Determination of Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Parameters of Doxycycline against Edwardsiella ictaluri in Yellow Catfish (Pelteobagrus fulvidraco)
Abstract
1. Introduction
2. Results
2.1. In Vitro Susceptibility Assay
2.2. Analytical Method Validation
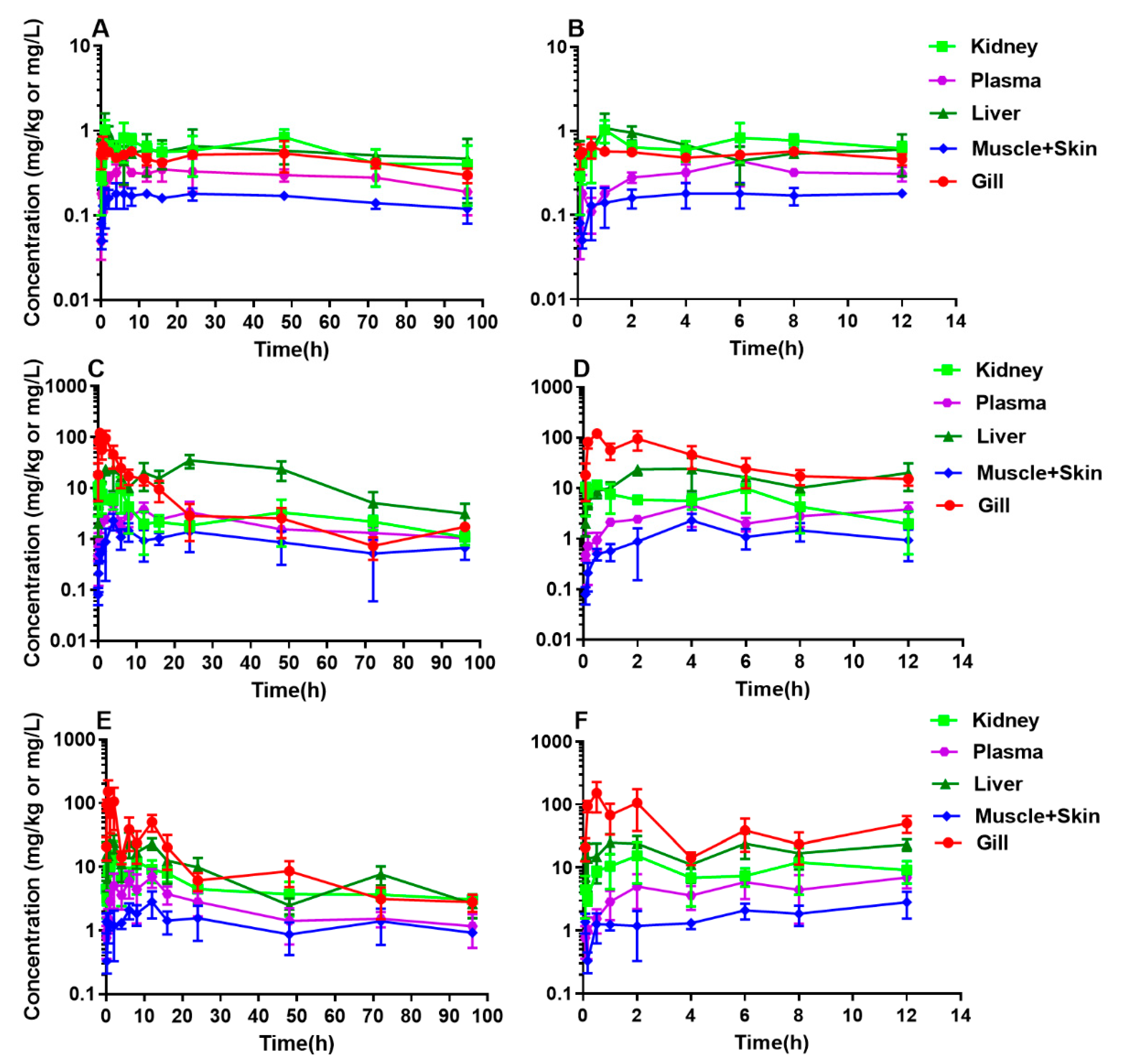
2.3. PK Profile of DC in Yellow Catfish
2.4. PK/PD Integration for DC in Plasma and Tissues
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Microorganism and Culture Medium
4.3. In Vitro Susceptibility Testing
4.4. Fish and Diet
4.5. Drug Administration and Sampling
4.6. Sample Preparation and Instrument Analysis
4.7. Calibration Curves and Recovery Rates
4.8. Pharmacokinetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CFFA (Ed.) China Fishery Statistics Yearbook 2018; China Agriculture Press: Beijing, China, 2018. [Google Scholar]
- Geng, Y.; Wang, K.; Chen, D.; Huang, J. Edwardsiella ictalurid and Edwardsiellosis. Fish. Sci. Technol. Inf. 2009, 36, 236–239. [Google Scholar]
- Liao, Y. Study on Pathomorphology and Pathogen Distribution of Edwardsiella ictalurid in Yellow Catfish. Master Thesis, Sichuan Agricultural University, Yaan, China, 2012. [Google Scholar]
- Done, H.Y.; Venkatesan, A.K.; Halden, R.U. Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J. 2015, 17, 513–524. [Google Scholar] [CrossRef]
- Cunha, B.A.; Sibley, C.M.; Ristuccia, A.M. Doxycycline. Ther. Drug Monit. 1982, 4, 115–135. [Google Scholar] [CrossRef]
- Salminen, L. Penetration of ocular compartments by tetracyclines. II. An experimental study with doxycycline. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1977, 204, 201–207. [Google Scholar] [CrossRef]
- Thompson, S.; Townsend, R. Pharmacological agents for soft tissue and bone infected with MRSA: Which agent and for how long? Injury 2011, 42 (Suppl. 5), S7–S10. [Google Scholar] [CrossRef]
- Riviere, J.E.; Papich, M.G. Tetracycline Antibiotics. In Veterinary Pharmacology and Therapeutics, 10th ed.; Riviere, J.E., Pahich, M.G., Eds.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2018. [Google Scholar]
- Arthur, J.R.; Lavilla-Pitogo, C.R.; Subasinghe, R.P. Use of chemicals in aquaculture in Asia. In Proceedings of the Meeting on the Use of Chemicals In Aquaculture in Asia, Iloilo, Philippines, 20–22 May 1996. [Google Scholar]
- Liu, R.; Lian, Z.; Hu, X.; Lü, A.; Sun, J.; Chen, C.; Liu, X.; Song, Y.; Yiksung, Y. First report of Vibrio vulnificus infection in grass carp Ctenopharyngodon idellus in China. Aquaculture 2019, 499, 283–289. [Google Scholar] [CrossRef]
- Deng, B.; Fu, L.; Zhang, X.; Zheng, J.; Peng, L.; Sun, J.; Zhu, H.; Wang, Y.; Li, W.; Wu, X.; et al. The Denitrification Characteristics of Pseudomonas stutzeri SC221-M and Its Application to Water Quality Control in Grass Carp Aquaculture. PLoS ONE 2014, 9, e114886. [Google Scholar] [CrossRef] [PubMed]
