Antibiotic Resistant Bloodstream Infections in Pediatric Patients Receiving Chemotherapy or Hematopoietic Stem Cell Transplant: Factors Associated with Development of Resistance, Intensive Care Admission and Mortality
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
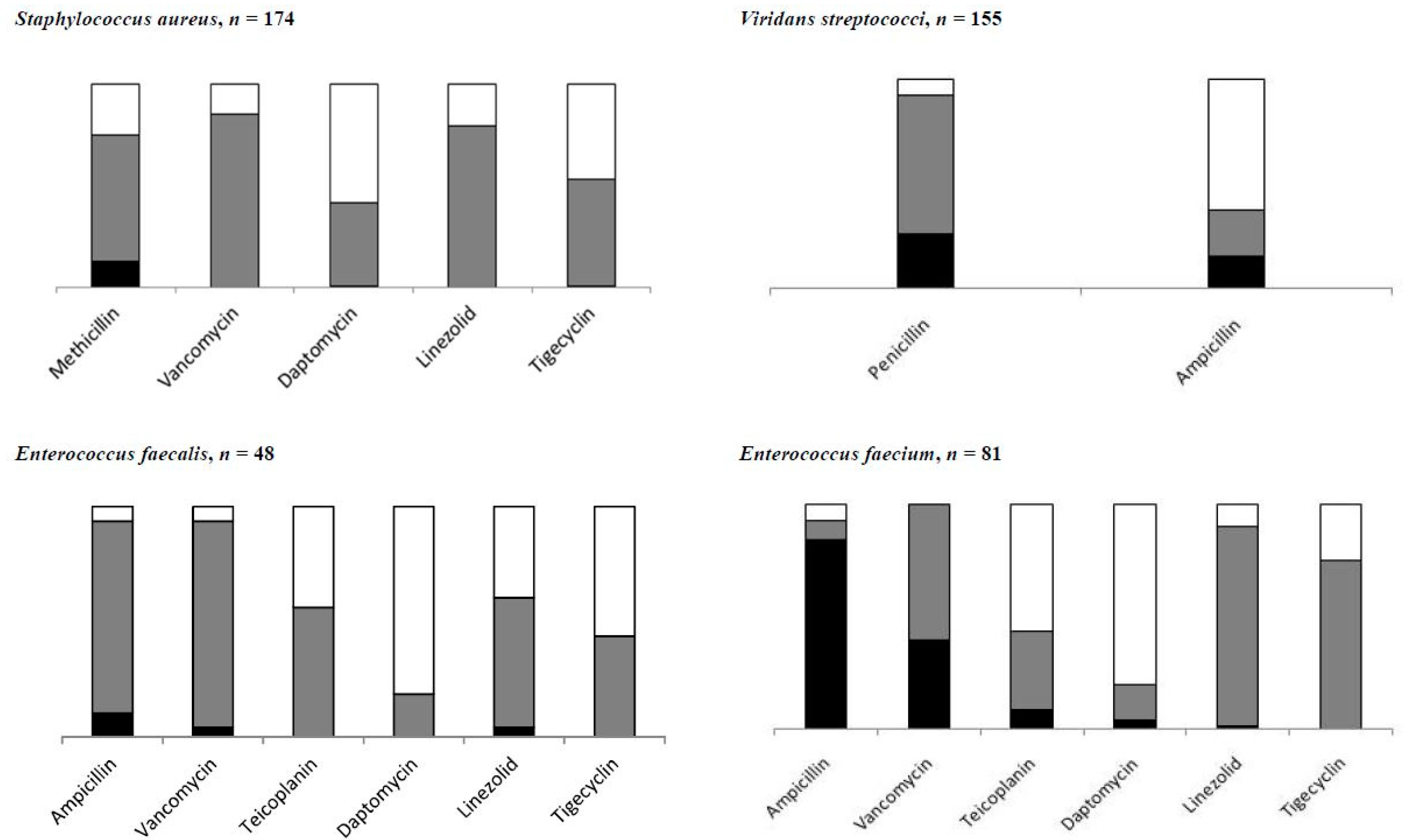
3. Results
3.1. Resistance to Anti-Infectives
3.2. Admission in Intensive Care Unit and Mortality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dupont, H.L.; Spink, W.W. Infections Due to Gram-Negative Organisms: An Analysis Of 860 Patients with Bacteremia at the University of Minnesota Medical Center, 1958. Medicine 1969, 48, 307–332. [Google Scholar] [CrossRef]
- Mikulska, M.; Viscoli, C.; Orasch, C.; Livermore, D.M.; Averbuch, D.; Cordonnier, C.; Akova, M. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J. Infect. 2014, 68, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.D.; Lehrnbecher, T.; Phillips, R.; Dupuis, L.L.; Sung, L. Strategies for Empiric Management of Pediatric Fever and Neutropenia in Patients with Cancer and Hematopoietic Stem-Cell Transplantation Recipients: A Systematic Review of Randomized Trials. J. Clin. Oncol. 2016, 34, 2054–2060. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Robinson, P.; Fisher, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Groll, A.H.; Haeusler, G.M.; Santolaya, M.; et al. Guideline for the Management of Fever and Neutropenia in Children with Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef]
- Castagnola, E.; Caviglia, I.; Pescetto, L.; Bagnasco, F.; Haupt, R.; Bandettini, R. Antibiotic susceptibility of Gram-negatives isolated from bacteremia in children with cancer. Implications for empirical therapy of febrile neutropenia. Futur. Microbiol. 2015, 10, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Viale, P.; Viscoli, C.; Trecarichi, E.M.; Tumietto, F.; Marchese, A.; Spanu, T.; Ambretti, S.; Ginocchio, F.; Cristini, F.; et al. Predictors of Mortality in Bloodstream Infections Caused by Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: Importance of Combination Therapy. Clin. Infect. Dis. 2012, 55, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Radhakrishnan, V.; Vijayakumar, V.; Ramamoorthy, J.; Ganesan, P.; Dhanushkodi, M.; Ganesan, T.S.; Sagar, T.G. Prevalence of multi-drug resistant organisms in stool of paediatric patients with acute leukaemia and correlation with blood culture positivity: A single institution experience. Pediatr. Blood Cancer 2018, 65, 6740. [Google Scholar] [CrossRef]
