Abstract
In Tehuacán-Cuicatlán valley (Mexico), studies have been carried out on the essential oils of medicinal plants with antimicrobial activity and it was found that they present compounds in common such as: α-pinene, β-pinene, carvacrol, eugenol, limonene, myrcene, ocimene, cineole, methyl salicylate, farnesene, and thymol. The goal of this study was to assess the antimicrobial activity of essential oils’ compounds. The qualitative evaluation was carried out by the Kirby Baüer agar diffusion technique in Gram-positive bacteria (11 strains), Gram-negative bacteria (18 strains), and yeasts (8 strains). For the determination of the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), the agar dilution method was used. All the evaluated compounds presented antimicrobial activity. The compounds eugenol and carvacrol showed the largest inhibition zones. Regarding yeasts, the compounds ocimene, cineole, and farnesene did not show any activity. The compounds eugenol, carvacrol, and thymol presented the lowest MIC; bactericidal effect was observed at MIC level for S. aureus 75MR, E. coli 128 MR, and C albicans CUSI, for different compounds, eugenol, carvacrol, and thymol. Finally, this study shows that the essential oils of plants used by the population of Tehuacán-Cuicatlán valley share compounds and some of them have antibacterial and fungicidal activity.
1. Introduction
One of the major health concerns today in the world is the emergence of new microbial strains resistant to the chemical substances used for their control [1]. During the last years, new strains resistant to commonly used antibiotics have appeared; for example, Candida auris is today an emergent and extremely dangerous strain difficult to control [2,3], as well as some other strains of Enterobacter aerogenes, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Streptococcus pneumoniae, and Vibrio cholerae, among others, causing serious health problems that are difficult to control and causing global concerns on the subject.
On the other hand, the essential oils of plants have been developed as a commercial and pharmacological alternative for the treatment of various diseases, and their importance is based on the selective effects towards resistant strains that are recently reported. For example, it has been observed that clove oil, for which main component is eugenol, has antibacterial activity affecting respiratory metabolism, structural changes of DNA, and cell membrane permeability [4,5].
Therefore, it is important to carry out studies that validate the use of essential oils and to know the components that confer antimicrobial activity.
The International Union for the Conservation of Nature has decreed that Tehuacán-Cuicatlán valley [comprises the region from Puebla to Oaxaca State, México] is a center of mega diversity and endemism [6]. The valley is an area with high tradition in the use of medicinal plants [7]. Various studies have been carried out on the essential oils of some species such as: Lantana achyranthifolia Desf. (Verbenaceae) [8,9], Lippia graveolens H.B.K. (Verbenaceae) [9,10], Cordia curassavica (Boraginaceae) [11,12], and Gymnolaena oaxacana (Greenman) Rydb. (Asteraceae) [13], among others, and it was found that they contain many compounds in common among them: α-pinene, β-pinene, carvacrol, eugenol, limonene, myrcene, ocimene, cineole, methyl salicylate, farnesene, and thymol. Some studies have been carried out on these compounds, among which we can mention thymol [14,15], carvacrol [16,17], α-pinene [18], and eugenol [19,20,21] and it was observed that they present antimicrobial activity in both Gram-positive and Gram-negative strains affecting respiratory metabolism, structural changes of DNA, and cell membrane permeability.
In this way, this study aims to evaluate the antimicrobial activity of several compounds in the essential oils of plants used in the Mexican Traditional Medicine of the Valley of Tehuacán-Cuicatlán, Puebla, that include α-pinene, β-pinene, carvacrol, eugenol, limonene, myrcene, ocimene, cineole, methyl salicylate, farnesene, and thymol.
2. Results
2.1. Qualitative Evaluation of Antibacterial Activity
The results obtained in the evaluations of the antibacterial activity are shown in Table 1, Table 2 and Table 3.

Table 1.
Antimicrobial activity of some compounds in Gram-positive strains.

Table 2.