- Shireman, J.V.; Colle, D.E.; Rottman, R.W. Incidence and treatment of columnaris disease in grass carp brood stock. Prog. Fish-Cult. 1976, 38, 116–117. [Google Scholar] [CrossRef]
- Song, X.; Zhao, J.; Bo, Y.; Liu, Z.; Wu, K.; Gong, C. Aeromonas hydrophila induces intestinal inflammation in grass carp (Ctenopharyngodon idella): An experimental model. Aquaculture 2014, 434, 171–178. [Google Scholar] [CrossRef]
- Yang, F.; Li, Z.L.; Shan, Q.; Zeng, Z.L. Pharmacokinetics of doxycycline in tilapia (Oreochromis aureus × Oreochromis niloticus) after intravenous and oral administration. J. Vet. Pharmacol. Ther. 2014, 37, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Li, M.; Fu, Y.; Zhang, X.; Ai, X.; Lin, Z. Tissue residue depletion kinetics and withdrawal time estimation of doxycycline in grass carp, Ctenopharyngodon idella, following multiple oral administrations. Food Chem. Toxicol. 2019, 131, 110592. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Li, M.; Fu, Y.; Zhang, X.; Dong, J.; Liu, Y.; Zhou, S.; Ai, X.; Lin, Z. Effect of temperature on plasma and tissue kinetics of doxycycline in grass carp (Ctenopharyngodon idella) after oral administration. Aquaculture 2019, 511, 734204. [Google Scholar] [CrossRef]
- Zhang, N.; Gu, X.; Ye, X.; Wu, X.; Zhang, B.; Zhang, L.; Shen, X.; Jiang, H.; Ding, H. The PK/PD Interactions of Doxycycline against Mycoplasma gallisepticum. Front. Microbiol. 2016, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Wang, Y.; Sajid, A.; Ahmed, S.; Li, X. Integration of pharmacokinetic-pharmacodynamic for dose optimization of doxycycline against Haemophilus parasuis in pigs. J. Vet. Pharmacol. Ther. 2018, 41, 706–718. [Google Scholar] [CrossRef]
- Yang, F.; Sun, N.; Zhao, Z.S.; Wang, G.Y.; Wang, M.F. Pharmacokinetics of doxycycline after a single intravenous, oral or intramuscular dose in Muscovy ducks (Cairina moschata). Br. Poult. Sci. 2015, 56, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Riond, J.L.; Riviere, J.E. Pharmacokinetics and metabolic inertness of doxycycline in young pigs. Am. J. Vet. Res. 1990, 51, 1271–1275. [Google Scholar] [PubMed]
- Fabre, J.; Pitton, J.S.; Kunz, J.P.; Rozbroj, S.; Hungerbühler, R.M. Distribution and excretion of doxycycline in man. Chemotherapy 1966, 11, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Certara. Phoenix WinNonlin—Nonparametric Superposition. 2019. Available online: https://onlinehelp.certara.com/phoenix/8.2/topics/nonparasuper.htm (accessed on 20 March 2021).