- Satlin, M.J.; Walsh, T.J. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistantEnterococcus: Three major threats to hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2017, 19, e12762. [Google Scholar] [CrossRef]
- Averbuch, D.; Tridello, G.; Hoek, J.; Mikulska, M.; Akan, H.; Segundo, L.Y.S.; Pabst, T.; Özçelik, T.; Klyasova, G.; Donnini, I.; et al. Antimicrobial Resistance in Gram-Negative Rods Causing Bacteremia in Hematopoietic Stem Cell Transplant Recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin. Infect. Dis. 2017, 65, 1819–1828. [Google Scholar] [CrossRef]
- Haeusler, G.M.; Mechinaud, F.; Daley, A.J.; Starr, M.; Shann, F.; Connell, T.G.; Bryant, P.A.; Donath, S.; Curtis, N. Antibiotic-resistant Gram-negative Bacteremia in Pediatric Oncology Patients—Risk Factors and Outcomes. Pediatr. Infect. Dis. J. 2013, 32, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Gudiol, C.; Vidal, G.C.; Tubau, F.; Contra, A.; Boix, L.; Domenech, D.E.; Calvo, M.; Carratalà, J. Epidemiology, antibiotic therapy and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in cancer patients. Support. Care Cancer 2014, 22, 603–610. [Google Scholar] [CrossRef]
- Girmenia, C.; Gitmo, T.; Rossolini, G.M.; Piciocchi, A.; Bertaina, A.; Pisapia, G.; Pastore, D.; Sica, S.; Severino, A.; Cudillo, L.; et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: A nationwide retrospective survey from Italy. Bone Marrow Transplant. 2014, 50, 282–288. [Google Scholar] [CrossRef]
- Spychala, Z.O.; Wachowiak, J.; Kwiatkowska, G.O.; Gietka, A.; Baginska, D.B.; Semczuk, K.; Fangrat, D.K.; Czyzewski, K.; Dziedzic, M.; Wysocki, M.; et al. Prevalence, Epidemiology, Etiology, and Sensitivity of Invasive Bacterial Infections in Pediatric Patients Undergoing Oncological Treatment: A Multicenter Nationwide Study. Microb. Drug Resist. 2021, 27, 53–63. [Google Scholar] [CrossRef] [PubMed]
- EUCAST v 10.0 Recommendations. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 8.1; EUCAST. Available online: http://www.eucast.org (accessed on 4 March 2021).