Antimicrobial activity of some compounds in Gram-negative strains.

Table 3.
Antimicrobial activity of some compounds in yeast strains.
As it can be seen, bacterial strains (Gram-positive and Gram-negative bacteria) and yeasts were sensitive to the tested compounds, and the ones that showed the highest inhibition zones for Gram-positive and Gram-negative bacteria were carvacrol and eugenol; α-pinene, β-pinene, and myrcene were the compounds that presented the lowest inhibition zones (Table 1 and Table 2). Regarding yeasts, the ocimene, cineole, and farnesene compounds did not show activity on any yeast strain (Table 3).
Considering the size of the inhibition zone with respect to the bacterial group (Gram-positive and Gram-negative) and yeast, an analysis of variance (ANOVA) was carried out. It can be observed that there are no significant differences (p < 0.05), i.e., the compounds inhibited the growth of both bacterial and yeast types.
2.2. Quantitative Evaluation
Once the antimicrobial activity of the different compounds had been verified, the minimum inhibitory concentrations (MIC) were determined. The results obtained are presented in Table 4, Table 5 and Table 6.

Table 4.
Minimal inhibitory concentrations of some compounds in Gram-positive strains.

Table 5.
Minimal inhibitory concentrations of some compounds in Gram-negative strains.

Table 6.
Minimal inhibitory concentrations of some compounds in yeast strains.
The compounds carvacrol and eugenol presented the smallest minimum inhibitory concentrations for the bacterial strains (Table 4 and Table 5), i.e., low concentrations were required to drastically inhibit growth (0.03 to 0.75 mg /mL). The α-pinene and β-pinene compounds presented MICs above 4 mg /mL, these being the highest values for both bacterial types. The compounds carvacrol, eugenol, and thymol presented the lowest MICs in yeast (Table 6), and low concentrations of 0.062 to 0.25 mg/mL were required to drastically inhibit the growth of challenged populations. The other compounds that showed activity presented concentrations greater than or equal to 2 mg/mL.
The eugenol (0.125–0.250 mg/mL), carvacrol (0.03 mg/mL), and thymol (0.03–0.250 mg/mL) compounds were those that presented the lowest concentrations in yeasts (Table 6).
2.3. Time-Killing Curves
Once the MIC, MBC and MFC parameters were established, the effects of the compounds with the highest antimicrobial activity on a Gram-positive bacterium (S. aureus 75 MR), a Gram-negative bacterium (E. coli 128 MR) and a yeast (C albicans CUSI), for the compounds eugenol (Figure 1a–c), carvacrol (Figure 2a–c), and thymol (Figure 3a–c) were evaluated (the strains were chosen for being multi-resistant and the compounds for presenting the lowest MIC values).
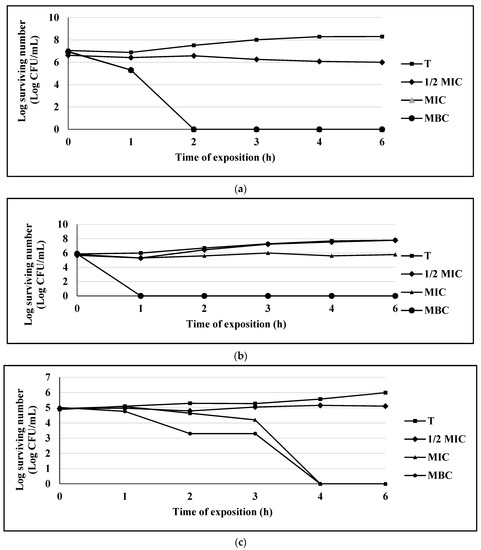
Figure 1.
