Is Dosing of Ethambutol as Part of a Fixed-Dose Combination Product Optimal for Mechanically Ventilated ICU Patients with Tuberculosis? A Population Pharmacokinetic Study
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Data
2.2. Pharmacokinetic Model Selection and Evaluation
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Patients and Study Design
4.3. Drug Assay
4.4. Population Pharmacokinetic Modelling
4.5. Model Evaluation
4.6. Dose Simulations and Target Attainment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Neves, C.P.; Costa, A.G.; Safe, I.P.; Brito, A.D.S.; Jesus, J.S.; Kritski, A.L.; Lacerda, M.V.G.; Viveiros, M.; Cordeiro-Santos, M. The role of mini-bronchoalveolar lavage fluid in the diagnosis of pulmonary tuberculosis in critically ill patients. BMC Infect. Dis. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Zahar, J.-R.; Azoulay, E.; Klement, E.; De Lassence, A.; Lucet, J.-C.; Regnier, B.; Schlemmer, B.; Bedos, J.-P. Delayed treatment contributes to mortality in ICU patients with severe active pulmonary tuberculosis and acute respiratory failure. Intensiv. Care Med. 2001, 27, 513–520. [Google Scholar] [CrossRef]
- Hagan, G.; Nathani, N. Clinical review: Tuberculosis on the intensive care unit. Crit. Care 2013, 17, 240. [Google Scholar] [CrossRef]
- Balkema, C.A.; Irusen, E.M.; Taljaard, J.J.; Koegelenberg, C.F.N. Tuberculosis in the intensive care unit: A prospective obser-vational study. Int. J. Tuberc. Lung Dis. 2014, 18, 824–830. [Google Scholar] [CrossRef]
- Koegelenberg, C.F.N.; Balkema, C.A.; Jooste, Y.; Taljaard, J.J.; Irusen, E.M. Validation of a severity-of-illness score in patients with tuberculosis requiring intensive care unit admission. S. Afr. Med. J. 2015, 105, 398. [Google Scholar] [CrossRef][Green Version]
- Ferreira, M.D.; Das Neves, C.P.; De Souza, A.B.; Beraldi-Magalhães, F.; Migliori, G.B.; Kritski, A.L.; Cordeiro-Santos, M. Predictors of mortality among intensive care unit patients coinfected with tuberculosis and HIV. J. Bras. Pneumol. 2018, 44, 118–124. [Google Scholar] [CrossRef]
- Zimmerman, M.; Lestner, J.; Prideaux, B.; O’Brien, P.; Dias-Freedman, I.; Chen, C.; Dietzold, J.; Daudelin, I.; Kaya, F.; Blanc, L.; et al. Ethambutol Partitioning in Tuberculous Pulmonary Lesions Explains Its Clinical Efficacy. Antimicrob. Agents Chemother. 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Fox, W.; Ellard, G.A.; Mitchison, D.A. Studies on the treatment of tuberculosis undertaken by the British Medical Research Coun-cil tuberculosis units, 1946–1986, with relevant subsequent publications. Int. J. Tuberc. Lung Dis. 1999, 3, 231–279. [Google Scholar]
- Sundell, J.; Bienvenu, E.; Birgersson, S.; Äbelö, A.; Ashton, M. Population Pharmacokinetics and Pharmacogenetics of Ethambutol in Adult Patients Coinfected with Tuberculosis and HIV. Antimicrob. Agents Chemother. 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Akhloufi, H.; Hulscher, M.; Melles, D.C.; Prins, J.M.; Van Der Sijs, H.; Verbon, A. Development of operationalized intravenous to oral antibiotic switch criteria. J. Antimicrob. Chemother. 2016, 72, 543–546. [Google Scholar] [CrossRef]
- Lienhardt, C.; Cook, S.V.; Burgos, M.; Yorke-Edwards, V.; Rigouts, L.; Anyo, G.; Kim, S.-J.; Jindani, A.; Enarson, D.A.; Nunn, A.J.; et al. Efficacy and Safety of a 4-Drug Fixed-Dose Combination Regimen Compared with Separate Drugs for Treatment of Pulmonary Tuberculosis. JAMA 2011, 305, 1415–1423. [Google Scholar] [CrossRef]
- Koegelenberg, C.F.N.; Nortje, A.; Lalla, U.; Enslin, A.; Irusen, E.M.; Rosenkranz, B.; Seifart, H.I.; Bolliger, C.T. The pharmacokinetics of enteral antituberculosis drugs in patients requiring intensive care. S. Afr. Med. J. 2013, 103, 394. [Google Scholar] [CrossRef]