- The Clinical & Laboratory Standards Institute (CLSI). Global Laboratory Standards for a Healthier World. Available online: https://clsi.org (accessed on 4 March 2021).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models (Analytical Methods for Social Research); Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- MacFadden, D.; Coburn, B.; Shah, N.; Robicsek, A.; Savage, R.; Elligsen, M.; Daneman, N. Utility of prior cultures in predicting antibiotic resistance of bloodstream infections due to Gram-negative pathogens: A multicentre observational cohort study. Clin. Microbiol. Infect. 2018, 24, 493–499. [Google Scholar] [CrossRef]
- Giacometti, A.; Siquini, F.M.; Cirioni, O.; Petroni, S.; Scalise, G. Imipenem and meropenem induced resistance to beta-lactam antibiotics inPseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 315–318. [Google Scholar] [CrossRef]
- McLaughlin, M.; Advincula, M.R.; Malczynski, M.; Qi, C.; Bolon, M.; Scheetz, M.H. Correlations of Antibiotic Use and Carbapenem Resistance in Enterobacteriaceae. Antimicrob. Agents Chemother. 2013, 57, 5131–5133. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Fisher, B.T.; Gaur, A.H.; Dvorak, C.C.; Luna, D.V.; Dang, H.; Chen, L.; Green, M.; Nieder, M.L.; Fisher, B.; et al. Effect of Levofloxacin Prophylaxis on Bacteremia in Children with Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation. JAMA 2018, 320, 995–1004. [Google Scholar] [CrossRef]
- Calitri, C.; Ruberto, E.; Castagnola, E. Antibiotic prophylaxis in neutropenic children with acute leukemia: Do the presently available data really support this practice? Eur. J. Haematol. 2018, 101, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Mesini, A.; Bandettini, R.; Faraci, M.; Castagnola, E. Antibacterial prophylaxis of febrile neutropenia is not effective in the pre-engraftment period in pediatric allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2020, 22, 3340. [Google Scholar] [CrossRef]
- Widjajanto, P.H.; Sumadiono, S.; Cloos, J.; Purwanto, I.; Sutaryo, S.; Veerman, A.J. Randomized double blind trial of ciprofloxacin prophylaxis during induction treatment in childhood acute lymphoblastic leukemia in the WK-ALL protocol in Indonesia. J. Blood Med. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Fisher, B.T.; Phillips, B.; Alexander, S.; A Ammann, R.; Beauchemin, M.; Carlesse, F.; Castagnola, E.; Davis, B.L.; Dupuis, L.L.; et al. Guideline for Antibacterial Prophylaxis Administration in Pediatric Cancer and Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2020, 71, 226–236. [Google Scholar] [CrossRef]