- Antovska, P.; Ugarkovic, S.; Petruševski, G.; Stefanova, B.; Manchevska, B.; Petkovska, R.; Makreski, P. Development and experimental design of a novel controlled-release matrix tablet formulation for indapamide hemihydrate. Pharm. Dev. Technol. 2017, 22, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Matsuzaki, T.; Oka, R.; Baba, K.; Noda, T.; Yoshida, Y.; Sato, K.; Kiyota, K.; Mizutare, T.; Yoshida, R. The antiviral effects of baloxavir marboxil against influenza A virus infection in ferrets. Influenza Other Respi. Viruses 2020, 14, 710–719. [Google Scholar] [CrossRef]
- Zeng, D.; Sun, M.; Lin, Z.; Li, M.; Gehring, R.; Zeng, Z. Pharmacokinetics and pharmacodynamics of tildipirosin against pasteurella multocida in a murine lung infection model. Front. Microbiol. 2018, 9, 1038. [Google Scholar] [CrossRef] [PubMed]
- Delis, G.A.; Koutsoviti-Papadopoulou, M.; Siarkou, V.I.; Kounenis, G.; Batzias, G.C. Pharmacodynamics of amoxicillin against Mannheimia haemolytica and Pasteurella multocida and pharmacokinetic/pharmacodynamic (PK/PD) correlation in sheep. Res. Vet. Sci. 2010, 89, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, F.S.; Ali, B.H.; Landoni, M.F.; Lees, P. Pharmacokinetics and PK-PD modelling of danofloxacin in camel serum and tissue cage fluids. Vet. J. 2003, 165, 104–118. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, J.X.; Wang, J.; Ma, L.S.; Yang, F.H.; Yin, Y.; Huang, R.; Liu, S.G.; Li, L.C.; Zheng, G.M. Pharmacokinetic/pharmacodynamic relationship of enrofloxacin against Aeromonas hydrophila in crucian carp (Carassius auratus gibelio). J. Vet. Pharmacol. Ther. 2018, 41, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Buyck, J.; Plesiat, P.; Traore, H.; Vanderbist, F.; Tulkens, P.; Van Bambeke, F. Increased susceptibility of Pseudomonas aeruginosa to macrolides and ketolides in eukaryotic cell culture media and biological fluids due to decreased expression of oprm and increased outer-membrane permeability. Clin. Infect. Dis. 2012, 55, 534–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunha, B.A.; Domenico, P.; Cunha, C.B. Pharmacodynamics of doxycycline. Clin. Microbiol. Infect. 2000, 6, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Kabbara, S.; Vo, G.; Schilling, A.N.; Coyle, E.A. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.H.; Brown, G.H.; Peterson, M.L.; Rotschafer, J.C. Application of fluoroquinolone pharmacodynamics. J. Antimicrob. Chemother. 2000, 46, 669–683. [Google Scholar] [CrossRef]
- Mckellar, Q.A.; Sanchez Bruni, S.F.; Jones, D.G. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Ther. 2004, 27, 503–514. [Google Scholar] [CrossRef]