- Mehta, K.; Ravimohan, S.; Pasipanodya, J.G.; Srivastava, S.; Modongo, C.; Zetola, N.M.; Weissman, D.; Ivaturi, V.; Gumbo, T.; Bisson, G.P.; et al. Optimizing ethambutol dosing among HIV/tuberculosis co-infected patients: A population pharmacokinetic modelling and simulation study. J. Antimicrob. Chemother. 2019, 74, 2994–3002. [Google Scholar] [CrossRef]
- Chideya, S.; Winston, C.A.; Peloquin, C.A.; Bradford, W.Z.; Hopewell, P.C.; Wells, C.D.; Reingold, A.L.; Kenyon, T.A.; Moeti, T.L.; Tappero, J.W. Isoniazid, Rifampin, Ethambutol, and Pyrazinamide Pharmacokinetics and Treatment Outcomes among a Predominantly HIV-Infected Cohort of Adults with Tuberculosis from Botswana. Clin. Infect. Dis. 2009, 48, 1685–1694. [Google Scholar] [CrossRef]
- World Health Organization. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ängeby, K.; Juréen, P.; Kahlmeter, G.; Hoffner, S.E.; Schön, T. Challenging a dogma: Antimicrobial susceptibility testing breakpoints for Mycobacterium tuberculosis. Bull. World Health Organ. 2012, 90, 693–698. [Google Scholar] [CrossRef]
- Denti, P.; Jeremiah, K.; Chigutsa, E.; Faurholt-Jepsen, D.; PrayGod, G.; Range, N.; Castel, S.; Wiesner, L.; Hagen, C.M.; Christian, M.H.; et al. Pharmacokinetics of Isoniazid, Pyrazinamide, and Ethambutol in Newly Diagnosed Pulmonary TB Patients in Tanzania. PLoS ONE 2015, 10, e0141002. [Google Scholar] [CrossRef]
- Court, R.; Chirehwa, M.T.; Wiesner, L.; de Vries, N.; Harding, J.; Gumbo, T.; Maartens, G.; McIlleron, H. Effect of tablet crushing on drug exposure in the treatment of multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2019, 23, 1068–1074. [Google Scholar] [CrossRef]
- Tostmann, A.; Mtabho, C.M.; Semvua, H.H.; Boogaard, J.V.D.; Kibiki, G.S.; Boeree, M.J.; Aarnoutse, R.E. Pharmacokinetics of First-Line Tuberculosis Drugs in Tanzanian Patients. Antimicrob. Agents Chemother. 2013, 57, 3208–3213. [Google Scholar] [CrossRef][Green Version]
- Tappero, J.W.; Bradford, W.Z.; Agerton, T.B.; Hopewell, P.; Reingold, A.L.; Lockman, S.; Oyewo, A.; Talbot, E.A.; Kenyon, T.A.; Moeti, T.L.; et al. Serum Concentrations of Antimycobacterial Drugs in Patients with Pulmonary Tuberculosis in Botswana. Clin. Infect. Dis. 2005, 41, 461–469. [Google Scholar] [CrossRef]
- Alsultan, A.; Peloquin, C.A. Therapeutic Drug Monitoring in the Treatment of Tuberculosis: An Update. Drugs 2014, 74, 839–854. [Google Scholar] [CrossRef]
- Gumbo, T. New Susceptibility Breakpoints for First-Line Antituberculosis Drugs Based on Antimicrobial Pharmacokinetic/Pharmacodynamic Science and Population Pharmacokinetic Variability. Antimicrob. Agents Chemother. 2010, 54, 1484–1491. [Google Scholar] [CrossRef]
- Peets, E.A.; Sweeney, W.M.; Place, V.A.; Buyske, D.A. The Absorption, Excretion, and Metabolic Fate of Ethambutol in Man1. Am. Rev. Respir. Dis. 1965, 91, 51–58. [Google Scholar] [CrossRef]
- Roberts, J.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Jönsson, S.; Davidse, A.; Wilkins, J.; Van Der Walt, J.-S.; Simonsson, U.S.H.; Karlsson, M.O.; Smith, P.; McIlleron, H. Population Pharmacokinetics of Ethambutol in South African Tuberculosis Patients. Antimicrob. Agents Chemother. 2011, 55, 4230–4237. [Google Scholar] [CrossRef][Green Version]
- Swaminathan, S.; Pasipanodya, J.G.; Ramachandran, G.; Kumar, A.K.H.; Srivastava, S.; Deshpande, D.; Nuermberger, E.; Gumbo, T. Drug Concentration Thresholds Predictive of Therapy Failure and Death in Children with Tuberculosis: Bread Crumb Trails in Random Forests. Clin. Infect. Dis. 2016, 63, S63–S74. [Google Scholar] [CrossRef]
- McIlleron, H.; Wash, P.; Burger, A.; Norman, J.; Folb, P.I.; Smith, P. Determinants of Rifampin, Isoniazid, Pyrazinamide, and Ethambutol Pharmacokinetics in a Cohort of Tuberculosis Patients. Antimicrob. Agents Chemother. 2006, 50, 1170–1177. [Google Scholar] [CrossRef]
- Brasil. Boletim Epidemiológico; Secretaria de Vigilância em Saúde, Ministério da Saúde: Brasilia, Brazil, 2020.