- Castagnola, E.; Tatarelli, P.; Mesini, A.; Baldelli, I.; La Masa, D.; Biassoni, R.; Bandettini, R. Epidemiology of carbapenemase-producing Enterobacteriaceae in a pediatric hospital in a country with high endemicity. J. Infect. Public Health 2019, 12, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gyssens, I.C.; Kern, W.V.; Livermore, D.M. The role of antibiotic stewardship in limiting antibacterial resistance among hematology patients. Haematology 2013, 98, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Strymish, J.; Elliman, B.W.; Itani, K.M.F.; Williams, S.; Gupta, K. A Clinical History of Methicillin-Resistant Staphylococcus aureus Is a Poor Predictor of Preoperative Colonization Status and Postoperative Infections. Infect. Control. Hosp. Epidemiol. 2012, 33, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Disease Control. Surveillance Report. Surveillance of Antimicrobial Consumption in Europe. Available online: http://www.ecdc.europa.eu/en/publications/Publications/antimicrobial-antibiotic-consumption-ESAC-report-2010-data.pdf (accessed on 4 March 2021).
- Van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Mikulska, M.; Viscoli, C. Recent advances in the pharmacological management of infections due to multidrug-resistant Gram-negative bacteria. Expert Rev. Clin. Pharmacol. 2018, 11, 1219–1236. [Google Scholar] [CrossRef]
- Wi, Y.M.; Quaintance, G.K.E.; Schuetz, A.N.; Ko, K.S.; Peck, K.R.; Song, J.H.; Patel, R. Activity of Ceftolozane-Tazobactam against Carbapenem-Resistant, Non-Carbapenemase-Producing Pseudomonas aeruginosa and Associated Resistance Mechanisms. Antimicrob. Agents Chemother. 2017, 62. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M.; Mesini, A.; Castagnola, E. Optimal Management of Complicated Infections in the Pediatric Patient: The Role and Utility of Ceftazidime/Avibactam. Infect. Drug Resist. 2020, 13, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Ang, J.Y.; Arrieta, A.C.; Larson, K.B.; Rizk, M.L.; Caro, L.; Yang, S.; Yu, B.; Johnson, M.G.; Rhee, E.G. Pharmacokinetics and Safety of Single Intravenous Doses of Ceftolozane/Tazobactam in Children with Proven or Suspected Gram-Negative Infection. Pediatr. Infect. Dis. J. 2018, 37, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.B.; Patel, Y.T.; Willavize, S.; Bradley, J.S.; Rhee, E.G.; Caro, L.; Rizk, M.L. Ceftolozane-Tazobactam Population Pharmacokinetics and Dose Selection for Further Clinical Evaluation in Pediatric Patients with Complicated Urinary Tract or Complicated Intra-abdominal Infections. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Hanretty, A.M.; Kaur, I.; Evangelista, A.T.; Moore, W.S.; Enache, A.; Chopra, A.; Cies, J.J. Pharmacokinetics of the Meropenem Component of Meropenem-Vaborbactam in the Treatment ofKPC-ProducingKlebsiella pneumoniaeBloodstream Infection in a Pediatric Patient. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, e87–e91. [Google Scholar] [CrossRef] [PubMed]