- Ahmad, I.; Huang, L.; Hao, H.; Sanders, P.; Yuan, Z. Application of PK/PD modeling in veterinary field: Dose optimization and drug resistance prediction. Biomed. Res. Int. 2016, 2016, 5465678. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, C.; Xie, C.; Jin, J.; Huang, Y. Dietary available phosphorus affected growth performance, body composition, and hepatic antioxidant property of juvenile yellow catfish Pelteobagrus fulvidraco. Sci. World J. 2012, 2012, 987570. [Google Scholar] [CrossRef]
- Xu, N.; Fu, Y.; Chen, F.; Liu, Y.; Dong, J.; Yang, Y.; Zhou, S.; Yang, Q.; Ai, X. Sulfadiazine pharmacokinetics in grass carp (Ctenopharyngodon idellus) receiving oral and intravenous administrations. J. Vet. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Fu, Y.; Cheng, B.; Liu, Y.; Yang, Q.; Dong, J.; Yang, Y.; Zhou, S.; Song, Y.; Ai, X. The pharmacokinetics of doxycycline in channel catfish (Ictalurus punctatus) following intravenous and oral administrations. Front. Vet. Sci. 2020, 7, 577234. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Dong, J.; Zhou, W.; Liu, Y.; Ai, X. Determination of doxycycline, 4-epidoxycycline, and 6-epidoxycycline in aquatic animal muscle tissue by an optimized extraction protocol and ultra-performance performance liquid chromatography with ultraviolet detection. Anal. Lett. 2019, 52, 452–464. [Google Scholar] [CrossRef]
| Tissues or Plasma | Spiked Level (µg/kg or µg/L) | Recovery (%) | Within-Day RSD (%) | Between-Day RSD (%) |
|---|---|---|---|---|
| Muscle and Skin | 50 | 77.3 | 4.4 | 5.1 |
| 500 | 83.7 | 3.8 | 4.6 | |
| 5000 | 80.2 | 4.7 | 5.8 | |
| Liver | 50 | 71.4 | 3.1 | 4.9 |
| 500 | 72.7 | 4.0 | 6.2 | |
| 5000 | 67.2 | 4.3 | 5.8 | |
| Kidney | 50 | 68.2 | 5.2 | 7.3 |
| 500 | 71.7 | 2.7 | 4.5 | |
| 5000 | 79.7 | 2.2 | 3.7 | |
| Gill | 50 | 80.3 | 3.5 | 5.5 |
| 500 | 81.7 | 2.8 | 4.1 | |
| 5000 | 72.6 | 3.7 | 5.6 | |
| Plasma | 50 | 82.7 | 4.5 | 5.7 |
| 500 | 82.5 | 3.9 | 6.3 | |
| 5000 | 77.2 | 4.7 | 6.1 |
| Parameters | Unit | Gill | Kidney | Liver | Muscle and Skin | Plasma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 10 | 20 | 40 | 10 | 20 | 40 | 10 | 20 | 40 | 10 | 20 | 40 | ||
| λz | 1/h | 0.012 | 0.043 | 0.029 | 0.015 | 0.023 | 0.005 | 0.005 | 0.042 | 0.021 | 0.008 | 0.008 | 0.005 | 0.007 | 0.009 | 0.014 |
| T1/2 λz | h | 56.55 | 16.27 | 23.53 | 45.15 | 29.60 | 143.26 | 142.52 | 16.49 | 32.6 | 90.28 | 82.31 | 147.18 | 106.38 | 80.81 | 51.34 |
| Tmax | h | 0.50 | 0.50 | 0.50 | 1.00 | 0.50 | 2.00 | 1.00 | 24.00 | 1.00 | 6.00 | 4.00 | 12.00 | 6.00 | 4.00 | 12.00 |
| Cmax | mg/kg (L) | 0.66 | 120.74 | 151.94 | 1.03 | 11.64 | 15.40 | 1.08 | 34.81 | 24.95 | 0.18 | 2.30 | 2.84 | 0.44 | 4.67 | 6.99 |
| AUC0–96 | h*mg/L | 44.69 | 708.96 | 1207.18 | 57.23 | 260.95 | 471.78 | 54.65 | 1615.75 | 790.04 | 15.22 | 86.52 | 126.48 | 27.86 | 192.10 | 223.56 |
