Anti-Herpes Simplex Virus Efficacy of Silk Cocoon, Silkworm Pupa and Non-Sericin Extracts
Abstract
:1. Introduction
2. Results
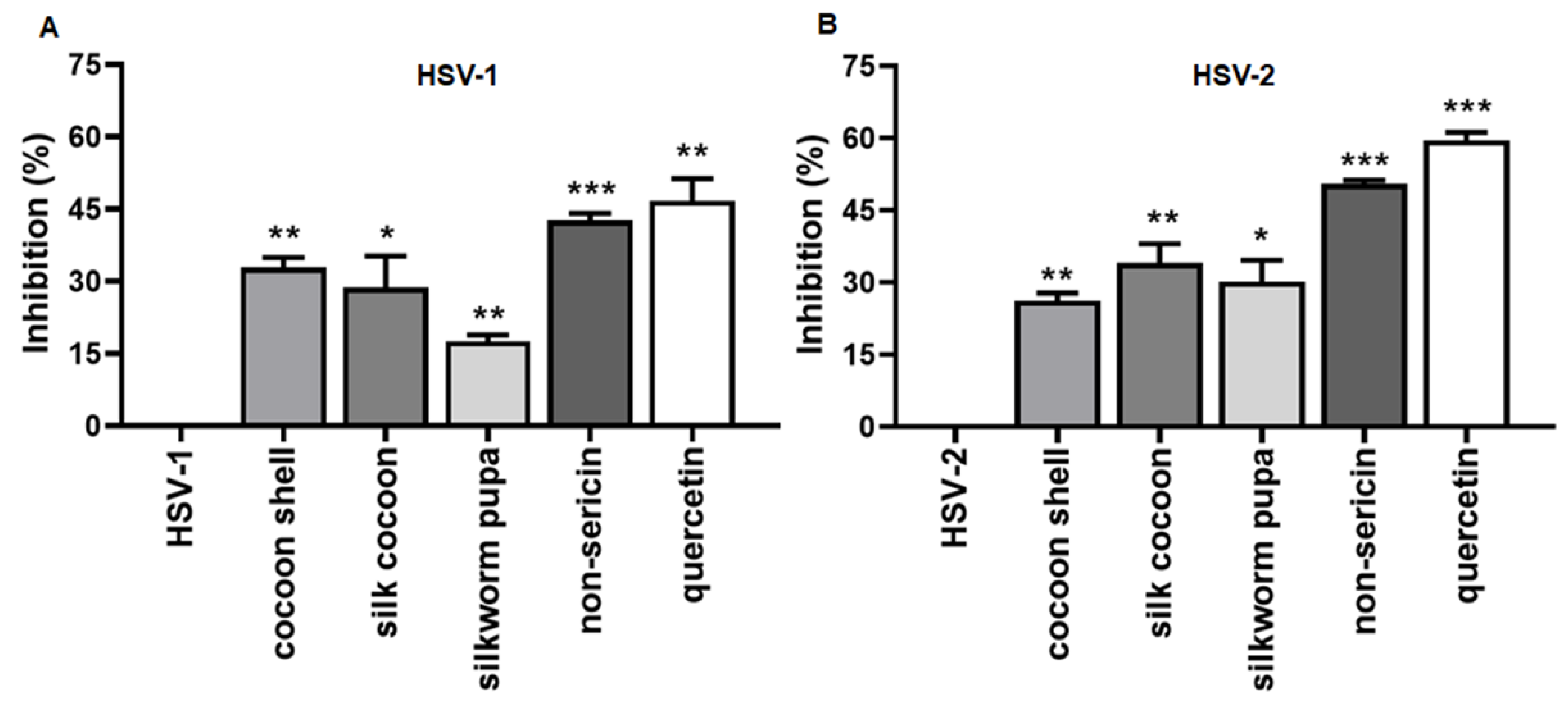
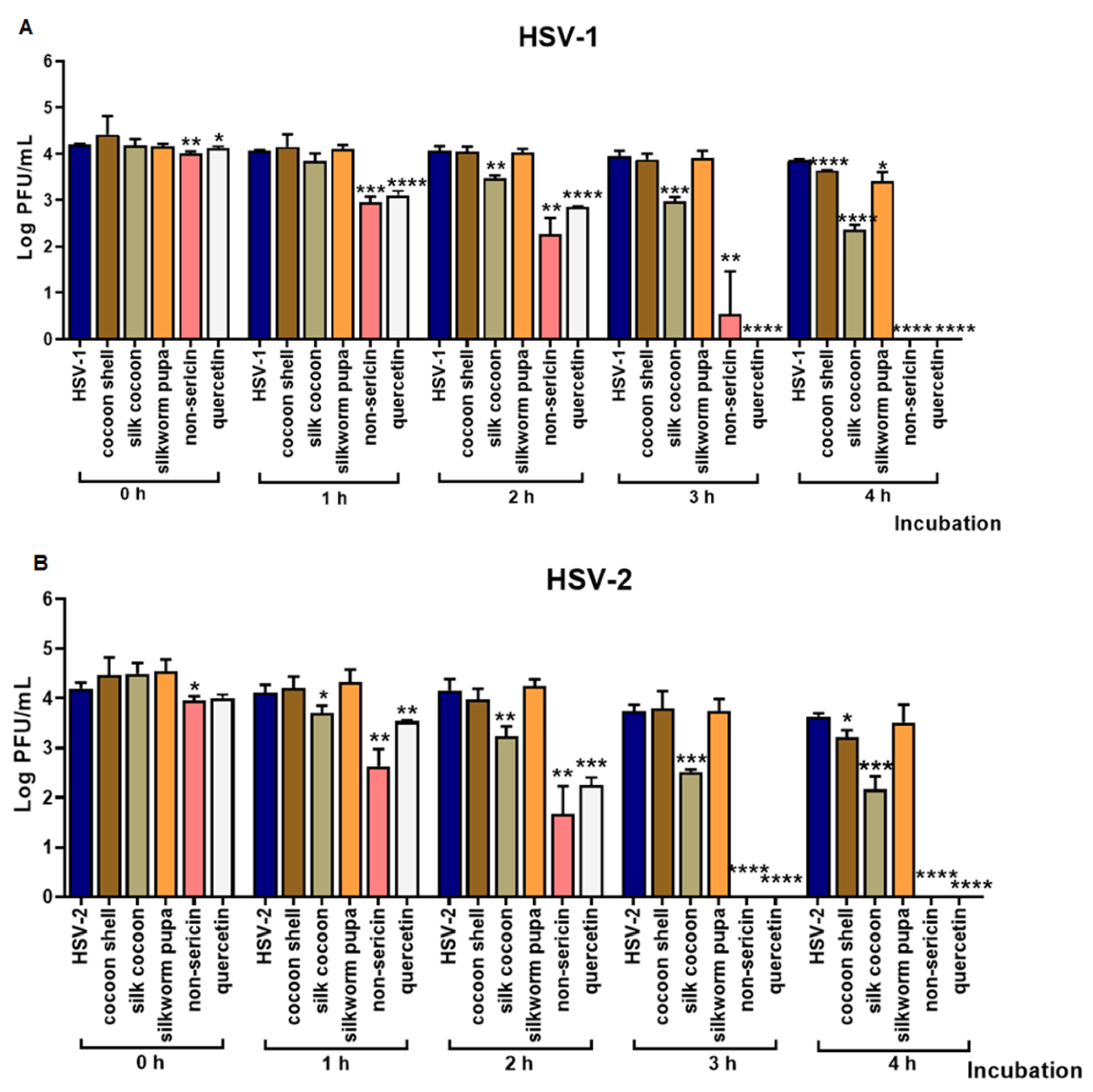
2.1. The Inhibitory Effect of Silk Cocoon and Silkworm Pupa Extracts against HSV-1 and HSV-2 Infection via Blocking Binding Activity in Vero Cell
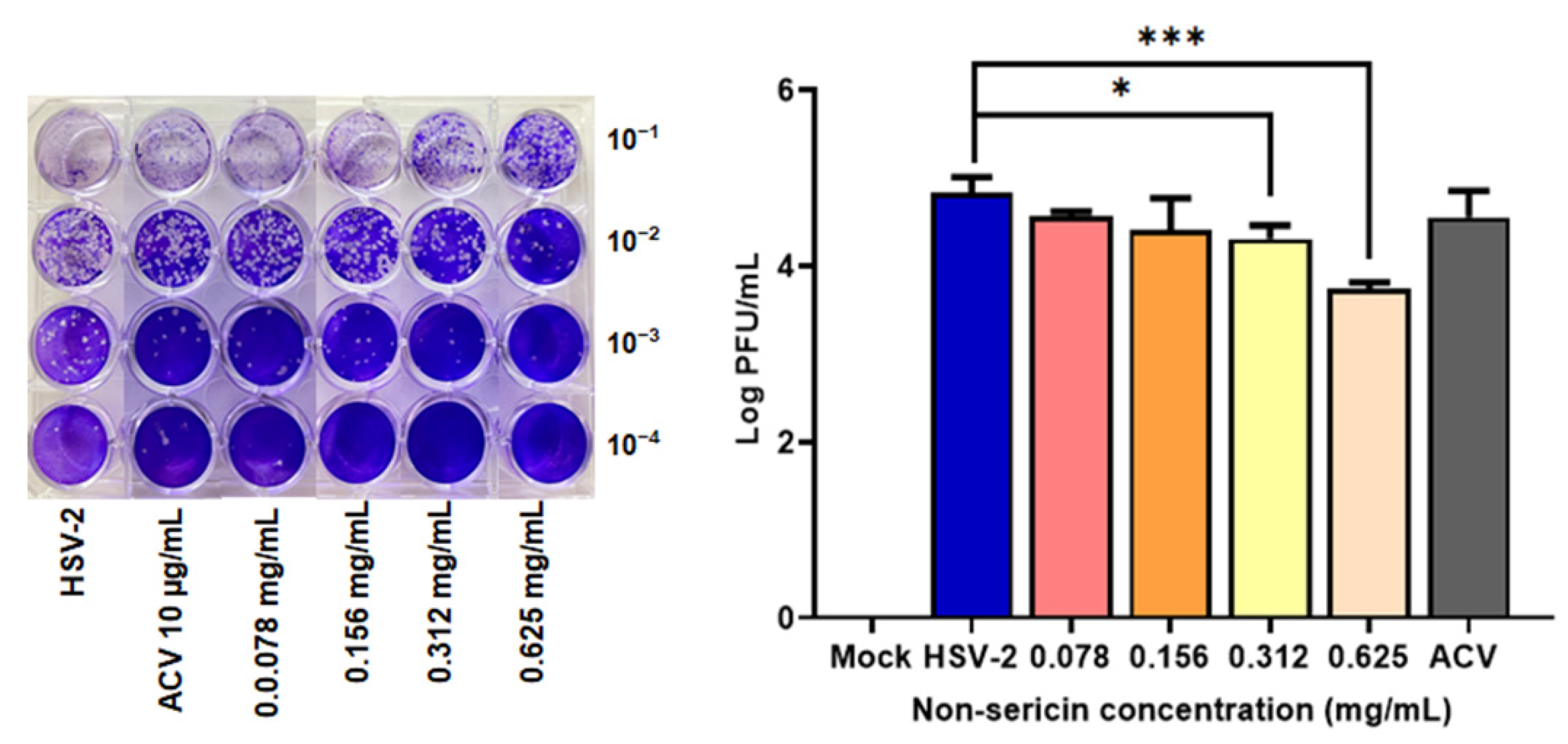
2.2. Non-Sericin Extract Reduced HSV-2 Infection in HeLa Cell
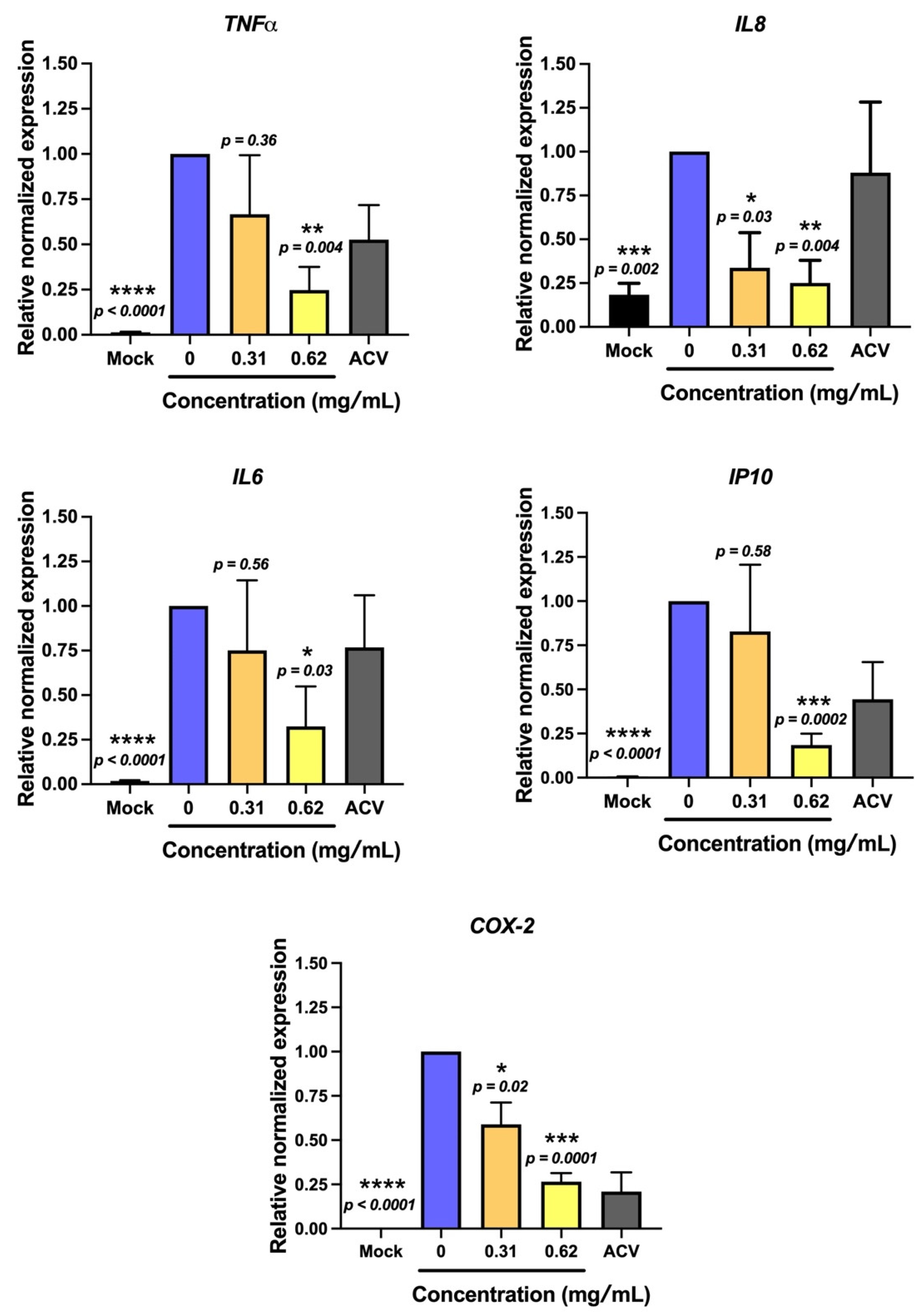
2.3. Non-Sericin Extract Protected HSV-2-Induced Cell Death and Inflammation in HeLa Cells
2.4. Bioactive Compounds in Silk Cocoon Extracts
3. Discussion
4. Materials and Methods
4.1. Silk Cocoon and Silkworm Pupa Extraction
4.2. Cell Culture and Virus Propagation
4.3. Anti-Herpes Simplex Virus Activity
4.3.1. Cytotoxicity of Silk Extract on Vero Cell
4.3.2. Viral-Host Cell Receptor Binding Activity Assays
4.3.3. Direct Virus Inactivation Assay
4.4. Antiviral Effect of Non-Sericin Extract in Human Cervical Adenocarcinoma Cell Line (Hela)
4.4.1. Anti HSV-2 Efficacy of Non-Sericin Extracts in Hela Cells
4.4.2. Effect of HSV Infection on HeLa Cell Viability and Protective Effect of Non-Sericin Extracts
4.4.3. Effect of HSV Infection on Inducing Inflammation in HeLa and the Inhibitory Effect of Non-Sericin Extracts
4.5. The Antioxidant Activity and Quantification of Total Phenolic Compounds of Silk Cocoon and Pupa Extracts
4.5.1. Investigation of Antioxidant Activity of Silk Cocoon and Pupa Extracts
4.5.2. Investigation of Total Phenolic Compound
4.5.3. Investigation of Total Flavonoid Compound
4.5.4. High-Performance Liquid Chromatography (HPLC)
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchi, S.; Trombetta, C.M.; Gasparini, R.; Temperton, N.; Montomoli, E. Epidemiology of herpes simplex virus type 1 and 2 in Italy: A seroprevalence study from 2000 to 2014. J. Prev. Med. Hyg. 2017, 58, E27–E33. [Google Scholar]