- Alamarat, Z.I.; Babic, J.; Tran, T.T.; Wootton, S.H.; Dinh, A.Q.; Miller, W.R.; Hanson, B.; Wanger, A.; Gary, J.L.; Arias, C.A.; et al. Long-Term Compassionate Use of Cefiderocol To Treat Chronic Osteomyelitis Caused by Extensively Drug-Resistant Pseudomonas aeruginosa and Extended-Spectrum-β-Lactamase-Producing Klebsiella pneumoniae in a Pediatric Patient. Antimicrob. Agents Chemother. 2019, 64. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; International Society of Antimicrobial Chemotherapy (ISAC); Gould, I.M.; Lee, W.S.; Hsueh, P.R. New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship. Drugs 2019, 79, 705–714. [Google Scholar] [CrossRef]
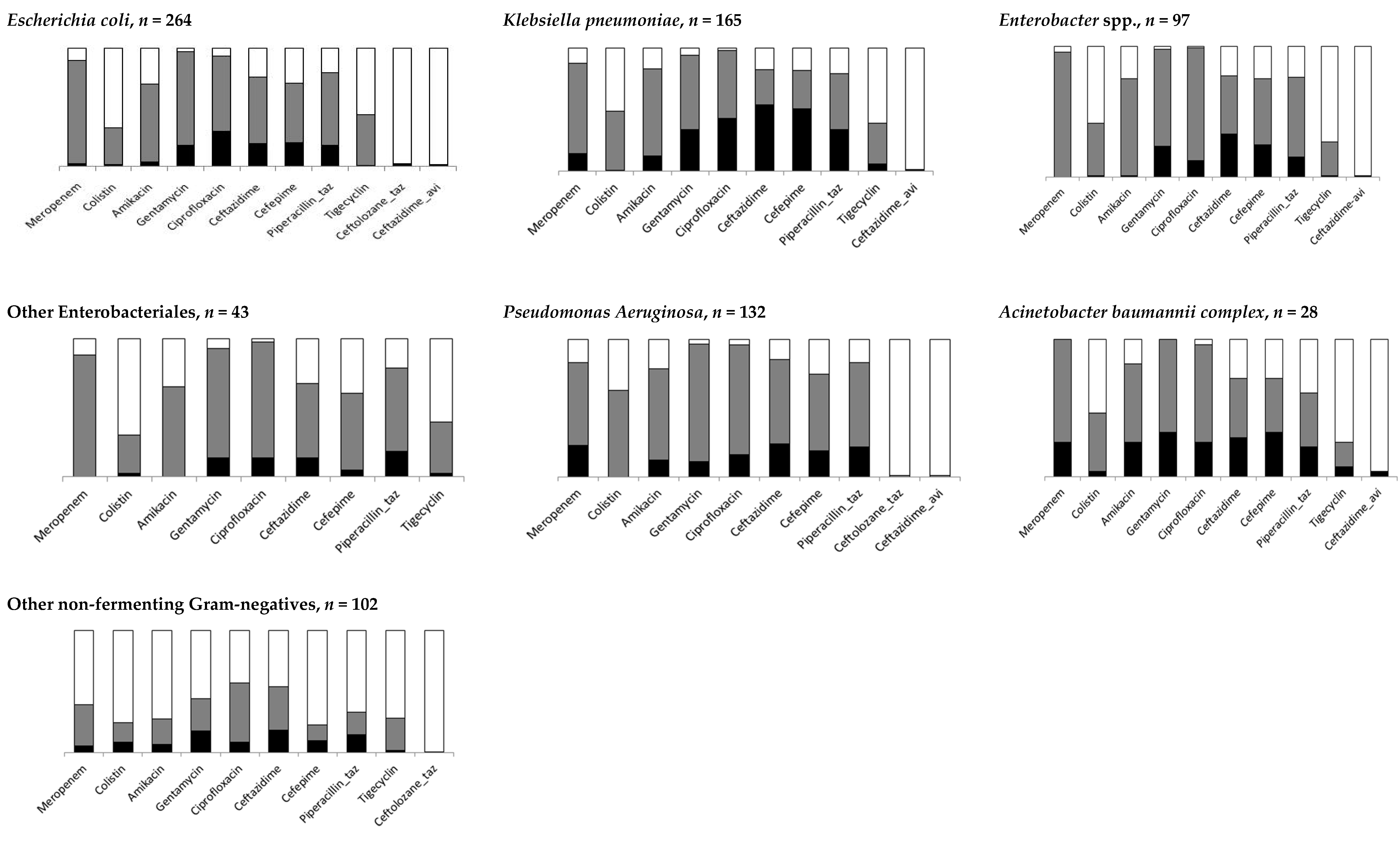
| Resistant, n | % Resistance (95% CI) | |
|---|---|---|
| Gram-negatives, n = 797 | ||
| Meropenem | 72 | 9.0 (3.7–20.5) |
| Amikacin | 60 | 7.5 (3.1–17.0) |
| Gentamycin | 173 | 21.7 (11.8–36.5) |
| Ceftazidime | 235 | 29.5 (14.2–51.4) |
| Cefepime | 206 | 25.8 (10.0–52.4) |
| Piperacillin-tazobactam | 174 | 21.8 (16.8–27.8) |
| Ciprofloxacin | 203 | 25.5 (14.2–41.4) |
| S.aureus, n = 131 | ||
| Methicillin | 22 | 16.8 (7.9–32.1) |
| Enterococci, n = 127 | ||
| Ampicillin | 73 | 57.5 (28.0–82.4) |
| Vancomycin | 34 | 26.8 (13.4–46.4) |
| Viridans streptococci | ||
| Penicillin, n = 143 | 40 | 28.0 (18.7–39.6) |
| Ampicillin, n = 58 | 24 | 41.4 (28.1–56.1) |
| Odds Ratio (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Factors | Meropenem * | Amikacin ** | Gentamycin * | Ciprofloxacin ** | Ceftazidime * | Cefepime ** | Piperacillin-Tazobactam * |
| Sex, p-value | 0.768 | 0.981 | 0.327 | 0.204 | 0.316 | 0.296 | 0.013 |
| Male vs. female | 0.9 (0.4–1.9) | 1.0 (0.5–1.8) | 1.3 (0.7–2.3) | 1.3 (0.9–1.9) | 0.7 (0.4–1.3) | 1.3 (0.8–2.0) | 0.6 (0.3–0.9) |
| Age at bloodstream infections, years, p-value | 0.9 (0.8–1.1), 0.062 | 1.0 (0.9–1.1), 0.383 | 1.0 (0.9–1.1), 0.344 | 1.0 (1.0–1.1), 0.017 | 1.0 (0.9–1.1), 0.324 | 1.0 (0.9–1.1), 0.120 | 1.0 (0.9–1.1), 0.654 |
| Underlying disease, p-value | 0.038 | 0.015 | 0.706 | 0.061 | 0.195 | 0.438 | 0.476 |
| NMD vs. HM | 4.0 (1.1–14.0) | 3.5 (1.5–8.0) | 1.5 (0.6–3.7) | 2.0 (1.0–3.7) | 1.4 (0.5–3.5) | 1.2 (0.6–2.4) | 1.6 (0.7–3.5) |
| ST vs. HM | 0.8 (0.3–2.4) | 1.0 (0.4–2.4) | 1.0 (0.5–2.1) | 0.8 (0.5–1.4) | 0.6 (0.3–1.2) | 0.7 (0.4–1.3) | 1.2 (0.7–2.2) |
| Allogeneic stem cell transplant, p-value | 0.533 | 0.611 | 0.199 | 0.773 | 0.927 | 0.443 | 0.382 |
| Yes vs. no | 1.3 (0.5–3.3) | 0.8 (0.4–1.8) | 1.8 (0.9–3.7) | 0.9 (0.6–1.6) | 1.0 (0.5–2.1) | 0.8 (0.4–1.4) | 1.3 (0.7–2.4) |
| Relapse/ progression, p-value | 0.279 | 0.438 | 0.150 | 0.099 | 0.218 | 0.008 | 0.467 |
| Yes vs. no | 1.6 (0.7–3.9) | 1.3 (0.7–2.6) | 1.6 (0.8–3.0) | 1.4 (0.9–2.2) | 1.5 (0.8–2.9) | 2.0 (1.2–3.3) | 1.2 (0.7–2.1) |
| BSI, p-value | 0.768 | 0.298 | 0.668 | 0.392 | 0.216 | 0.280 | 0.028 |
| Single agent vs. polymicrobial | 0.8 (0.1–4.0) | 0.5 (0.1–1.8) | 0.8 (0.3–2.1) | 1.4 (0.6–3.4) | 0.5 (0.2–1.4) | 0.6 (0.2–1.5) | 0.4 (0.2–0.9) |
| Previous antibacterial exposure (prophylaxis/therapy)1, p-value | <0.001 | <0.001 | 0.138 | 0.008 | 0.009 | 0.215 | <0.001 |