(a) Survival curve of E. coli exposed to eugenol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.125 mg/mL (½ MIC), 0.25 mg/mL (MIC), and 0.5 mg/mL (MBC); the control group (T) only contains the bacterial culture. (b) Survival curve of S. aureus 75MR exposed to eugenol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.25 mg/mL (½ MIC), 0.5 mg/mL (MIC), and 1.0 mg/mL (MBC); the control group (T) only contains the bacterial culture. (c) Survival curve of C. albicans CUSI exposed to eugenol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.125 mg/mL (½ MIC), 0.25 mg/mL (MIC), and 0.5 mg/mL (MBC); the control group (T) only contains the bacterial culture.
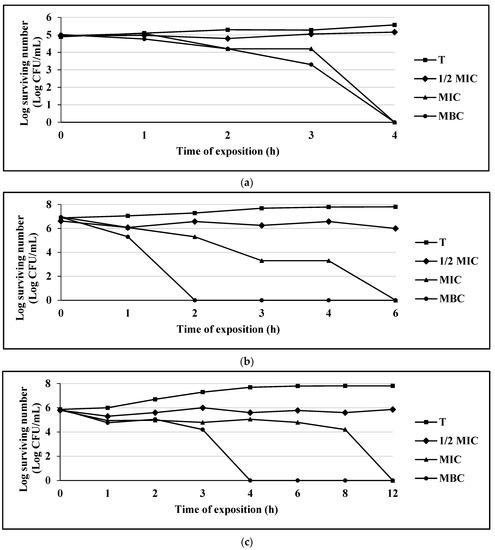
Figure 2.
(a) Survival curve of E. coli exposed to carvacrol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.062 mg/mL (½ MIC), 0.125 mg/mL (MIC), and 0.25 mg/mL (MBC); the control group (T) only contains the bacterial culture. (b) Survival curve of S. aureus 75MR exposed to carvacrol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.062 mg/mL (½ MIC), 0.125 mg/mL (MIC), and 0.25 mg/mL (MBC); the control group (T) only contains the bacterial culture. (c) Survival curve of C. albicans CUSI exposed to carvacrol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.01 mg/mL (½ MIC), 0.03 mg/mL (MIC), and 0.062 mg/mL (MBC); the control group (T) only contains the bacterial culture.
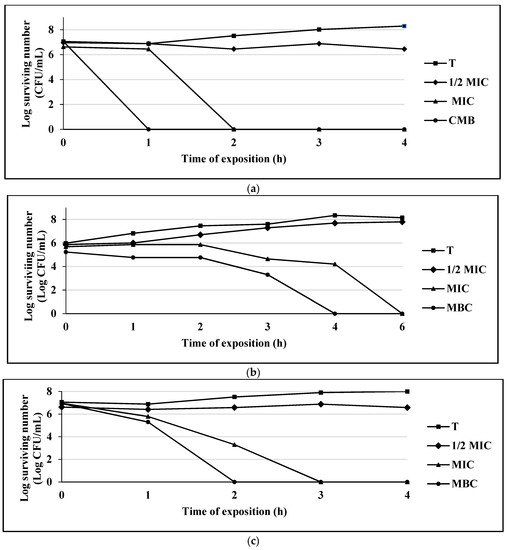
Figure 3.
(a) Survival curve of E. coli exposed to thymol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.125 mg/mL (½ MIC), 0.25 mg/mL (MIC), and 0.50 mg/mL (MBC); the control group (T) only contains the bacterial culture. (b) Survival curve of S. aureus 75MR exposed to thymol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.25 mg/mL (½ MIC), 0.50 mg/mL (MIC), and 1.0 mg/mL (MBC); the control group (T) only contains the bacterial culture. (c) Survival curve of C. albicans CUSI exposed to thymol. Data are represented as the mean ± S.E. (n = 3). The concentrations used were: 0.01 mg/mL (½ MIC), 0.03 mg/mL (MIC), and 0.062 mg/mL (MBC); the control group (T) only contains the bacterial culture.