- Srivastava, S.; Musuka, S.; Sherman, C.; Meek, C.; Leff, R.; Gumbo, T. Efflux-Pump–Derived Multiple Drug Resistance to Ethambutol Monotherapy inMycobacterium tuberculosisand the Pharmacokinetics and Pharmacodynamics of Ethambutol. J. Infect. Dis. 2010, 201, 1225–1231. [Google Scholar] [CrossRef]
- Van Oosterhout, J.J.; Dzinjalamala, F.K.; Dimba, A.; Waterhouse, D.J.; Davies, G.R.; Zijlstra, E.E.; Molyneux, M.E.; Molyneux, E.; Ward, S. Pharmacokinetics of Antituberculosis Drugs in HIV-Positive and HIV-Negative Adults in Malawi. Antimicrob. Agents Chemother. 2015, 59, 6175–6180. [Google Scholar] [CrossRef][Green Version]
- Nagai, K.; Horita, N.; Sato, T.; Yamamoto, M.; Nagakura, H.; Kaneko, T. Age, Dehydration, Respiratory Failure, Orientation Disturbance and Blood Pressure Score Predicts In-hospital Mortality in HIV-negative Non-multidrug-resistant Smear-positive Pulmonary Tuberculosis in Japan. Sci. Rep. 2016, 6, 21610. [Google Scholar] [CrossRef]
- Horita, Y.; Alsultan, A.; Kwara, A.; Antwi, S.; Enimil, A.; Ortsin, A.; Dompreh, A.; Yang, H.; Wiesner, L.; Peloquin, C.A. Evaluation of the Adequacy of WHO Revised Dosages of the First-Line Antituberculosis Drugs in Children with Tuberculosis Using Population Pharmacokinetic Modeling and Simulations. Antimicrob. Agents Chemother. 2018, 62, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hasenbosch, R.E.; Alffenaar, J.W.C.; Koopmans, S.A.; Kosterink, J.G.W.; Van Der Werf, T.S.; Van Altena, R. Ethambutol-induced opti-cal neuropathy: Risk of overdosing in obese subjects. Int. J. Tuberc. Lung Dis. 2008, 12, 967–971. [Google Scholar] [PubMed]