| Standard regimen vs. none | 5.1 (1.5–17.4) | 4.5 (1.8–11.4) | 2.1 (1.1–4.2) | 1.7 (1.0–2.8) | 2.2 (1.1–4.6) | 1.7 (0.9–2.9) | 3.3 (1.7–6.3) |
| Carbapenems vs. none | 31.5 (5.1–193.4) | 7.3 (2.6–20.1) | 2.0 (0.8–4.7) | 2.5 (1.4–4.7) | 3.8 (1.4–10.2) | 1.9 (0.9–3.8) | 3.4 (1.5–7.4) |
| Fluoroquinolones/β-lactams/Combination 2/Others vs. none | 5.8 (1.3–25.7) | 2.2 (0.7–6.9) | 1.4 (0.6–3.3) | 2.1 (1.1–3.7) | 1.4 (0.6–3.5) | 1.4 (0.7–2.9) | 2.3 (1.1–4.9) |
| Neutropenia, p-value | 0.016 | 0.494 | 0.485 | 0.427 | 0.872 | 0.190 | 0.048 |
| Yes vs. no | 3.1 (1.1–8.9) | 1.3 (0.6–2.7) | 1.3 (0.7–2.4) | 1.2 (0.8–1.9) | 0.9 (0.5–1.8) | 1.4 (0.8–2.4) | 1.7 (1.0–3.0) |
| Previous colonization, p-value | 0.120 | 0.257 | 0.895 | 0.891 | 0.421 | 0.653 | 0.035 |
| No vs. yes | 2.2 (0.8–5.7) | 1.6 (0.7–3.5) | 1.0 (0.5–2.2) | 1.0 (0.6–1.8) | 1.4 (0.6–2.9) | 1.1 (0.6–2.0) | 2.0 (1.0–3.8) |
| Previous infection, p-value | 0.161 | 0.040 | 0.280 | <0.001 | <0.001 | <0.001 | 0.031 |
| No vs. yes | 1.9 (0.8–5.0) | 2.2 (1.1–4.6) | 1.5 (0.7–3.3) | 2.6 (1.5–4.4) | 3.9 (1.7–8.9) | 3.6 (1.8–6.9) | 2.0 (1.1–3.7) |
| Random effect, variance component, centre | 2.0 (0.4–9.1) | 1.6 (0.4–6.2) | 1.1 (0.3–3.7) | 1.8 (0.7–4.7) | 4.4 (1.3–14.6) | 8.4 (2.9–24.9) | 0.7 (0.2–2.6) |
| Random effect, variance component, patient | 1.8 (0.1–28.9) | NA | 2.9 (0.7–11.0) | NA | 2.7 (0.6–12.5) | NA | 1.3 (0.2–7.0) |
| LR test vs. logistic regression, p-value *** | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 |
| Odds Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Factors | Methicillin-Staphylococcus aureus, n = 131 * | Penicillin-Viridians Streptococci, n = 143 ** | Ampicillin-Viridians Streptococci, n = 58 ** | Ampicillin-Enterococcus (faecalis & faecium), n = 127 * | Vancomycin-Enterococcus(faecalis & faecium), n = 127 ** |
| Sex, p-value | 0.760 | 0.792 | 0.228 | 0.490 | 0.197 |
| Male vs. female | 1.2 (0.3–4.9) | 0.9 (0.4–2.0) | 0.4 (0.1–1.7) | 1.5 (0.5–4.5) | 1.9 (0.7–5.1) |
| Age at bloodstream infections, years, p-value | 1.0 (0.9–1.1), 0.868 | 0.9 (0.8–1.1), 0.107 | 0.9 (0.8–1.1), 0.118 | 1.0 (0.9–1.1), 0.790 | 1.0 (0.9–1.1), 0.790 |
| Underlying disease, p-value | 0.462 | 0.418 | 0.947 | 0.072 | 0.570 |
| NMD vs. HM | 1.4 (0.2–9.7) | 1.0 (0.1–9.8) | NA | 0.1 (0.0–1.2) | 0.4 (0.1–4.9) |
| ST vs. HM | 2.7 (0.5–13.6) | 0.4 (0.1–1.8) | 0.9 (0.1–5.8) | 0.4 (0.1–1.6) | 0.7 (0.2–2.9) |
| Allogeneic stem cell transplant, p-value | 0.324 | 0.693 | 0.461 | 0.046 | 0.796 |
| Yes vs. no | 2.9 (0.3–24.4) | 1.3 (0.4–4.6) | 2.2 (0.5–19.5) | 5.2 (0.9–29.2) | 1.2 (0.3–4.4) |
| Relapse/ progression, p-value | 0.278 | 0.890 | 0.498 | 0.282 | 0.550 |
| Yes vs. no | 0.3 (0.1–2.4) | 0.9 (0.3–2.7) | 0.5 (0.1–3.1) | 2.0 (0.6–7.4) | 1.3 (0.5–3.6) |
| BSI, p-value | 0.176 | 0.447 | 0.879 | 0.895 | 0.925 |
| Single agent vs. polymicrobial | 0.1 (0.0–2.4) | 0.6 (0.2–2.0) | 1.2 (0.2–8.5) | 1.1 (0.2–6.1) | 0.9 (0.2–4.8) |