| AUC_%extrap | % | 35.30 | 5.40 | 7.29 | 31.48 | 15.00 | 57.32 | 63.85 | 4.42 | 13.57 | 50.54 | 47.75 | 60.82 | 51.71 | 38.42 | 28.01 |
| Vz_F | L/kg | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 26.60 | 7.47 | 9.54 |
| Cl_F | L/h/kg | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.17 | 0.06 | 0.13 |
| Tissues and Plasma | Time Intervals (h) | Oral Doses (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| 10 | 20 | 40 | |||||
| AUC0–96/MIC (h) | %T ˃ MIC (%) | AUC0–96/MIC (h) | %T ˃ MIC (%) | AUC0–96/MIC (h) | %T ˃ MIC (%) | ||
| Gill | 96 | 89.37 | 43.99 | 1471.91 | 100.00 | 2414.36 | 100.00 |
| 24 | 234.19 | 35.78 | 5037.98 | 96.00 | 7744.17 | 100.00 | |
| 12 | 425.79 | 21.94 | 9768.54 | 100.00 | 14677.36 | 100.00 | |
| Kidney | 96 | 114.47 | 69.44 | 521.90 | 100.00 | 943.55 | 99.99 |
| 24 | 304.91 | 65.56 | 1433.19 | 96.00 | 2707.67 | 99.99 | |
| 12 | 555.15 | 34.85 | 2660.07 | 94.84 | 4990.22 | 63.72 | |
| Liver | 96 | 109.30 | 78.02 | 3231.50 | 99.98 | 1580.08 | 100.00 |
| 24 | 284.60 | 71.70 | 9277.55 | 96.00 | 4548.55 | 100.00 | |
| 12 | 516.17 | 34.03 | 17116.13 | 23.59 | 8467.98 | 60.95 | |
| Muscle and Skin | 96 | 30.43 | NA | 173.03 | 99.47 | 252.95 | 99.89 |
| 24 | 79.25 | NA | 479.16 | 96.00 | 666.29 | 95.56 | |
| 12 | 143.61 | NA | 878.61 | 43.24 | 1213.31 | 40.54 | |
| Plasma | 96 | 55.72 | NA | 384.21 | 99.90 | 447.12 | 99.94 |
| 24 | 146.31 | 25.95 | 1087.35 | 96.00 | 1331.33 | 99.94 | |
| 12 | 265.29 | 12.99 | 1999.88 | 35.16 | 2461.86 | 53.31 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Li, M.; Ai, X.; Lin, Z. Determination of Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Parameters of Doxycycline against Edwardsiella ictaluri in Yellow Catfish (Pelteobagrus fulvidraco). Antibiotics 2021, 10, 329. https://doi.org/10.3390/antibiotics10030329
Xu N, Li M, Ai X, Lin Z. Determination of Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Parameters of Doxycycline against Edwardsiella ictaluri in Yellow Catfish (Pelteobagrus fulvidraco). Antibiotics. 2021; 10(3):329. https://doi.org/10.3390/antibiotics10030329
Chicago/Turabian StyleXu, Ning, Miao Li, Xiaohui Ai, and Zhoumeng Lin. 2021. "Determination of Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Parameters of Doxycycline against Edwardsiella ictaluri in Yellow Catfish (Pelteobagrus fulvidraco)" Antibiotics 10, no. 3: 329. https://doi.org/10.3390/antibiotics10030329
APA StyleXu, N., Li, M., Ai, X., & Lin, Z. (2021). Determination of Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Parameters of Doxycycline against Edwardsiella ictaluri in Yellow Catfish (Pelteobagrus fulvidraco). Antibiotics, 10(3), 329. https://doi.org/10.3390/antibiotics10030329







