mcg/mL: 25.3% | No data |
| Chung E 2018 [18] | Age (PMA and PNA) PMA ≤29 w + PNA 0–14 d PMA ≤29 w + PNA >14 d PMA 30–36 w + PNA 0–14 d PMA 30–36 w + PNA >14 d PMA 37–44 w + PNA 0–7 d PMA 37–44 w + PNA >7 d PMA ≥ 45 w + PNA All | N/A | 10 to 15 mg/kg e/18 h 10 to 15 mg/kg e/12 h 10 to 15 mg/kg e/12 h 10 to 15 mg/kg e/8 h 10 to 15 mg/kg e/12 h 10 to 15 mg/kg e/8 h 10 to 15 mg/kg e/6 h | n = 85 10–20 mcg/mL: 60.7% (n = 52) | n = 85 <10 mcg/mL: 39.3% | No data |
| Radu L 2018 [19] | Age (PMA and PNA) PMA ≤29 w + PNA 0–21 d PMA ≤29 w + PNA >21 d PMA 30–36 w + PNA 0–14 d PMA 30–36 w + PNA >14 d PMA 37–44 w + PNA 0–7 d PMA 37–44 w + PNA >7 dPMA ≥ 45 w + PNA All | N/A | 15 mg/kg e/18 h 15 mg/kg e/12 h 15 mg/kg e/12 h 15 mg/kg e/8 h 15 mg/kg e/12 h 15 mg/kg e/8 h 15 mg/kg e/6 h | 38.71% (n = 12) 50% (n = 2) 78.57% (n = 11) 68.97% (n = 20) 41.67% (n = 5) 46.43% (n = 13) N/A (n = 0) Total: 53.4% (n = 63) | I: 61.25%; S: 0% I: 50%; S: 0% I: 21.43% I:10.3%; S:20.7% I: 50%; S: 8.3% I:39.3%; S:14.3% N/A | No data |
| Petrie K 2015 [20] | Age (PMA) <29 w 29–35 w >35 w | N/A | 15 mg/kg e/24 h 15 mg/kg e/12 h 15 mg/kg e/8 h | Level 10–15 mcg/mL: 13% (n = 11) | < 10 mcg/mL: 81% > 15 mcg/mL: 6% | No data |
| Reilly AM 2019 [21] | Age (PMA and PNA) Old PMA <28 w + PNA 0–14 d PMA < 28 w + PNA >14 d PMA 28–33 w + PNA 0–14 d PMA 28–33 w + PNA >14 d PMA 34–37 w + PNA 0–7 d PMA 34–37 w + PNA >7 d PMA >37 w + PNA 0–7 d PMA >37 w + PNA >7 d New PMA <28 w + PNA 0–14 d PMA < 28 w + PNA >14 d PMA 28–33 w + PNA 0–14 d PMA 28–33 w + PNA >14 d PMA 34–37 w + PNA 0–7 d PMA 34–37 w + PNA >7 d PMA >37 w + PNA 0–7 d PMA >37 w + PNA >7 d | N/A | Old 15–20 mg/kg e/24 h 15 mg/kg e/18 h 15 mg/kg e/18 h 10–15 mg/kg e/8–12 h 10 mg/kg e/12 h 10 mg/kg e/8 h 10 mg/kg e/12 h 10 mg/kg e/12 h New 12.5 mg/kg e/12 h 12.5 mg/kg e/8 h 12.5 mg/kg e/8 h 10 mg/kg e/6 h 12.5 mg/kg e/8 h 12.5 mg/kg e/6 h 15 mg/kg e/8 h 15 mg/kg e/6 h | All: 28.6% (n = 26) 10% (n = 1) 25% (n = 2) 17% (n = 2) 47% (n = 17) 0% (n = 0) 25% (n = 2) 0% (n = 0) 20% (n = 2) All: 62% (n = 75) 64% (n = 21) 40% (n = 4) 45% (n = 10) 74% (n = 29) 0% (n = 0) 80% (n = 8) 50% (n = 1) 50% (n = 2) | I: 69.2% (n = 63); S: 2.2% (n = 2) I: 90%; S: 0% I: 75%; S: 0% I: 83%; S: 0% I: 47%; S: 6% I: 100%; S: 0% I: 75%; S: 0% I: 100; S: 0% I: 80%; S: 0% I: 9% (n = 11); S: 29% (n = 35) I: 15%; S: 21% I: 10%; S: 50% I: 9%; S: 45% I: 8%; S: 18% I: 0%; S: 100% I: 0%; S: 20% I: 0%; S: 50% I: 0%; S: 50% | Old guideline: Nephrotoxicity: 7.7% New guideline: Nephrotoxicity: 8.3%. No differences were observed between groups |