- Tweed, C.D.; Crook, A.M.; Amukoye, E.I.; Dawson, R.; Diacon, A.H.; Hanekom, M.; McHugh, T.D.; Mendel, C.M.; Meredith, S.K.; Murphy, M.E.; et al. Toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Infect. Dis. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Egelund, E.; Alsultan, A.; Peloquin, C. Optimizing the clinical pharmacology of tuberculosis medications. Clin. Pharmacol. Ther. 2015, 98, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Chigutsa, E.; Pasipanodya, J.G.; Visser, M.E.; Van Helden, P.D.; Smith, P.J.; Sirgel, F.A.; Gumbo, T.; McIlleron, H. Impact of Nonlinear Interactions of Pharmacokinetics and MICs on Sputum Bacillary Kill Rates as a Marker of Sterilizing Effect in Tuberculosis. Antimicrob. Agents Chemother. 2014, 59, 38–45. [Google Scholar] [CrossRef]
- Gumbo, T.; Pasipanodya, J.G.; Romero, K.; Hanna, D.; Nuermberger, E. Forecasting Accuracy of the Hollow Fiber Model of Tuberculosis for Clinical Therapeutic Outcomes. Clin. Infect. Dis. 2015, 61, S25–S31. [Google Scholar] [CrossRef]
- Kanji, S.; Hayes, M.; Ling, A.; Shamseer, L.; Chant, C.; Edwards, D.J.; Edwards, S.; Ensom, M.; Foster, D.R.; Hardy, B.; et al. Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clin. Pharmacokinet. 2015, 54, 783–795. [Google Scholar] [CrossRef]
- Fachi, M.M.; Vilhena, R.O.; Boger, B.; Domingos, E.L.; Dos Santos, J.M.M.F.; Junkert, A.M.; Cobre, A.D.F.; Momade, D.R.O.; Beraldi-Magalhães, F.; De Liz, M.V.; et al. LC–QToF–MS method for quantification of ethambutol, isoniazid, pyrazinamide and rifampicin in human plasma and its application. Biomed. Chromatogr. 2020, 34, e4812. [Google Scholar] [CrossRef] [PubMed]