- Davison, A.J. Herpesvirus systematics. Vet. Microbiol. 2010, 143, 52–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez, F.J.; Farías, M.A.; Gonzalez-Troncoso, M.P.; Corrales, N.; Duarte, L.F.; Retamal-Díaz, A.; González, P.A. Experimental dissection of the lytic replication cycles of herpes simplex viruses in vitro. Front Microbiol. 2018, 9, 1–23. [Google Scholar] [CrossRef]
- Zaichick, S.V.; Bohannon, K.P.; Smith, G.A. Peripheral tissues, alphaherpesviruses and the cytoskeleton in neuronal infections. Viruses 2011, 3, 941–981. [Google Scholar] [CrossRef] [PubMed]
- Chayavichitsilp, P.; Buckwalter, J.V.; Krakowski, A.C.; Friedlander, S.F. Herpes Simplex. Pediatr. Rev. 2009, 30, 119–130. [Google Scholar] [CrossRef]
- Sanders, J.E.; Garcia, S.E. Pediatric Herpes Simplex Virus Infections: An Evidence-Based Approach to Treatment. Pediatr. Emerg. Med. Pract. 2014, 11, 1–19. [Google Scholar]
- Smith, T.T.; Whitley, R.J. SECTION 8 Clinical Microbiology: Viruses. Infect. Dis. 2017, 1426–1438. [Google Scholar]
- Lachmann, R. Herpes simplex virus latency. Expert Rev. Mol. Med. 2003, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–762. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Babar, M.; Najam-us-Sahar, S.Z.; Ashraf, M.; Kazi, A.G. Antiviral drug therapy exploiting medicinal plants. J. Antivir Antiretrovir. 2013, 5, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Wisskirchen, K.; Lucifora, J.; Michler, T.; Protzer, U. New pharmacological strategies to fight enveloped viruses. Trends Pharmacol. Sci. 2014, 35, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dev, P.; Kumar, R.V. Chapter 3 Biomedical Applications of Silkworm Pupae Proteins. In Biomedical Applications of Natural Proteins; Kumar, D., Kundapur, R., Eds.; Springer: New Delhi, India, 2015; pp. 40–49. [Google Scholar]