For the eugenol compound in the bacterial population of S. aureus 75MR, a bacteriostatic effect was observed at MIC level and a bactericidal effect in MBC at the first hour of exposure. For the populations of E. coli 128 MR and C. albicans, a bactericidal or fungicidal effect was observed on MIC level at 2 h and 4 h, respectively (Figure 1a–c).
Carvacrol completely inhibited bacterial growth at 4 h for the strain of E. coli 128 MR; for S. aureus 75MR, the growth inhibition occurred at 2 h, and for the yeast strain of C. albicans CUSI, at 4 h (Figure 2a–c).
The compound thymol completely inhibited bacterial growth at the first hour of exposure for E. coli 128 MR, while for S. aureus 75MR, it was at 4 h, and for the C. albicans strain CUSI, the inhibition occurred at 2 h (Figure 3a–c).
As shown in the figures, carvacrol and thymol compounds presented a bactericidal or fungicidal behavior on the bacterial and yeast growth curves at MIC level.
It can be of interest that these compounds are good antimicrobial agents, since at different concentrations, they interrupted the growth of a bacterial population of E. coli 128 MR (Gram-negative), S. aureus 75MR (Gram-positive), and C. albicans CUSI (yeast strain).
Regarding the sensitivity of yeast to the essential oil compound exposure, it was observed that they were more sensitive to carvacrol, presenting the lowest MIC values (0.03–0.06 mg/mL), followed by carvacrol (0.03–0.25 mg/mL), and finally eugenol (0.125–0.25 mg/mL).
3. Discussion
The results show that the use of plants and their essential oils used in traditional medicine in the Tehuacan-Cuicatlan valley Puebla can be validated, since several compounds have antimicrobial activity against Gram-positive, Gram-negative bacteria, and yeasts. Several essential oils compounds are lipophilic and can interact with bacterial cytoplasmic membranes, increasing membrane permeability [4,22,23,24,25].
The compounds eugenol, carvacrol, and thymol showed the highest antimicrobial activity against Gran positive bacteria, Gran negative bacteria, and yeast. Statistical analysis revealed no significant differences between the compounds and the different groups of microorganisms (bacteria and yeast), indicating that the essential oil compounds can act in different ways. Some authors mention that the sensitivity of Gram-negative bacteria to the different essential oil compounds is due to their outer membrane of lipopolysaccharides, which restricts the diffusion to hydrophobic compounds [15,26,27,28].
The structural differences between the compounds are also the cause of the different activities observed. Myrcene (7-methyl-3 -methylene-1,6-octadiene) and ocimene (3,7-dimethyl-1,3,6-octatriene) differ in the position of a double bond (C-3), causing one to present activity on yeast (myrcene) and the other not (ocimeno); carvacrol and thymol differ in the position of a hydroxyl group (in thymol is in ortho position, in carvacrol it is in meta position), causing the deterioration of enzyme systems by binding to the active sites of the enzymes responsible for producing energy and synthesis of structural components, and the destruction or inactivity of genetic material [17,29,30]. Thymol and carvacrol are lipophilic and can enter between the fatty acid chains that make up the lipid bilayers of the membrane, altering the fluidity and permeability of cell membranes; in the case of yeast, compounds such as carvacrol can affect the regulation and the functioning of membrane enzymes that catalyze the synthesis of a series of important polysaccharide components of the cell wall, such as β-glucans, chitin, and mannan, which are involved in cell growth and morphogenesis [14,15].
Gram-positive strains were more sensitive to carvacrol, presenting the lowest MIC values (0.03–0.125 mg/mL), followed by eugenol (0.25–0.75 mg/mL), and lastly thymol (0.25–1.0 mg/mL). Gram-negative bacterial strains were also more sensitive to carvacrol, presenting the lowest MIC values (0.06–0.25 mg/mL), followed by eugenol (0.06–0.5 mg/mL), and lastly thymol (0.25–0.5 mg/mL).