| Previous antibacterial exposure (prophylaxis/therapy), p-value | 0.004 | ||||
| Yes vs. no | NA | NA | 7.7 (1.7–35.5) | NA | NA |
| Previous antibacterial exposure (prophylaxis/therapy) 1, p-value | 0.068 | 0.489 | 0.396 | 0.083 | |
| Standard regimen vs. no one | 6.4 (1.1–39.5) | 1.8 (0.6–5.6) | NA | 1.1 (0.2–7.1) | 3.2 (0.4–25.5) |
| Carbapenem vs. no one | NA | 1.6 (0.4–6.1) | NA | 1.3 (0.2–8.2) | 8.7 (0.8–90.7) |
| Fluoroquinolones/β-lactams/Combination 2/Others vs. no one | 4.5 (0.8–26.7) | 2.2 (0.7–6.7) | NA | 3.6 (0.5–24.5) | 2.5 (0.2–25.2) |
| Neutropenia, p-value | 0.656 | 0.840 | 0.617 | 0.047 | 0.048 |
| Yes vs. no | 0.7 (0.2–3.1) | 1.1 (0.3–4.4) | 0.6 (0.1–4.1) | 3.7 (0.9–14.5) | 3.5 (1.1–11.1) |
| Previous colonization, p-value | 0.013 | 0.414 | 0.918 | 0.346 | 0.073 |
| Yes vs. no | 6.7 (1.4–31.3) | 0.3 (0.1–4.6) | 0.9 (0.1–10.8) | 2.2 (0.4–11.9) | 2.6 (0.9–7.4) |
| Previous infection, p-value | 0.556 | 0.725 | 0.021 | 0.726 | |
| Yes vs. no | NA | 1.5 (0.4–6.4) | 0.6 (0.1–7.9) | 5.7 (1.1–28.8) | 0.8 (0.2–2.7) |
| Random effect, variance component, centre | 2.7 (0.3–22.7) | NA | NA | 1.5 (0.3–7.0) | NA |
| LR test vs. logistic regression, p-value *** | 0.0255 | 0.5326 | 1.000 | 0.0002 | 0.6495 |
| Factors | Odds Ratio (95% Confidence Interval) | |
|---|---|---|
| ICU Admission * | Mortality * | |
| Sex, p-value | 0.302 | 0.724 |
| Male vs. female | 0.7 (0.4–1.3) | 0.9 (0.4–1.9) |
| Age at bloodstream infections, years, p-value | 1.0 (0.9–1.1), 0.210 | 0.9 (0.8–1.1), 0.068 |
| Underlying disease, p-value | 0.018 | 0.098 |
| NMD vs. HM | 0.8 (0.3–2.2) | 3.6 (1.0–13.4) |
| ST vs. HM | 0.3 (0.1–0.8) | 1.2 (0.4–3.3) |
| Relapse/ progression, p-valueYes vs. no | 0.2701.5 (0.7–2.9) | 0.0045.3 (1.7–16.5) |
| Allogeneic stem cell transplant phase, p-value | 0.5778 | 0.089 |
| Pre-engraftment vs. no allogenic-HSCT | 1.3 (0.5–3.0) | 0.8 (0.2–2.9) |
| Acute GvHD vs. no allogenic-HSCT | 2.1 (0.5–8.9) | 1.7 (0.3–10.8) |
| Chronic GvHD vs. no allogenic-HSCT | 1.8 (0.3–11.3) | 7.0 (0.9–51.6) |
| Post-engraftment vs. no allogenic-HSCT | 0.6 (0.1–2.1) | 4.2 (1.0–17.8) |
| Neutropenia, p-value | 0.023 | 0.327 |
| Yes vs. no | 2.5 (1.2–5.3) | 1.6 (0.6–4.1) |
| Previous antibacterial exposure (prophylaxis/therapy)1, p-value | 0.267 | 0.002 |
| Fluoroquinolones vs. no one/β-lactams | 1.4 (0.3–6.8) | 2.5 (0.3–21.0) |
| Standard regimen vs. no one/β-lactams | 1.4 (0.7–2.8) | 0.9 (0.3–2.7) |
| Carbapenem vs. no one/β-lactams | 2.8 (1.2–6.7) | 3.8 (1.1–13.5) |
| Combination 2 vs. no one/β-lactams | 1.1 (0.1–8.4) | 9.1 (1.1–77.8) |
| Others vs. no one/β-lactams | 2.0 (0.6–6.6) | 8.2 (1.6–41.9) |
| Previous colonization, p-value | 0.193 | 0.265 |
| Yes vs. no | 0.6 (0.2–1.3) | 1.8 (0.6–5.3) |
| Previous infection, p-value | 0.835 | 0.095 |
| Yes vs. no | 0.9 (0.4–2.0) | 2.7 (0.8–8.9) |
| BSI, p-value | 0.936 | 0.441 |