- Chappell, W.R.; Mordenti, J. Extrapolation of Toxicological and Pharmacological Data from Animals to Humans; Elsevier: Amsterdam, The Netherlands, 1991; Volume 20, pp. 1–116. [Google Scholar]
- Bachmann, K.; Pardoe, D.; White, D. Scaling basic toxicokinetic parameters from rat to man. Environ. Health Perspect. 1996, 104, 400–407. [Google Scholar] [CrossRef]
- Ritschel, W.; Vachharajani, N.; Johnson, R.; Hussain, A. The allometric approach for interspecies scaling of pharmacokinetic parameters. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1992, 103, 249–253. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. “PubChem Annotation Record for ETHAMBUTOL, Source: Hazardous Sub-stances Data Bank (HSDB)” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3078#section=Absorption-Distribution-and-Excretion-(Complete) (accessed on 28 November 2021).
- Alghamdi, W.A.; Al-Shaer, M.H.; Peloquin, C.A. Protein Binding of First-Line Antituberculosis Drugs. Antimicrob. Agents Chemother. 2018, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.D.; Ramachandran, G.; Swaminathan, S. The challenges of pharmacokinetic variability of first-line anti-TB drugs. Expert Rev. Clin. Pharmacol. 2016, 10, 47–58. [Google Scholar] [CrossRef] [PubMed]


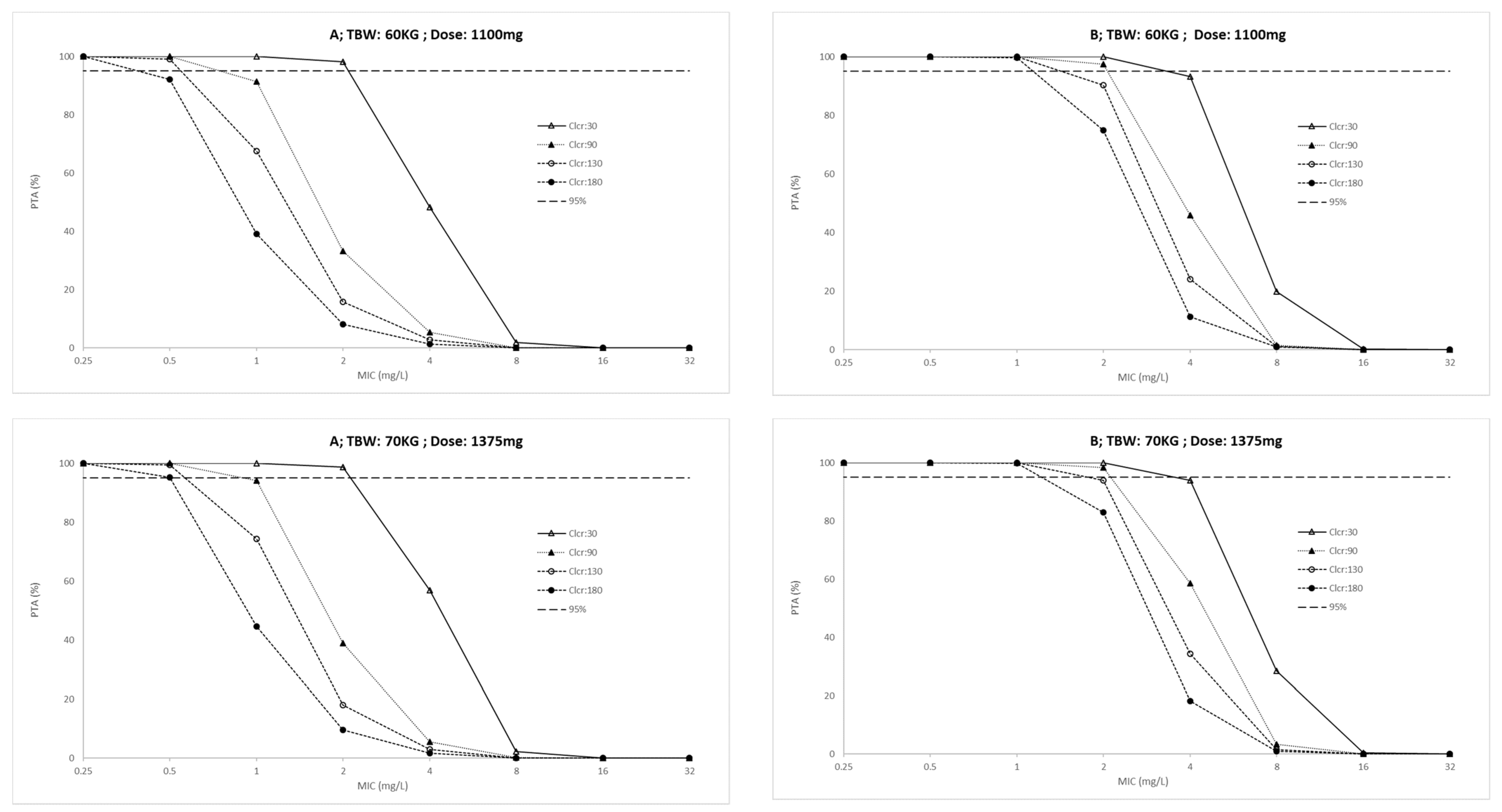
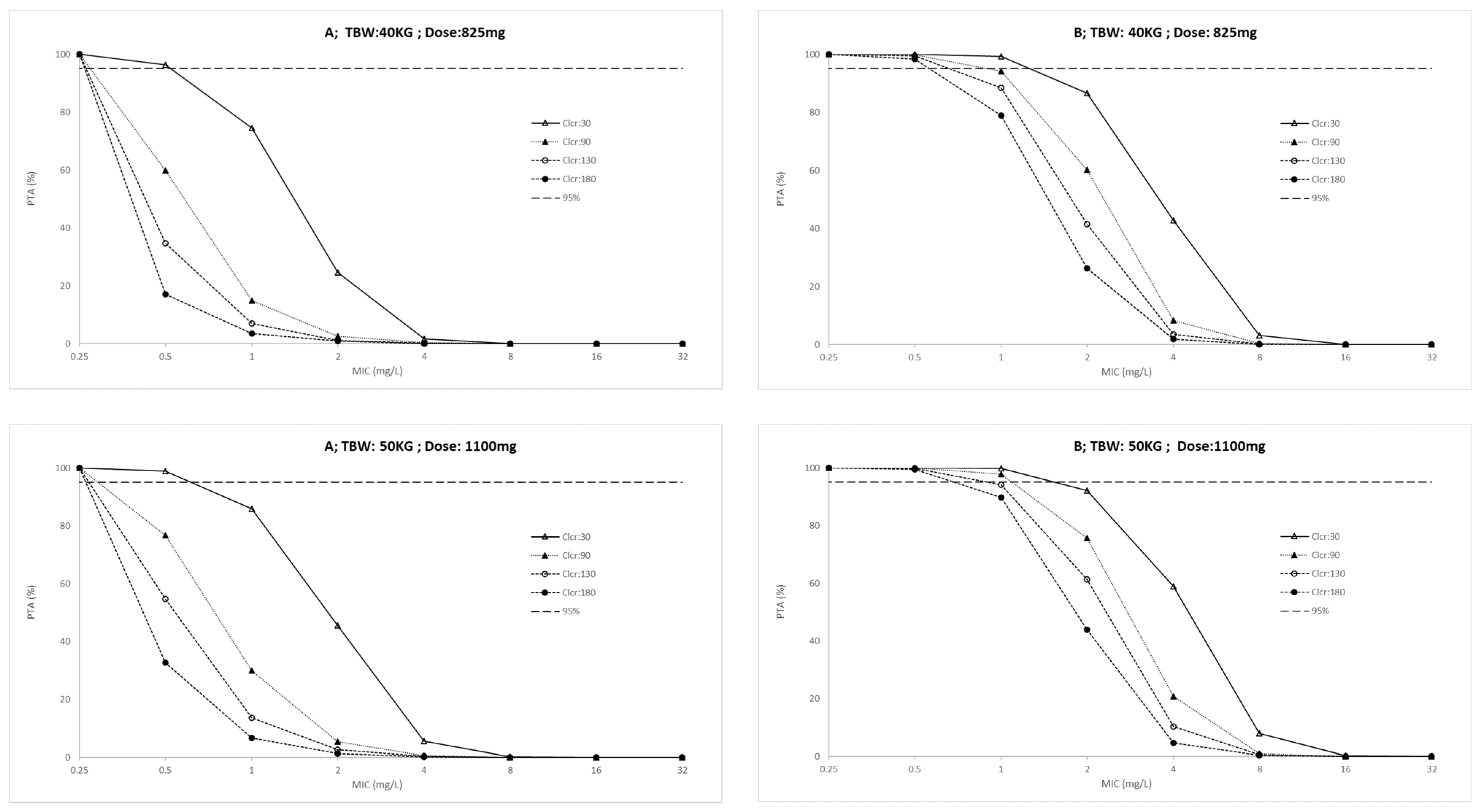
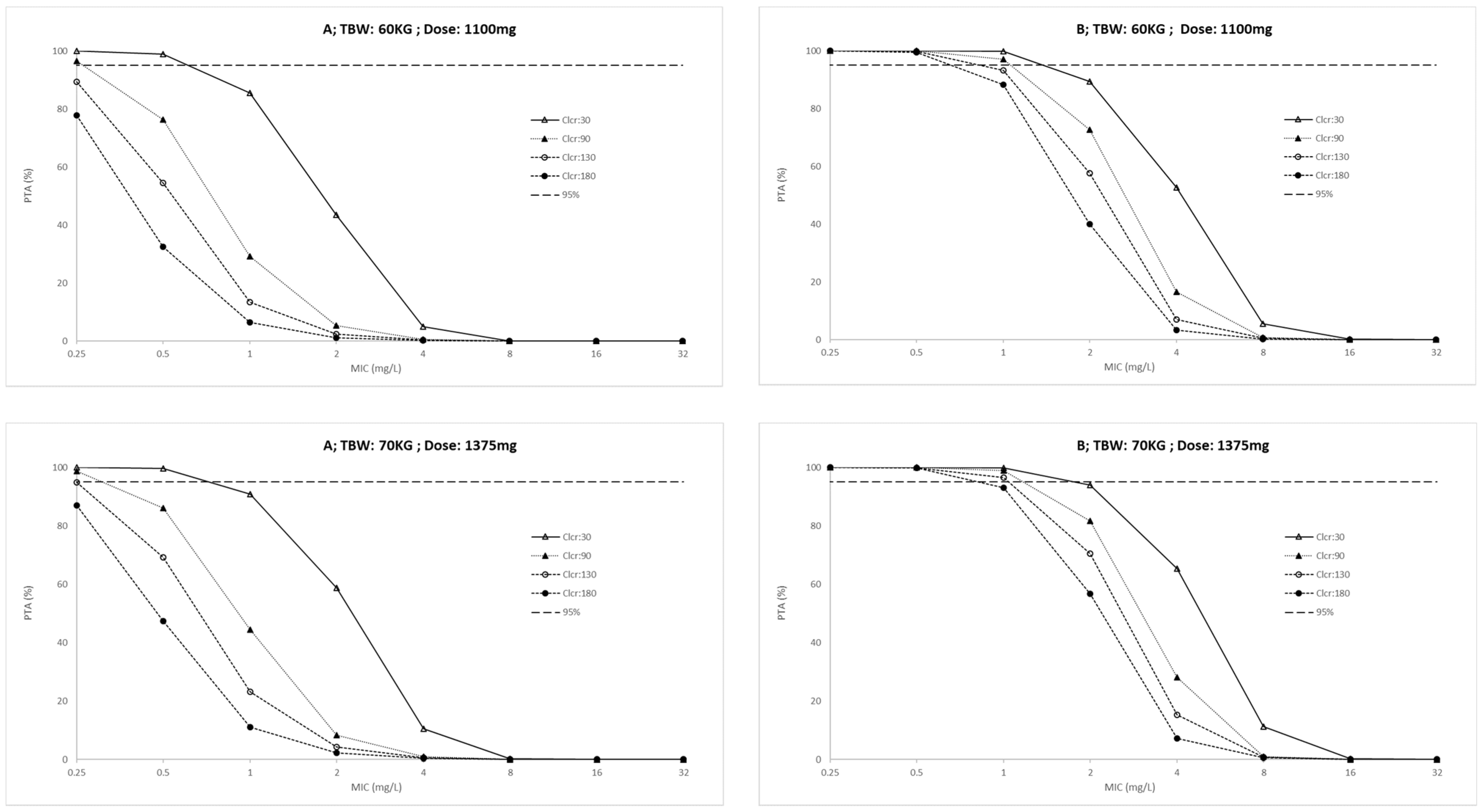
| Characteristic | ICU; n = 10 (IQR) | Outpatients; n = 20 (IQR) | p-Value |
|---|---|---|---|
| Age (year) | 31.0 (29–40) | 39.5 (32.7–46.2) | 0.13 a |
| Gender (Male/Female) | 8/2 | 16/4 | 1.00 b |
| Weight (kg) | 51.2 (46.2–58.6) | 58.35 (53.2–67) | 0.06 a |
| SOFA score | 10 (6.25–12.0) | - | |
| APACHE II score | 20.5 (17.5–26.5) | 6.5 (9–18.3) | <0.0001 a |