In regards to time-killing curves, it was observed that the effect of eugenol, at MIC level on the populations of E. coli 128 MR and C. albicans CUSI, showed a bactericidal and fungicidal effect at 2 and 4 h, respectively. Eugenol showed a bactericidal effect against S. aureus 75MR after 1 h of contact. Carvacrol and thymol showed a bactericidal and fungicidal effect at MIC level. This behavior is characteristic of a multiple mechanism of action [31].
Although the antimicrobial properties of essential oils and their components have been reviewed in the past, the mechanism of action has not been studied in detail [32]. Components of essential oils such as thymol and carvacrol have previously been reported to have properties that cause membrane disruption in E. coli and S. typhimurium [12,33]. On the other hand, it was shown that eugenol can disintegrate in the membrane and increase its permeability, and thus causes the death of the organism [4,34].
One of the main contributions of this work was to verify the antimicrobial activity of some essential oils compounds present in plants used in traditional medicine, validating their use in the treatment of diseases of possible infectious origin.
The determination of the specific antimicrobial action of essential oil compounds is important to define possible synergistic activities against resistant microorganisms. In addition, the determination of the specific antimicrobial activity of essential oil compounds is important to define possible synergistic activities against resistant strains, as demonstrated with the thyme essential oil compounds [35,36,37].
4. Materials and Methods
4.1. Essential Oils Compound
The compounds: α-pinene 98% (# cat. 147524), β-pinene 99% (# cat. 112089), carvacrol 98% (# cat. 282197), eugenol 99% (# cat. E51791), limonene 97% (# cat. 183164), myrcene 90% (# cat. M100005), ocimene 90% (cat. # W353901-1006), cineole 99% (cat. # C80601), methyl salicylate 98% (cat. # W274518), farnesene 90% (cat. # W383902), and thymol 98.5% (cat. # T0501) were obtained from Sigma-Aldrich (Toluca, Mexico).
4.2. Microbial Strains
The following strains of bacteria were used: Enterobacter agglomerans ATCC 27155, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, E. coli ATCC 53218, E. coli 10, 28, 128, and 1249 MR multi-resistant strains isolated from clinical cases (donated by the clinical analysis laboratory of the CUSI of the FES Iztacala, UNAM). Pseudomonas aeruginosa ATCC 27853, Salmonella typhi ATCC 19430, Staphylococcus aureus ATCC 12398, S. aureus ATCC 29213 (donated by the Microbiology Laboratory of FES-Cuautitlán), S. aureus 48, 75, and 83 MR multiresistant strains isolated from clinical cases (donated by the clinical analysis laboratory of the CUSI of the FES Iztacala, UNAM). Bacillus subtillis, Enterobacter aerogenes, S. epidermidis, Yersinia enterocolitica (donated by the Clinical Analysis Laboratory of FES Iztacala), Y. enterocolitica (isolated from a clinical case and donated by Hospital Angeles Metropolitano), Proteus mirabilis, Serratia marcences, Streptococcus pneumoniae, Vibrio cholerae (isolated from a clinical case), V. cholerae INDRE 206 (isolated from polluted water), and V. cholerae (clinical strain pertaining to 01 group, Inaba serotype, El Tor biotype, and enterotoxin producer). These strains were maintained at 4 °C in Mueller Hinton agar (Bioxon), submitted to sensitivity tests (multidiscs Bigaux) and were subcultured twice prior the bioassays. The yeasts tested were: Candida albicans ATCC 10231, C. albicans ATCC 14065, C. albicans isolated from a clinical case (donated by the Clinical Analysis Laboratory of FES Iztacala), C. albicans, C. glabrata, and C. tropicalis (isolated from a clinical case and donated by Hospital Angeles Metropolitano). The stock culture was maintained on potato dextrose agar (PDA) and subcultured twice prior the bioassays.