- Zhang, Y.Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Wongputtaraksa, T.; Ratanavaraporn, J.; Pichyangkura, R.; Damrongsakkul, S. Surface modification of Thai silk fibroin scaffolds with gelatin and chitooligosaccharide for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2307–2315. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhang, Y.Q. Processing and Characterization of Silk Sericin from Bombyx mori and Its Application in Biomaterials and Biomedicines. Mater. Sci. Eng. 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Prommuak, C.; De-Eknamkul, W.; Shotipruk, A. Extraction of flavonoids and carotenoids from Thai silk waste and antioxidant activity of extracts. Sep. Purif. Technol. 2008, 62, 444–448. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates 2016. Bull. World Health Organ. 2020, 98, 315. [Google Scholar] [CrossRef]
- Whitley, R.J.; Roizman, B. Herpes Simplex Virus Infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Kaewkorn, W.; Limpeanchob, N.; Tiyaboonchai, W.; Pongcharoen, S.; Sutheerawattananonda, M. Effects of Silk Sericin on the Proliferation and Apoptosis of Colon Cancer Cells. Biol. Res. 2012, 45, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Kunz, R.I.; Brancalhão, R.M.; Ribeiro, L.D.; Natali, M.R. Silkworm sericin: Properties and biomedical applications. Biomed Res. Int. 2016, 14, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, J.; Mondal, I.H.; Sheikh, R.K.; Habib, A. Extraction, Structural and Functional Properties of Silk Sericin Biopolymer from Bombyx mori Silk Cocoon Waste. Int. J. Text. Sci. 2019, 9, 1–5. [Google Scholar] [CrossRef]
- Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Schuksz, M.; Fuster, M.M.; Brown, J.R.; Crawford, B.E.; Ditto, D.P.; Lawrence, R.; Glass, C.; Wang, L.; Tor, Y.; Esko, J.D. Surfen, a small molecule antagonist of heparan sulfate. Proc. Natl. Acad. Sci. USA 2008, 105, 13075–13080. [Google Scholar] [CrossRef] [Green Version]
- Aksyuk, A.A.; Newcomb, W.W.; Cheng, N.; Winkler, D.C.; Fontana, J.; Heymann, J.B.; Steven, A.C. Subassemblies and Asymmetry in Assembly of Herpes Simplex Virus Procapsid. Mbio 2015, 6, e01525-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luganini, A.; Nicoletto, S.F.; Pizzuto, L.; Pirri, G.; Giuliani, A.; Landolfo, S.; Gribaudo, G. Inhibition of Herpes Simplex Virus Type 1 and Type 2 Infections by Peptide-Derivatized Dendrimers. Antimicrob. Agents Chemother. 2011, 55, 3231–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gescher, K.; Kühn, J.; Lorentzen, E.; Hafezi, W.; Derksen, A.; Deters, A.; Hensel, A. Proanthocyanidin-enriched extract from Myrothamnus flabellifolia Welw. exerts antiviral activity against herpes simplex virus type 1 by inhibition of viral adsorption and penetration. J. Ethnopharmacol. 2011, 134, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Nana, H.M.; Ngane, R.A.N.; Gatsing, D.; Tiabou Tchinda, A.T.; Rovito, R.; Cagno, V.; Cagliero, C.; Boyom, F.F.; Rubiolo, F.; et al. In Vitro Anti-Herpes Simplex Virus Activity of Crude Extract of the Roots of Nauclea Latifolia Smith (Rubiaceae). BMC Complementary Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Jin, F.; Zhuo, C.; He, Z.; Wang, H.; Liu, W.; Zhang, R.; Wang, Y. Anti-herpes simplex virus activity of polysaccharides from Eucheuma gelatinae. World J. Microb. Biot. 2015, 31, 1–8. [Google Scholar] [CrossRef]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 Activity of Terminalia Chebula Retz Extract and Its Constituents, Chebulagic and Chebulinic Acids. BMC Complementary Altern. Med. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichling, J.; Neuner, A.; Sharaf, M.; Harkenthl, M.; Schnitzler, P. Antiviralactivity of Rhus aromatic (fragrant sumac) extracts against two types of hepes simplex viruses in cell culture. Pharmazie 2009, 64, 538–541. [Google Scholar] [PubMed]
- Lückemeyer, D.D.; Müller, V.D.M.; Moritz, M.I.G.; Stoco, P.H.; Schenkel, E.P.; Barardi, C.R.M.; Reginatto, F.H.; Simões, C.M.O. Effects of Ilex paraguariensis A.St.Hil.(yerba mate) on herpes simplex virus type 1 and 2 replication. Phytother. Res. 2012, 26, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, R.; Zhou, J.; Wang, M.; Yin, Z.; Cheng, A. Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections. Viruses 2016, 8, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidry, J.T.; Scott, R.S. The interaction between human papillomavirus and other viruses. Virus Res. 2017, 231, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Zhang, M.; Fu, M.; Luo, S.; Hu, Q. Herpes simplex virus type 2 immediate early protein ICP27 inhibits IFN-β production in mucosal epithelial cells by antagonizing IRF3 activation. Front. Immunol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Tamura, Y.; Nakajima, K.; Nagayasu, K.; Takabayashi, C. Flavonoid 5-Glucosides from the Cocoon Shell of the Silkworm, Bombyx mori. Phytochemistry 2002, 59, 275–278. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Izumi, H.; Kanamoto, T.; Tamada, Y.; Nakashima, H. Sulfated fibroin, a novel sulfated peptide derived from silk, inhibits human immunodeficiency virus replication in vitro. Biosci. Biotechnol. Biochem. 2000, 68, 1664–1670. [Google Scholar] [CrossRef]
- Jacob, J.R.; Mansfield, K.; You, J.E.; Tennant, B.C.; Kim, Y.H. Natural Iminosugar Derivatives of 1-Deoxynojirimycin Inhibit Glycosylation of Hepatitis Viral Envelope Proteins. J. Microbiol. 2007, 45, 43–440. [Google Scholar]