| Single agent vs. polymicrobial | 0.9 (0.3–2.8) | 0.5 (0.1–2.6) |
| Gram-negatives antibiotic resistance, p-value | <0.001 | 0.167 |
| Gram-negatives resistant to 1 antibiotic 3 vs. susceptible | 0.3 (0.1–0.8) | 3.4 (0.8–13.9) |
| Gram-negatives resistant to 2 or 3 antibiotics 3 vs. susceptible | 0.7 (0.3–1.9) | 3.7 (1.0–13.7) |
| Gram-negatives resistant to 4 or 5 antibiotics 3 vs. susceptible | 18.0 (3.7–87.2) | 4.5 (1.0–20.0) |
| Not applicable vs. susceptible | 0.8 (0.4–1.6) | 2.3 (0.8–6.5) |
| ICU for bloodstream infection, p-value Yes vs. no | NA | <0.001,44.4 (7.6–258.5) |
| Random effect, variance component, center | 1.8 (0.5–6.6) | 0.8 (0.2–5.9) |
| Random effect, variance component, patient | 4.4 (0.9–20.2) | 3.8 (0.7–19.7) |
| LR test vs. logistic regression, p-value ** | <0.0001 | 0.0172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagnola, E.; Bagnasco, F.; Mesini, A.; Agyeman, P.K.A.; Ammann, R.A.; Carlesse, F.; Santolaya de Pablo, M.E.; Groll, A.H.; Haeusler, G.M.; Lehrnbecher, T.; et al. Antibiotic Resistant Bloodstream Infections in Pediatric Patients Receiving Chemotherapy or Hematopoietic Stem Cell Transplant: Factors Associated with Development of Resistance, Intensive Care Admission and Mortality. Antibiotics 2021, 10, 266. https://doi.org/10.3390/antibiotics10030266
Castagnola E, Bagnasco F, Mesini A, Agyeman PKA, Ammann RA, Carlesse F, Santolaya de Pablo ME, Groll AH, Haeusler GM, Lehrnbecher T, et al. Antibiotic Resistant Bloodstream Infections in Pediatric Patients Receiving Chemotherapy or Hematopoietic Stem Cell Transplant: Factors Associated with Development of Resistance, Intensive Care Admission and Mortality. Antibiotics. 2021; 10(3):266. https://doi.org/10.3390/antibiotics10030266
Chicago/Turabian StyleCastagnola, Elio, Francesca Bagnasco, Alessio Mesini, Philipp K. A. Agyeman, Roland A. Ammann, Fabianne Carlesse, Maria Elena Santolaya de Pablo, Andreas H. Groll, Gabrielle M. Haeusler, Thomas Lehrnbecher, and et al. 2021. "Antibiotic Resistant Bloodstream Infections in Pediatric Patients Receiving Chemotherapy or Hematopoietic Stem Cell Transplant: Factors Associated with Development of Resistance, Intensive Care Admission and Mortality" Antibiotics 10, no. 3: 266. https://doi.org/10.3390/antibiotics10030266
APA StyleCastagnola, E., Bagnasco, F., Mesini, A., Agyeman, P. K. A., Ammann, R. A., Carlesse, F., Santolaya de Pablo, M. E., Groll, A. H., Haeusler, G. M., Lehrnbecher, T., Simon, A., D’Amico, M. R., Duong, A., Idelevich, E. A., Luckowitsch, M., Meli, M., Menna, G., Palmert, S., Russo, G., ... Sung, L. (2021). Antibiotic Resistant Bloodstream Infections in Pediatric Patients Receiving Chemotherapy or Hematopoietic Stem Cell Transplant: Factors Associated with Development of Resistance, Intensive Care Admission and Mortality. Antibiotics, 10(3), 266. https://doi.org/10.3390/antibiotics10030266