| Zhao W 2013 [22] | Age (GA and PNA) GA ≥24<27 w + ≤7 d GA ≥24<27 w + >7 d GA ≥27<30 w + ≤7 d GA ≥27<30 w + >7 d GA ≥30<32 w + ≤7 d GA ≥30<32 w + >7 d GA ≥32 w + ≤7 d GA ≥32 w + >7 d 2) n/A 3) N/A | a) 1)10 10 10 10 15 15 15 15 2)15 3) No b) Validation Loading: Target × Vd | NRF: 20 IRF: 15 NRF: 20 IRF: 15 NRF: 25 IRF: 20 NRF: 25 IRF: 20 NRF: 30 IRF: 25 NRF: 25 IRF: 20 NRF: 30 IRF: 25 NRF: 30 IRF: 25 NRF: 35 IRF: 30 NRF: 30 NRF: 30 IRF: 20 b) Calculated based on variables (individualized) | a) Dose optimization: the results broken down by hospitals are not provided: Total 15–25 mcg/mL: 41.4% (n = 48 b) Validation: 15–25 mcg/mL: 70.7% (n = 41) | a) Dose optimization: The results broken down by hospitals are not provided: <15 mcg/mL: 34% (n = 40) >25 mcg/mL: 24% (n = 28) b) Validation: < 15 mcg/mL: 15.5% (n = 9) > 25 mcg/mL: 13.8% (n = 8) | No data |
| Matthijs de Hoog 1999 [23] | N/A | N/A | Retrospective: 15 mg/kg e/12 h Prospective: 10 mg/kg e/8 h | n = 108 T: 5–15 mcg/mL: 65.7% (n = 71) P: 20–40 mcg/mL: 77.8% (n = 84) n = 22: before the 5th dose T: 5–15 mcg/mL: 77.3% (n = 17) P: 20–40 mcg/mL: 86.4% (n = 19) | n = 108 T: <5 mcg/mL: 17.6% >15 mcg/mL: 16.7% P: <20 mcg/mL: 5.6% >40 mcg/mL: 16.7% n = 22: before the 5th dose T < 5 mcg/mL: 4.5% >15 mcg/mL: 18.2% P: <20 mcg/mL: 13.6% >40 mcg/mL: 0% | No data |
| Sinkeler FS 2014 [24] | Age (GA and/or PMA and/or PNA) PMA <26 w GA 26–37 w + PNA <7 d GA >37 w + PNA <7 d PNA >7 d | N/A | 15 mg/kg e/24 h 10 mg/kg e/12 h 15 mg/kg e/12 h 20 mg/kg e/12 h | n = 112 10–15 mcg/mL: 33.04% (n = 37) | n = 112 <10 mcg/mL: 47.32% >15 mcg/mL: 19.64% | No data |
| Madigan T 2015 [25] | Control: unknown Intervention: weight and age (PNA) < 1.3 kg + <7 d <1.3 kg + ≥ 7 d ≥1.3 kg + <7 d ≥1.3 kg + ≥ 7 d | N/A N/A | Control: unknown Intervention: 15 mg/kg e/24 h 15 mg/kg e/12 h 15 mg/kg e/18 h 15 mg/kg e/8 h | Control: 10–20 mcg/mL: 4% (n = 1) Intervention: 10–20 mcg/mL: 34% (n = 10) | Control: <5 mcg/mL: 50% 5–10 mcg/mL: 46% > 20 mcg/mL: 0% Intervention: <5 mcg/mL: 24% 5–10 mcg/mL: 34% > 20 mcg/mL: 7% | Nephrotoxicity: 2 patients intervention group, 0 patients in control group. Failure hearing: Intervention group: 3/24 Control group 3/22 |
| Badran EF 2011 [26] | Age (PMA and PNA) PMA ≤29 w + PNA 0–14 d PMA ≤29 w + PNA >14 d PMA 30–36 w + PNA 0–14 d PMA 30–36 w + PNA >14 d PMA 37–44 w + PNA 0–7 d PMA 37–44 w + PNA >7 d PMA ≥ 45 w + PNA All | N/A | 10 mg/kg e/18 h 10 mg/kg e/12 h 10 mg/kg e/12 h 10 mg/kg e/8 h 10 mg/kg e/12 h 10 mg/kg e/8 h 10 mg e/6 h | Peak: 20–40 mcg/mL: 65.6% (n = 99) Trough: 5–10 mcg/mL: 51% (n = 77) | Peak: <20 mcg/mL: 29.1% > 40 mcg/mL: 5.3% Trough: <5 mcg/mL: 32.5% >10 mcg/mL: 16.6% | Nephrotoxicity and ototoxicity from vancomycin in this study are unlikely |