- Ponnuvel, K.M.; Nakazawa, H.; Furukawa, s.; Asaoka, A.; Ishibashi, J.; Tanaka, H.; Yamakawa, M. A lipase isolated from the silkworm Bombyx mori shows antiviral activity against nucleopolyhedrovirus. J. Virol. 2003, 77, 10725–10729. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, H.; Wei, B. Immune response of T cells during herpes simplex. J. Zhejiang Univ. Sci. B 2017, 18, 277–288. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wang, Y.-J.; Zhou, L.-X.; Zhuab, L.; Zhang, Y.-Q. Isolation and Bioactivities of a Non-Sericin Component from Cocoon Shell Silk Sericin of the Silkworm Bombyx mori. Food Funct. 2012, 3, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sakudoh, T.; Sezutsu, H.; Nakashima, T.; Kobayashi, I.; Fujimoto, H.; Uchino, K.; Banno, Y.; Iwano, H.; Maekawa, H.; Tamura, T.; et al. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the yellow blood gene. Proc. Natl. Acad. Sci. USA 2007, 104, 8941–8946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurioka, A.; Yamazaki, M. Purification and identification of flavonoids from the yellow green cocoon shell (Sasammayu) of the silkworm. Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 1396–1399. [Google Scholar] [CrossRef] [Green Version]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef]
- Hung, P.Y.; Ho, B.C.; Lee, S.Y.; Chang, S.Y.; Kao, C.L.; Lee, S.S.; Lee, C.N. Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, H.H.; Shin, Y.S.; Kang, H.; Cho, H. The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch. Pharmacal Res. 2017, 40, 623–630. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, J.E.; Song, Y.J. Antiviral activities of quercetin and isoquercitrin against human herpesviruses. Molecules 2020, 25, 2379. [Google Scholar] [CrossRef]