4.3. Antibacterial Activity
The antibacterial activity was measured by the disc diffusion method [38,39]. The microorganisms were grown overnight at 37 °C in 10 mL of Mueller Hinton Broth (Bioxon). The cultures were adjusted with sterile saline solution to obtain turbidity comparable to that of McFarland no. 0.5 standard (108 CFU/mL). Petri dishes containing Mueller Hinton agar (Bioxon) were inoculated with these microbial suspensions. Discs of filter paper (Whatman no. 5) of 5-mm diameter were impregnated with 4 µL of each compound (α-pinene 3.4 mg, β-pinene 3.4 mg, carvacrol 3.9 mg, eugenol 4.3 mg, limonene 3.4 mg, myrcene 3.2 mg, ocimene 3.3 mg, cineole 3.7 mg, methyl salicylate 4.7 mg, farnesene 3.4 mg) and placed on the agar surface (for thymol, a solution was prepared at a concentration of 0.980 g/mL, discs were impregnated with 4 µL = 3.9 mg). Discs impregnated with chloramphenicol (25 µg) were used as positive controls. The plates were incubated overnight at 37 °C and the resulting inhibition zones were measured with a vernier. Each experiment was performed in triplicate. The estimation of the minimal inhibitory concentration (MIC) was carried out by the broth dilution method [40,41]. Ten dilutions of each compound (0.03–4.0 mg/mL) in broth were evaluated. The tubes were inoculated with 106 CFU/mL microorganism suspensions. MIC values were taken as the lowest essential oil concentration that prevents visible bacterial growth after 24 h of incubation at 37 °C and MBC as the lowest concentration that completely inhibited bacterial growth. Chloramphenicol was used as reference standard and compound-free plates as controls. Each experiment was repeated at least three times. The time-killing curve assay was performed using appropriate concentrations of the compounds (corresponding to ½MIC, MIC, and MBC) in 10 mL of broth. One control tube was prepared without compound. Each tube was inoculated with 0.1 mL of a microbial suspension (106 CFU/mL) and incubated at 37 °C for 24 h. Of each tube, 50 µL aliquots were taken in ranges of 2 h. Aliquots were plated on Muller–Hinton agar and incubated for 24 h at 37 °C. Finally, the number of CFU was determined. Death kinetics was expressed in log10 reduction time kill plots [42].
4.4. Antifungal Activity
The evaluation of antifungal activity in yeast for each compound was evaluated following the same methodology as for the antibacterial test, but with potato dextrose agar and nystatin as a positive control [40].
4.5. Statistics
All experiments were performed in triplicate. The mean and standard deviation of the three experiments were determined. Statistical analysis of the differences between mean values obtained for experimental groups was done by an analysis of variance (ANOVA multifactorial model), where p-values of 0.05 or less were considered statistically significant [43].
5. Conclusions
There are no significant differences in the antimicrobial activity of the compounds on the different bacterial groups and yeasts. The compounds carvacrol, eugenol, and thymol were those that showed the highest antimicrobial activity. Carvacrol was the component with the highest antibacterial activity, followed by eugenol and thymol. Carvacrol was the component with the highest antifungal activity, followed by thymol and lastly eugenol. The compounds ocimene, cineole, and farnesene did not show activity on any yeast strain. A bactericidal effect was observed at concentrations equal to or greater than MIC for eugenol, thymol, and carvacrol. Finally, this study shows that the essential oils of plants used by the population of Tehuacán-Cuicatlán valley share compounds and some of them have antibacterial and fungicidal activity.