| Vasoactive drugs, n(%) | 8 (80%) | - | |
| HIV, n(%) | 09 (90%) | 15 (75%) | 0.64 b |
| Creatinine Clearance (mL/min) | 92.3 (36.0–129.1) | 113.88 (26.5–157.9) | 0.30 a |
| Albumin (g/dL) | 3.1 (2.22–3.6) | 3.5 (3.1–4.1) | 0.04 a |
| ICU (IQR) | Outpatients (IQR) | p-Value | |
|---|---|---|---|
| Cmax (mg/L) | 2.33 (1.2–3.1) | 1.11 (0.9–1.5) | 0.04 |
| AUC0–24 (mg.h/L) | 19.61 (5.4–34) | 5.52 (4.7–7.9) | 0.06 |
| Tmax (h) | 2.0 (2–4) | 2.6 (2–4) | 0.58 |
| ICU (n = 10) | Outpatients (n = 20) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Mean (SD) | Median | %CV | Mean (SD) | Median | %CV | |
| Clearance (L/h) | 1.2 (1.5) | 0.9 | 120.9 | 17.5 (13.3) | 11.1 | 75.8 | <0.0001 |
| Volume (L) | 64.8 (11.7) | 61.1 | 18.1 | 137.2 (55.1) | 170.4 | 40.1 | 0.002 |
| Ka1 (h−1) | 0.72 (0.05) | 0.7 | 7.4 | 0.35 (0.12) | 0.3 | 35.5 | <0.001 |
| Ka2 (h−1) | 0.75 (0.10) | 0.8 | 13.8 | 0.39 (0.18) | 0.4 | 44.9 | <0.001 |
| F | 0.80 (0.06) | 0.8 | 7.9 | 0.14 (0.13) | 0.1 | 87.1 | <0.001 |
| Q (L/h) | 7.3 (3.5) | 6.6 | 48.6 | 2.66 (2.02) | 3.8 | 75.9 | <0.001 |
| Vp (L) | 348.6 (30.1) | 361.6 | 8.6 | 343.3 (78.2) | 400.0 | 22.8 | 0.2 |
| Daily Dose (mg) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC(0–24)/MIC | Cmax/MIC | ||||||||||||||||
| ICU Patients | Outpatients | ICU Patients | Outpatients | ||||||||||||||
| TBW kg | Clcr mL/min | 550 | 825 | 1100 | 1375 | 550 | 825 | 1100 | 1375 | 550 | 825 | 1100 | 1375 | 550 | 825 | 1100 | 1375 |
| 40 | 30 | 47.8 | 52.2 | 56.6 | - | - | - | - | - | 63.0 | 71.9 | 80.6 | - | - | - | - | - |
| 90 | 23.3 | 26.8 | 29.8 | - | 22.1 | 32.7 | 41.2 | - | 43.4 | 50.0 | 56.4 | - | 22.1 | 32.7 | 41.2 | - | |