| McDougal A 1995 [27] | Weight and age (PMA) 1) <0.8 kg + <27 w 2) 0.8–1.2 kg + 27–30 w 3) 1.2–2 kg + 31–36 w 4) >2 kg + ≥37 w | N/A | 1)18 mg/kg e/36 h 2)16 mg/kg e/24 h .3)18 mg/kg e/18 h 4)15 mg/kg e/12 h | 1) 0% (n = 0) 2) P: 62.5% (n = 16) T: 18.8% (n = 3) 3) P: 73.3% (n = 11) T: 20% (n = 3) 4) P: 46.2% (n = 6) T: 38.5% (n = 5) Total: Peak 75%; Trough 25% | 1) I: 0%; S: 0% 2) P: I:31.2%; S: 6.3% T: I: 81.3% 3) P: I: 26.7% T: 80% 4) P: I 38.5%; S: 15.4% T: I: 46.2%; S: 15.4% | No adverse effects. No bacteriologic treatment failure. |
| Patel AD 2013 [28] | Creatinine +/– age (PMA) Inter. Inf: <0.33 mg/dl 0.34–0.44 mg/dl 0.45–0.72 mg/dl 0.73–1.13 mg/dl >1.13 mg/dl Cont. Inf: <0.45 mg/dl + PMA ≥40 w <0.45 mg/dl + PMA <40 w 0.45–0.68 mg/dl + PMA All w >0.68 mg/dl + PMA All w | Inter. inf: No loading dose Cont. inf: 15 mg/kg | Inter. inf: 20 mg/kg e/8 h 15 mg/kg e/8 h 10 mg/kg e/8 h 10 mg/kg e/12 h 15 mg/kg and adjustment Cont. inf: 60 mg/kg/day 50 mg/kg/day 40 mg/kg/day 30 mg/kg/day 20 mg/kg/day | Inter. inf: 10–20 mcg/mL: 46% Cont. inf: Includes 60 mg/kg guideline: 15–25 mcg/mL: 68% (n = 41) No 60 mg/kg regimen 15–25 mcg/mL: 82% (n = 49) | Inter. inf: <10 mcg/mL: 20% Cont. inf: Includes 60 mg/kg guideline: >25 mcg/mL: 30% <15 mcg/mL: 2% No 60 mg/kg regimen >25 mcg/mL: 5% <15 mcg/mL: 13% | No adverse effects. and no problems with intravenous access. |
| Plan 2008 [29] | Creatinine 1) ≤ 1.02 mg/dl >1.02 mg/dl 2) ≤ 1.02 mg/dl >1.02 mg/dl | N/A | 1) 25 mg/kg/day 15 mg/kg/day 2) 30 mg/kg/day 20 mg/kg/day | 1) 10–25 mcg/mL: 74% (n = 54) 2) 10–25 mcg/mL: 75% (n = 54) Negativization of CoNS* (48 h) = Bacteriological efficacy: 71.3% (n = 57/80) 1) 69% (n = 27) 2) 73% (n = 30) Negativization of blood cultures at the end of treatment: 93% (n = 76) | 1) <10 mcg/mL: 24% >25 mcg/mL: 1.4% 2) < 10 mcg/mL: 5% >25 mcg/mL: 19% Positivity of CoNS (48 h): 28.7% (n = 23/80) Bacteriological inefficacy: 1) 31% (n = 12) 2) 27% (n = 11) Positivity of blood cultures at the end of treatment: 7% (n = 6) | Nephrotoxicity was not evaluated. Creatinine levels were measured at 48 h: similar in both groups: 64 (50–85) mmol/l Vs. 63 (49–85) mmol/l |
| Demirel 2015 [30] | Age (PMA and PNA) Group 1 PMA ≤29 w + PNA 0–14 d PMA ≤29 w + PNA >14 d PMA 30–36 w + PNA 0–14 d PMA 30–36 w + PNA >14 d PMA 37–44 w + PNA 0–7 d PMA 37–44 w + PNA >7 dPMA ≥ 45 w + PNA All Group 2 PMA ≤29 w + PNA 0–14 d PMA ≤29 w + PNA >14 d PMA 30–36 w + PNA 0–14 d PMA 30–36 w + PNA >14 d PMA 37–44 w + PNA 0–7 d PMA 37–44 w + PNA >7 d PMA ≥ 45 w + PNA All | Group 1: N/A Group 2: 10 mg/kg | Group 1 10 mg/kg e/18 h 10 mg/kg e/12 h 10 mg/kg e/12 h 10 mg/kg e/8 h 10 mg/kg e/12 h 10 mg/kg e/8 h 10 mg e/6 h Group 2: Total daily dose was calculated from the dosage of