Author Contributions
Investigation, S.C.-D., R.S.-P., M.Á.-R., S.M.-M., J.O.-M., J.G.A.-A., A.M.G.-B., C.L.C.-A., I.P.-C. and T.H.-D.; Supervision, T.H.-D.; Writing—review & editing, T.H.-D. All authors participated in the experimental development, writing, and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FESI-DIP-PAPCA-2013-1, UNAM.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Eckbo, E.J.; Wong, T.; Bharat, A.; Cameron-Lane, M.; Hoang, L.; Dawar, M.; Charles, M. First reported outbreak of the emerging pathogen Candida auris in Canada. Am. J. Infect. Control. 2021. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lozano, H.; Treviño-Rangel, R.D.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Coutinho, H.D.M.; et al. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Dávila, P.; Arizmendi, M.D.C.; Valiente-Banuet, A.; Villaseñor, J.L.; Casas, A.; Lira, R. Biological diversity in the Tehuacán-Cuicatlán Valley, Mexico. Biodivers. Conserv. 2002, 11, 421–442. [Google Scholar] [CrossRef]
- Hernandez, T.; Canales, M.; Avila, J.; Durán, A.; Caballero, J.; De Vivar, A.; Lira, R. Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitlán de las Salinas, Puebla (México). J. Ethnopharmacol. 2003, 88, 181–188. [Google Scholar] [CrossRef]
- Hernandez, T.; Canales, M.; Avila, J.; Garcia, A.; Martinez, A.; Caballero, J.; De Vivar, A.R.; Lira, R. Composition and antibacterial activity of essential oil of Lantana achyranthifolia Desf. (Verbenaceae). J. Ethnopharmacol. 2005, 96, 551–554. [Google Scholar] [CrossRef]
- Hernández, T.; Canales, M.; Avila, J.G.; García, A.M.; Meraz, S.; Duran, A. Antifungal activity of the essential oils of two Verbenaceae: Lantana achyranthifolia Desf. and Lippia graveolens H.B.K. of Zapotitlán de las Salinas, Puebla (México). BLACPMA 2008, 7, 203–207. [Google Scholar]
- Hernández, T.; Canales, M.; Avila, J.G.; García, A.M.; Caballero, J.; Romo de Vivar, A.; Lira, R. Composition and antibacterial activity of essential oil of Lippia graveolens HBK. (Verbenaceae). BLACPMA 2009, 8, 295–300. [Google Scholar]
- Hernandez, T.; Canales, M.; Teran, B.; Avila, O.; Durán, Á.; Garcia, A.M.; Hernandez, H.; Angeles-Lopez, O.; Fernandez-Araiza, M.; Avila, G. Antimicrobial activity of the essential oil and extracts of Cordia curassavica (Boraginaceae). J. Ethnopharmacol. 2007, 111, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Martim, J.K.; Maranho, L.T.; Costa-Casagrande, T.A. Review: Role of the chemical compounds present in the essential oil and in the extract of Cordia verbenacea DC as an anti-inflammatory, antimicrobial and healing product. J. Ethnopharmacol. 2021, 265, 113300. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, S.; Serrano, R.; Avila, M.; Meraz, S.; Orozco, J.; Peña, C.J.; García-Bores, A.M.; Avila, J.G.; Peñalosa, I.; Hernandez, T. Chemical Composition and Antimicrobial Activity of Gymnolaena oaxacana (Greenm.) Rydb. (Asteraceae) Essential Oil. J. Plant Sci. 2015, 3, 241–247. [Google Scholar] [CrossRef]
- Braga, P.C.; Dal Sasso, M.; Culici, M.; Alfieri, M. Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of Candida albicans. Fitoterapia 2007, 78, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Silva, E.R.; De Carvalho, F.O.; Teixeira, L.G.B.; Santos, N.G.L.; Felipe, F.A.; Santana, H.S.R.; Shanmugam, S.; Júnior, L.J.Q.; Araujo, A.A.d.S.; Nunes, P.S. Pharmacological Effects of Carvacrol in In vitro Studies: A Review. Curr. Pharm. Des. 2018, 24, 3454–3465. [Google Scholar] [CrossRef]
- Sieniawskaa, E.; Losb, R.; Baja, T.; Malmb, A.; Glowniak, K. Antimicrobial efficacy of Mutellina purpurea essential oil and α-pinene against Staphylococcus epidermidis grown in planktonic and biofilmcultures. Ind. Crop. Prod. 2013, 51, 152–157. [Google Scholar] [CrossRef]