| 120 | 15.5 | 18.5 | 21.2 | - | 16.9 | 27.0 | 34.9 | - | 36.2 | 43.2 | 48.6 | - | 16.9 | 27.0 | 34.9 | - | |
| 180 | 9.9 | 11.7 | 14.0 | - | 12.7 | 22.0 | 29.6 | - | 29.8 | 36.5 | 42.1 | - | 12.7 | 22.0 | 29.6 | - | |
| 50 | 30 | - | 48.8 | 53.1 | 57.0 | - | 44.3 | 54.1 | 54.1 | - | 65.0 | 73.3 | 81.9 | - | 44.3 | 54.1 | 54.1 |
| 90 | - | 24.9 | 27.8 | 30.7 | - | 30.8 | 39.2 | 46.0 | - | 46.5 | 52.7 | 58.2 | - | 30.8 | 39.2 | 46.0 | |
| 120 | - | 16.6 | 19.6 | 22.3 | - | 25.2 | 33.5 | 40.0 | - | 39.9 | 45.5 | 50.7 | - | 25.2 | 33.5 | 40.0 | |
| 180 | - | 10.7 | 12.4 | 14.8 | - | 20.7 | 28.0 | 34.0 | - | 39.1 | 39.1 | 44.2 | - | 20.7 | 28.0 | 34.0 | |
| 60 | 30 | - | 46.3 | 50.5 | 81.9 | - | 41.6 | 51.2 | 59.9 | - | 61.0 | 67.8 | 75.6 | - | 41.6 | 51.2 | 59.9 |
| 90 | - | 23.2 | 26.2 | 29.1 | - | 29.4 | 37.5 | 43.9 | - | 44.1 | 49.7 | 55.5 | - | 29.4 | 37.5 | 43.9 | |
| 120 | - | 15.2 | 18.3 | 21.0 | - | 24.0 | 31.8 | 38.3 | - | 37.4 | 43.3 | 21.0 | - | 24.0 | 31.8 | 38.3 | |
| 180 | - | 9.6 | 11.5 | 13.7 | - | 19.5 | 26.6 | 32.7 | - | 31.8 | 37.3 | 42.2 | - | 19.5 | 26.6 | 32.7 | |
| 70 | 30 | - | - | 48.1 | 52.7 | - | - | 49.1 | 57.1 | - | - | 64.2 | 71.3 | - | - | 49.1 | 57.1 |
| 90 | - | - | 25.2 | 27.9 | - | - | 35.8 | 42.3 | - | - | 47.4 | 53.3 | - | - | 35.8 | 42.3 | |
| 120 | - | - | 17.2 | 19.9 | - | - | 30.8 | 36.8 | - | - | 41.3 | 46.4 | - | - | 30.8 | 36.8 | |
| 180 | - | - | 10.6 | 12.8 | - | - | 25.5 | 31.7 | - | - | 35.7 | 40.5 | - | - | 25.5 | 31.