| Type of Extracts | Total Phenolic | Total Flavonoid | Antioxidant Activity | |
|---|---|---|---|---|
| (mg GAE/g Extract) | (µg QAE/g Extract) | DPPH Method ABTS Method | ||
| (mg GAE/g Extract) | (mg TEAC/g Extract) | |||
| Cocoon Shell | 8.18 ± 1.73 | 0.71 ± 002 | ND | ND |
| Silk Cocoon | 34.29 ± 2.03 | 0.57 ± 0.04 | 8.27 ± 0.90 | 40.45 ± 2.77 |
| Silkworm Pupa | 54.57 ± 3.16 * | 2.02 ± 0.17 | 60.57 ± 5.81 * | 275.06 ± 12.07 * |
| Non-Sericin | 36.47 ± 5.57 | 6.33 ± 0.39 * | 10.09 ± 0.81 | 46.01 ± 5.25 |
| Chemical Profile of Non-Sericin Extract | |
|---|---|
| Total gallic acid compounds (mg GAE/g extract) | 3.35 ± 0.55 |
| Total quercetin compounds (mg QAE/g extract) | 1.67 ± 0.15 |
| Total xanthophyll compounds (µg Xanthophyll/g extract) | 365.00 ± 13.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantakee, K.; Prangkio, P.; Panya, A.; Tragoolpua, Y. Anti-Herpes Simplex Virus Efficacy of Silk Cocoon, Silkworm Pupa and Non-Sericin Extracts. Antibiotics 2021, 10, 1553. https://doi.org/10.3390/antibiotics10121553
Jantakee K, Prangkio P, Panya A, Tragoolpua Y. Anti-Herpes Simplex Virus Efficacy of Silk Cocoon, Silkworm Pupa and Non-Sericin Extracts. Antibiotics. 2021; 10(12):1553. https://doi.org/10.3390/antibiotics10121553
Chicago/Turabian StyleJantakee, Kanyaluck, Panchika Prangkio, Aussara Panya, and Yingmanee Tragoolpua. 2021. "Anti-Herpes Simplex Virus Efficacy of Silk Cocoon, Silkworm Pupa and Non-Sericin Extracts" Antibiotics 10, no. 12: 1553. https://doi.org/10.3390/antibiotics10121553
APA StyleJantakee, K., Prangkio, P., Panya, A., & Tragoolpua, Y. (2021). Anti-Herpes Simplex Virus Efficacy of Silk Cocoon, Silkworm Pupa and Non-Sericin Extracts. Antibiotics, 10(12), 1553. https://doi.org/10.3390/antibiotics10121553