- Pandima, D.K.; Arif, N.S.; Sakthivel, R.; Karutha, P.S. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Constantino, J.A.; Delgado-Rastrollo, M.; Pacha-Olivenza, M.A.; Pérez-Giraldo, C.; Quiles, M.; González-Martín, M.L.; Gallardo-Moreno, A.M. Eficacia bactericida in vivo del farnesol sobre implantes de Ti6Al4V. Rev. SECOT 2016, 60, 260–266. [Google Scholar] [CrossRef]
- Feitosa, D.; Rodrigues, C.; Rose, I.; Oliveira, E.; Luiz, R.; Tavares, J.; Silvino, P.; MLS, Y.; Hs, R.; Datiane, C.; et al. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2020, 337, 127776. [Google Scholar] [CrossRef]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituent. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Guynot, M.E.; Ramos, A.J.; Setó, L.; Purroy, P.; Sanchis, V.; Marín, S. Antifungical activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products. J. Appl. Microbiol. 2003, 94, 893–899. [Google Scholar] [CrossRef]
- Solórzano, S.F.; Miranda, N.M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Pintore, G.; Usai, M.; Bradesi, P.; Juliano, C.; Boatto, G.; Tomi, F.; Chessa, M.; Cerri, R.; Casanova, J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils form Sardinia and Corsica. Flavour. Frag. J. 2002, 17, 15–19. [Google Scholar] [CrossRef]
- Vaara, M. Agents that increase the permeability of outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef]
- Farag, R.S.; Daw, Z.Y.; Hewedid, F.M.; El-Baroty, G.S.A. Antimicrobial Activity of some egyptyan specie essential oils. J. Food Prot. 1989, 52, 665–667. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Davis, B.D.; Dulbecco, R. Tratado de Microbiología, 4th ed.; Masson: Barcelona, Spain, 1996. [Google Scholar]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holly, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug Resistance: An Emerging Crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Logrieco, A.F.; Baruzzi, F. Unravelling the Antifungal Effect of Red Thyme Oil (Thymus vulgaris L.) Compounds in Vapor Phase. Molecules 2020, 25, 4761. [Google Scholar] [CrossRef]
- Vanden Berghe, D.A.; Vlietinck, A.J. Screening methods for antibacterial agents from higher plants. In Methods in Plant Biochemistry, Assays for Bioactivity; Dey, P.M., Harborne, J.B., Hostettman, K., Eds.; Academic Press: London, UK, 1991; pp. 47–69. [Google Scholar]
- Montero-Recalde, M.; Vayas, L.; Avilés-Esquivel, D.; Pazmiño, P.; Erazo-Gutierrez, V. Evaluación de dos métodos para medir la sensibilidad de inhibición de crecimiento de la cepa certificada de Staphylococcus aureus subsp. aureus. Rev. Inv. Vet. Perú. 2018, 29, 1543–1547. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. Tech. Rep. M100-S22; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012; 184p. [Google Scholar]
- Koneman, G.W.; Procop, D.L.; Church, G.S.H.; Janda, W.M.; Koneman, E.W.; Schreckenberger, P.C.; Woods, G.L. Koneman Diagnóstico Microbiológico: Texto y Atlas / Koneman Diagnóstico Microbiológico: Texto y Atlas, 7th ed.; Translation: León, R.B., Roig, F.G., Álvarez, L.M.M., Hmilowicz, A.A.R., Mondragón, A.R., Rojas, P.S., Reyes, R.I.V., Eds.; Jones & Barlet: Burlington, MA, USA, 2017. [Google Scholar]
- Avila, J.G.; Martinez, A.; Martinez, G.; Muñoz, J.L.; Arciniegas, A.; Romo de Vivar, A. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef]
- Durán, D.A.; Vargas, V.A.; Cisneros, C.A.E. Bioestadística. Facultad de Estudios Superiores Iztacala; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2004; pp. 109–114. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).