intermittent administration (cumulative dose) | Clinical failure: Group 1: (–) Group 2: 5.6% (n = 2) Tollner score: Group 1: –6 (–7/–4) Group 2: –4.5 (–6/–3) Patients with positive blood cultures at the beginning and became negative at 48 h: Group 1: 57.9% (n = 11) Group 2: 63.6% (n = 7) Plasma levels: 1) 5–10 mcg/mL: 34.1% (n = 14) 2)15–20 mcg/mL: 52.8% (n = 19) | Plasma levels: Group 1: a) <5 mcg/mL: 26.8% b) > 10 mcg/mL: 39% Group 2: a) < 15 mcg/mL: 41.7% b) >20 mcg/mL: 5.6% | No adverse effects in any groups. All the infants passed the hearing–screening tests. |
| Gwee A 2019 [31] | Inter. inf: age (PMA) <29 w 29–35 w 36–44 w >44 w Cont. inf: Cr + age (PMA) <0.45 mg/dl + PMA ≥40 w <0.45 mg/dl + PMA <40 w 0.45–0.68 mg/dl + PMA All w >0.68 mg/dl + PMA All w | Inter. inf: No loading dose Cont. inf: 15 mg/kg | Inter. inf: 15 mg/kg e/24 h 15 mg/kg e/12 h 15 mg/kg e/8 h 15 mg/kg e/6 h Cont. inf: 50 mg/kg/day 40 mg/kg/day 30 mg/kg/day 20 mg/kg/day | Inter. inf: 41,18% (n = 21) Cont. inf: 85% (n = 45) | Inter. inf: I: 47,06% S: 11,76% Cont. inf: I: 5,67% S: 9,43% | There were no differences in increased creatinine levels or toxicity between groups. |
| Database | Search Strategy |
|---|---|
| PubMed | (“vancomycin”[MeSH Terms] OR “vancomycin”[All Fields]) AND (“infant, newborn”[MeSH Terms] OR (“infant”[All Fields] AND “newborn”[All Fields]) OR “newborn infant”[All Fields] OR “neonates”[All Fields]) AND (“pharmacokinetics”[Subheading] OR “pharmacokinetics”[All Fields] OR “pharmacokinetics”[MeSH Terms]) |
| EMBASE | (“vancomycin”/exp OR vancomycin) AND neonates AND (“pharmacokinetics”/exp OR pharmacokinetics) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejías-Trueba, M.; Alonso-Moreno, M.; Herrera-Hidalgo, L.; Gil-Navarro, M.V. Target Attainment and Clinical Efficacy for Vancomycin in Neonates: Systematic Review. Antibiotics 2021, 10, 347. https://doi.org/10.3390/antibiotics10040347
Mejías-Trueba M, Alonso-Moreno M, Herrera-Hidalgo L, Gil-Navarro MV. Target Attainment and Clinical Efficacy for Vancomycin in Neonates: Systematic Review. Antibiotics. 2021; 10(4):347. https://doi.org/10.3390/antibiotics10040347
Chicago/Turabian StyleMejías-Trueba, Marta, Marta Alonso-Moreno, Laura Herrera-Hidalgo, and Maria Victoria Gil-Navarro. 2021. "Target Attainment and Clinical Efficacy for Vancomycin in Neonates: Systematic Review" Antibiotics 10, no. 4: 347. https://doi.org/10.3390/antibiotics10040347
APA StyleMejías-Trueba, M., Alonso-Moreno, M., Herrera-Hidalgo, L., & Gil-Navarro, M. V. (2021). Target Attainment and Clinical Efficacy for Vancomycin in Neonates: Systematic Review. Antibiotics, 10(4), 347. https://doi.org/10.3390/antibiotics10040347