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beraldi-Magalhaes, F.; Parker, S.L.; Sanches, C.; Sousa Garcia, L.; Souza Carvalho, B.K.; Fachi, M.M.; de Liz, M.V.; Pontarolo, R.; Lipman, J.; Cordeiro-Santos, M.; et al. Is Dosing of Ethambutol as Part of a Fixed-Dose Combination Product Optimal for Mechanically Ventilated ICU Patients with Tuberculosis? A Population Pharmacokinetic Study. Antibiotics 2021, 10, 1559. https://doi.org/10.3390/antibiotics10121559
Beraldi-Magalhaes F, Parker SL, Sanches C, Sousa Garcia L, Souza Carvalho BK, Fachi MM, de Liz MV, Pontarolo R, Lipman J, Cordeiro-Santos M, et al. Is Dosing of Ethambutol as Part of a Fixed-Dose Combination Product Optimal for Mechanically Ventilated ICU Patients with Tuberculosis? A Population Pharmacokinetic Study. Antibiotics. 2021; 10(12):1559. https://doi.org/10.3390/antibiotics10121559
Chicago/Turabian StyleBeraldi-Magalhaes, Francisco, Suzanne L. Parker, Cristina Sanches, Leandro Sousa Garcia, Brenda Karoline Souza Carvalho, Mariana Millan Fachi, Marcus Vinicius de Liz, Roberto Pontarolo, Jeffrey Lipman, Marcelo Cordeiro-Santos, and et al. 2021. "Is Dosing of Ethambutol as Part of a Fixed-Dose Combination Product Optimal for Mechanically Ventilated ICU Patients with Tuberculosis? A Population Pharmacokinetic Study" Antibiotics 10, no. 12: 1559. https://doi.org/10.3390/antibiotics10121559
APA StyleBeraldi-Magalhaes, F., Parker, S. L., Sanches, C., Sousa Garcia, L., Souza Carvalho, B. K., Fachi, M. M., de Liz, M. V., Pontarolo, R., Lipman, J., Cordeiro-Santos, M., & Roberts, J. A. (2021). Is Dosing of Ethambutol as Part of a Fixed-Dose Combination Product Optimal for Mechanically Ventilated ICU Patients with Tuberculosis? A Population Pharmacokinetic Study. Antibiotics, 10(12), 1559. https://doi.org/10.3390/antibiotics10121559







